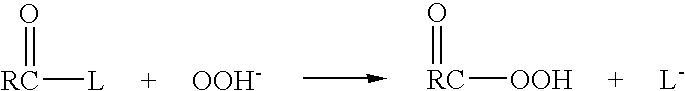Patents
Literature
202 results about "Boron trichloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Boron trichloride is the inorganic compound with the formula BCl₃. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water.
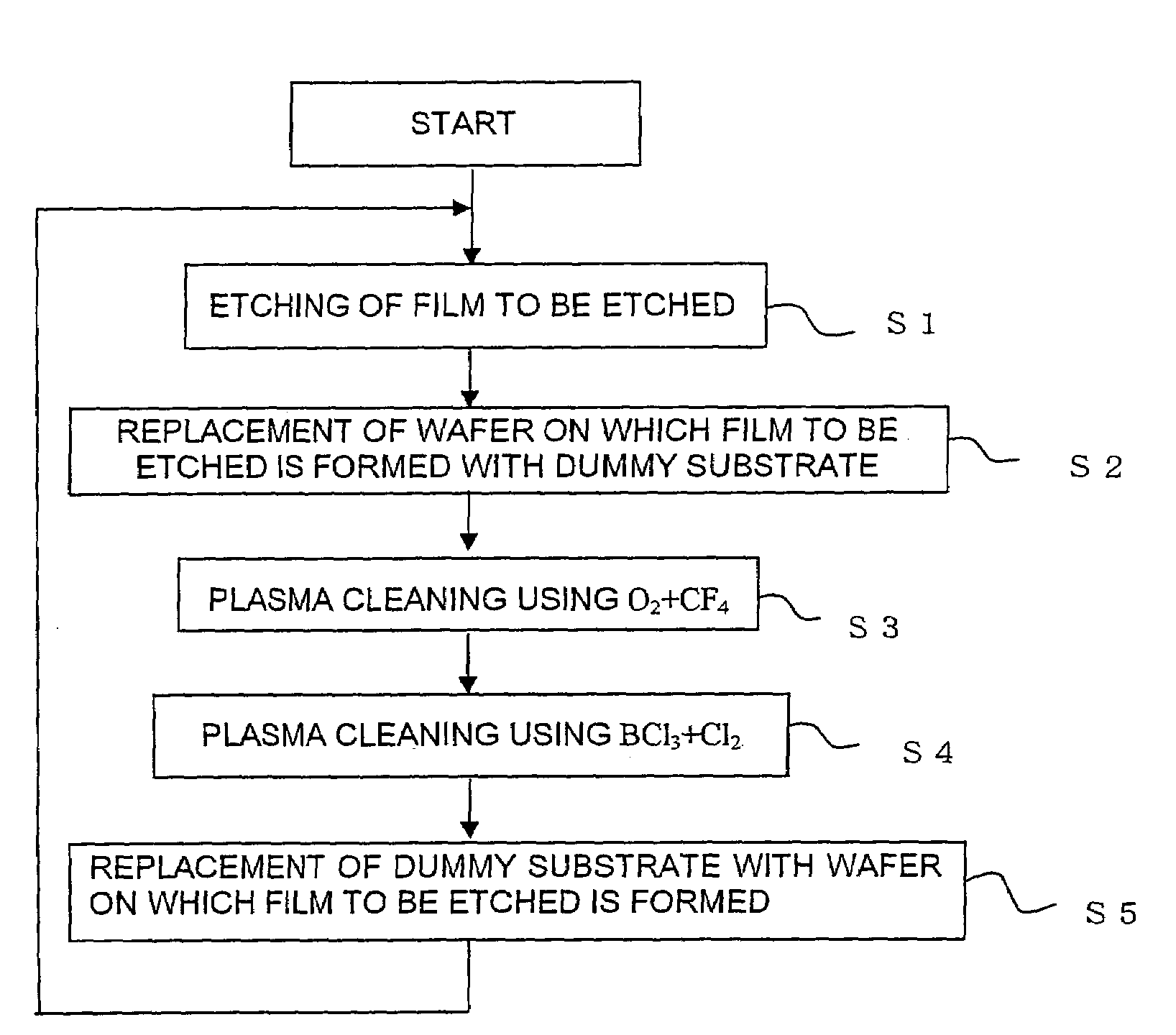
Method of cleaning etching apparatus
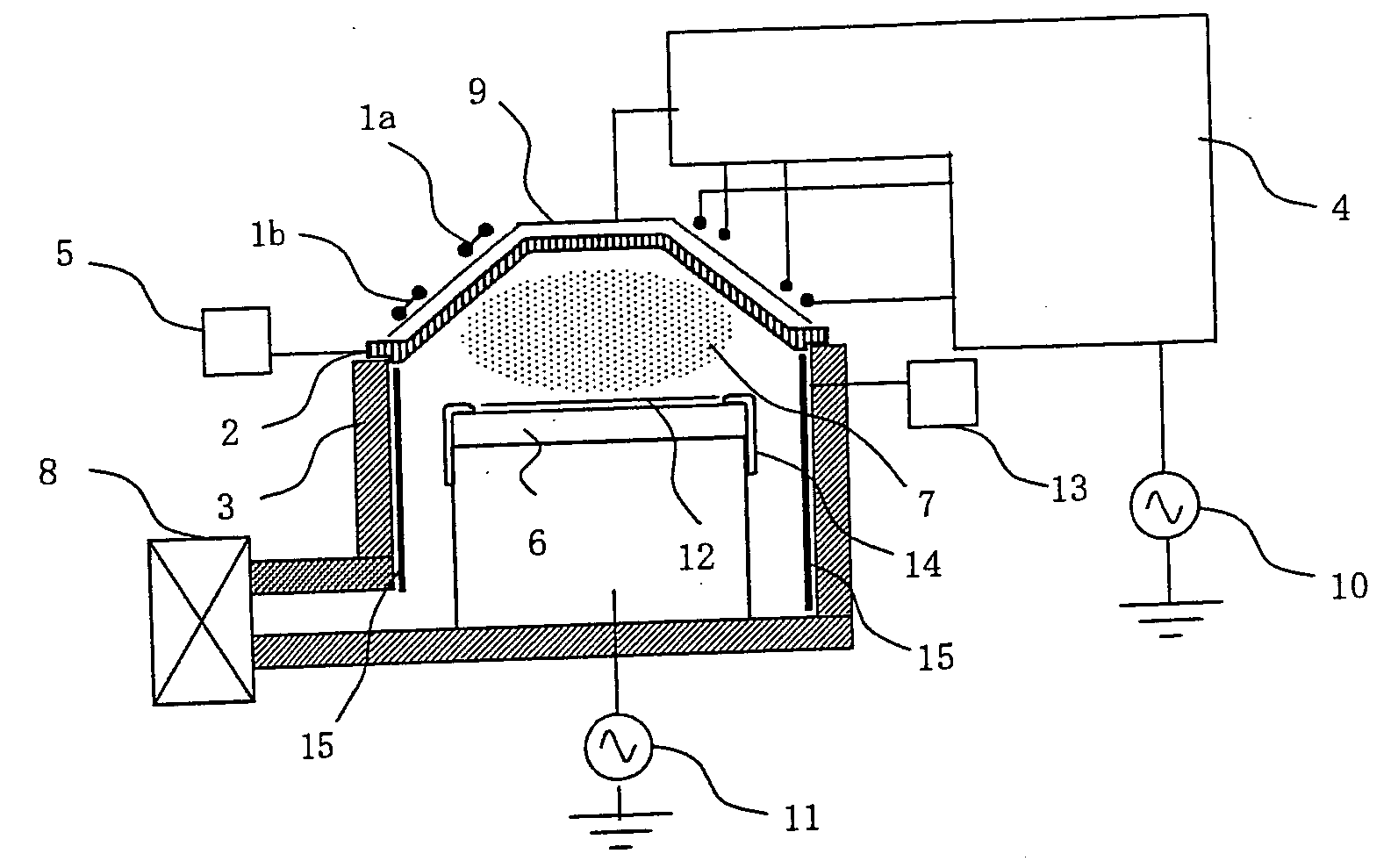
ActiveUS20060191555A1Clean interiorMaintain repeatabilityHollow article cleaningElectrostatic cleaningBoron trichlorideOxygen
To provide a cleaning method for an etching apparatus for a metal film that efficiently removes an etching residue deposited in an etching process chamber, assures the reproducibility of the etching performance, and keeps the etching process chamber in a low-dust-emission condition. Each time one workpiece with a metal film is etched (S1), the interior of the vacuum chamber is cleaned by replacing the workpiece with a dummy substrate (S2), performing a first step of plasma processing using oxygen (O2) and carbon tetrafluoride (CF4) to remove a carbon-based deposit pile (S3), and performing a second step of plasma processing using boron trichloride (BCl3) and chlorine (Cl2) to remove a residue that could not be removed by the first step and an etching residue of the metal film (S4).
Owner:HITACHI HIGH-TECH CORP
Reactive formulations for a neutralization of toxic industrial chemicals
InactiveUS7125497B1Efficiently neutralizedHydrogen peroxideLiquid degasificationBoron trichlorideMalathion
Decontamination formulations for neutralization of toxic industrial chemicals, and methods of making and using same. The formulations are effective for neutralizing malathion, hydrogen cyanide, sodium cyanide, butyl isocyanate, carbon disulfide, phosgene gas, capsaicin in commercial pepper spray, chlorine gas, anhydrous ammonia gas; and may be effective at neutralizing hydrogen sulfide, sulfur dioxide, formaldehyde, ethylene oxide, methyl bromide, boron trichloride, fluorine, tetraethyl pyrophosphate, phosphorous trichloride, arsine, and tungsten hexafluoride.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Method of patterning lead zirconium titanate and barium strontium titanate
In an embodiment of the present invention, a method is provided of patterning PZT layers or BST layers. For example, a PZT layer or a BST layer is plasma etched through a high-temperature-compatible mask such as a titanium nitride (TiN) mask, using a plasma feed gas comprising as a primary etchant boron trichloride (BCl3) or silicon tetrachloride (SiCi4). Although BCl3 or SiCl4 may be used alone as the etchant plasma source gas, it is typically used in combination with an essentially inert gas. Preferably the essentially inert gas is argon. Other potential essentially inert gases which may be used include xenon, krypton, and helium. In some instances O2 or N2, or Cl2, or a combination thereof may be added to the primary etchant to increase the etch rate of PZT or BST relative to adjacent materials, such as the high-temperature-compatible masking material. A TiN masking material can easily be removed without damaging underlying oxides. The selectivity of PZT or BST relative to TiN is very good, with the ratio of the etch rate of the PZT film to the etch rate of the TiN mask typically being better than 20:1. In addition, the etch rate for PZT using a BCl3-comprising plasma source gas is typically in excess of 2,000 Å per minute. A substrate bias power is applied to direct ions produced from the BCl3 or SiCl4 toward the surface to be etched. The bias power is controlled to avoid sputtering of a conductive layer or layers in contact with the PZT layer, so that the surface of the etched PZT is not contaminated by a conductive material, which can cause the semiconductor device which includes the patterned PZT to short out.
Owner:APPLIED MATERIALS INC
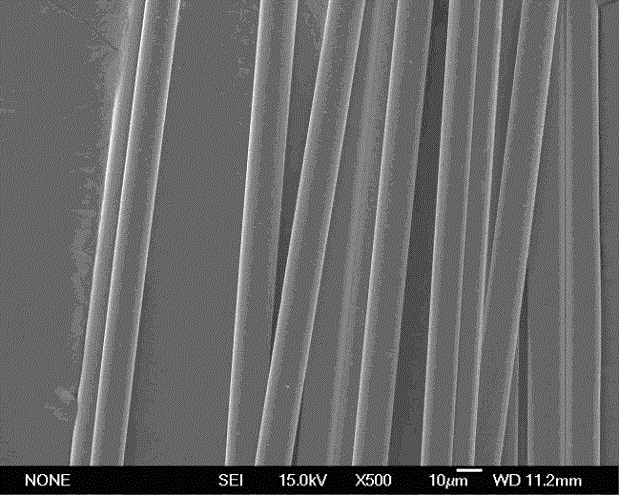
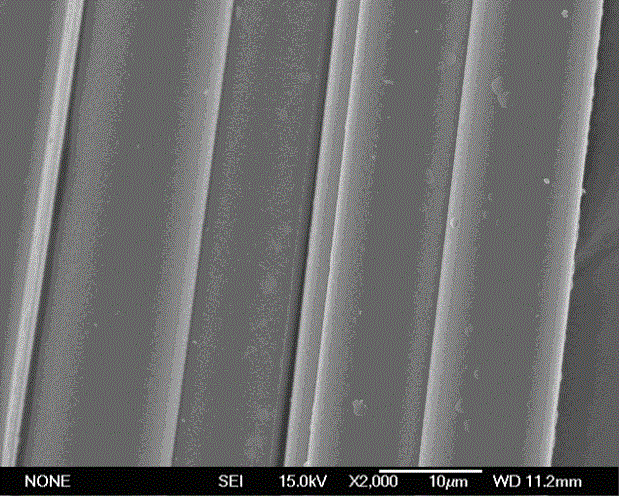
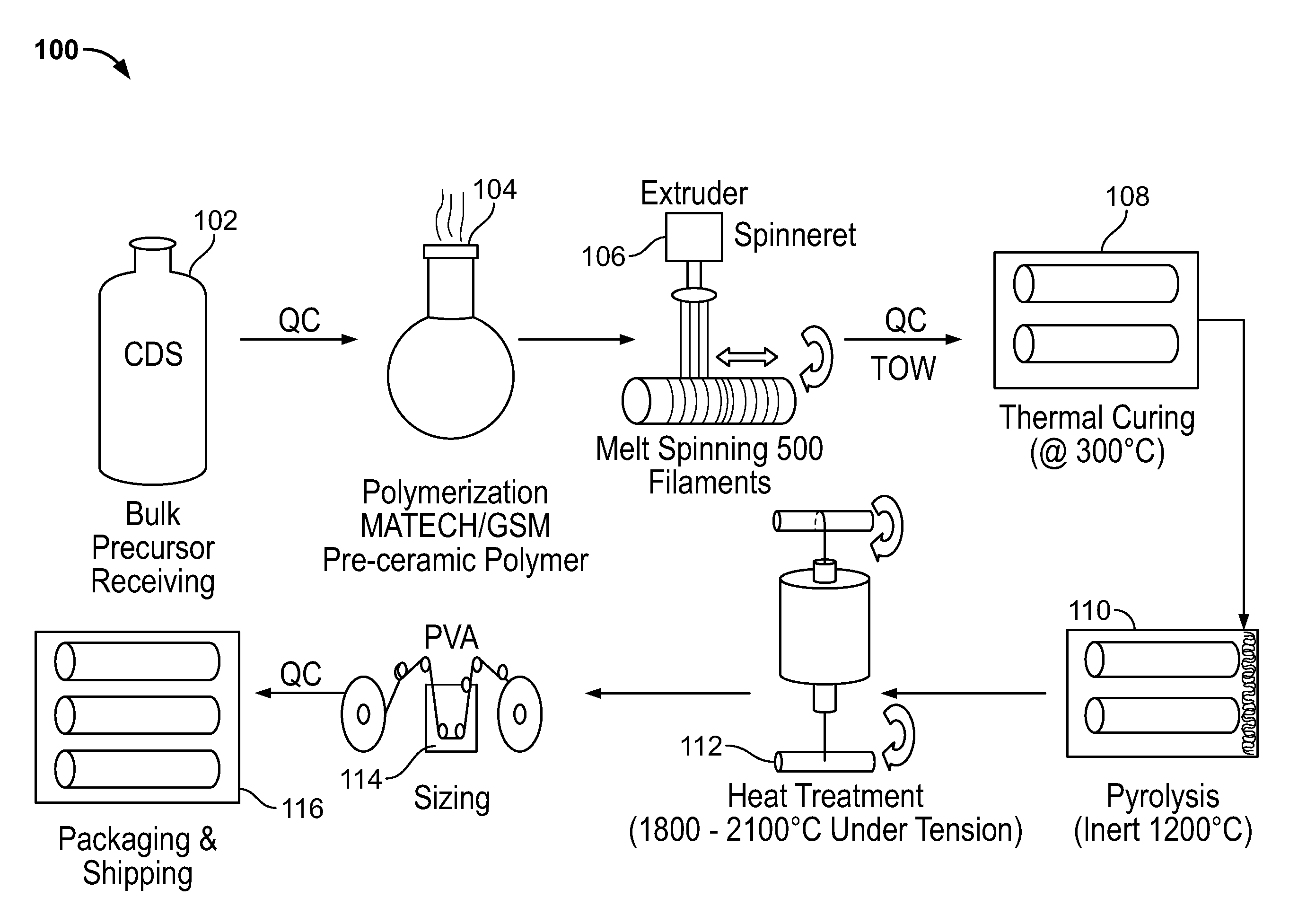
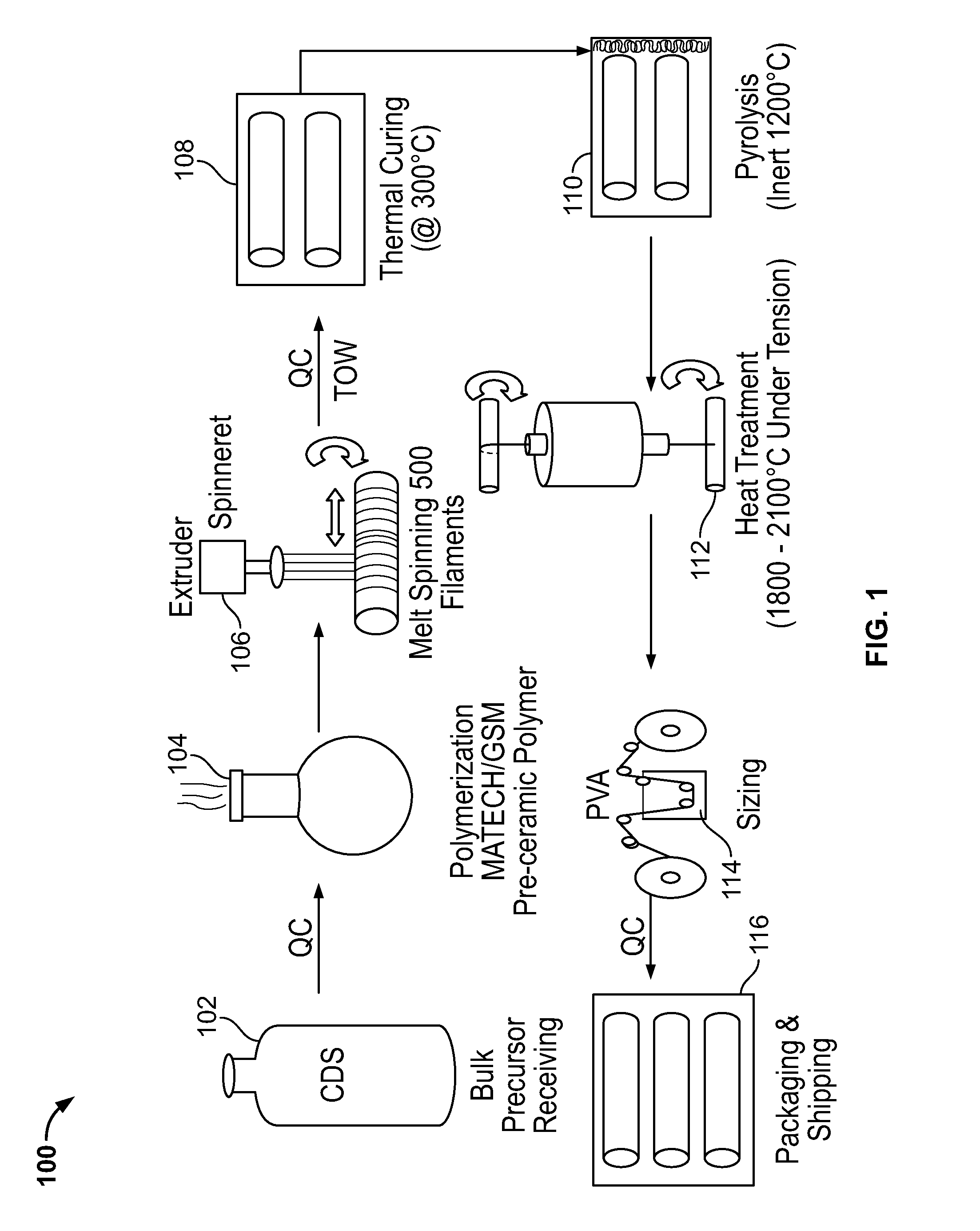
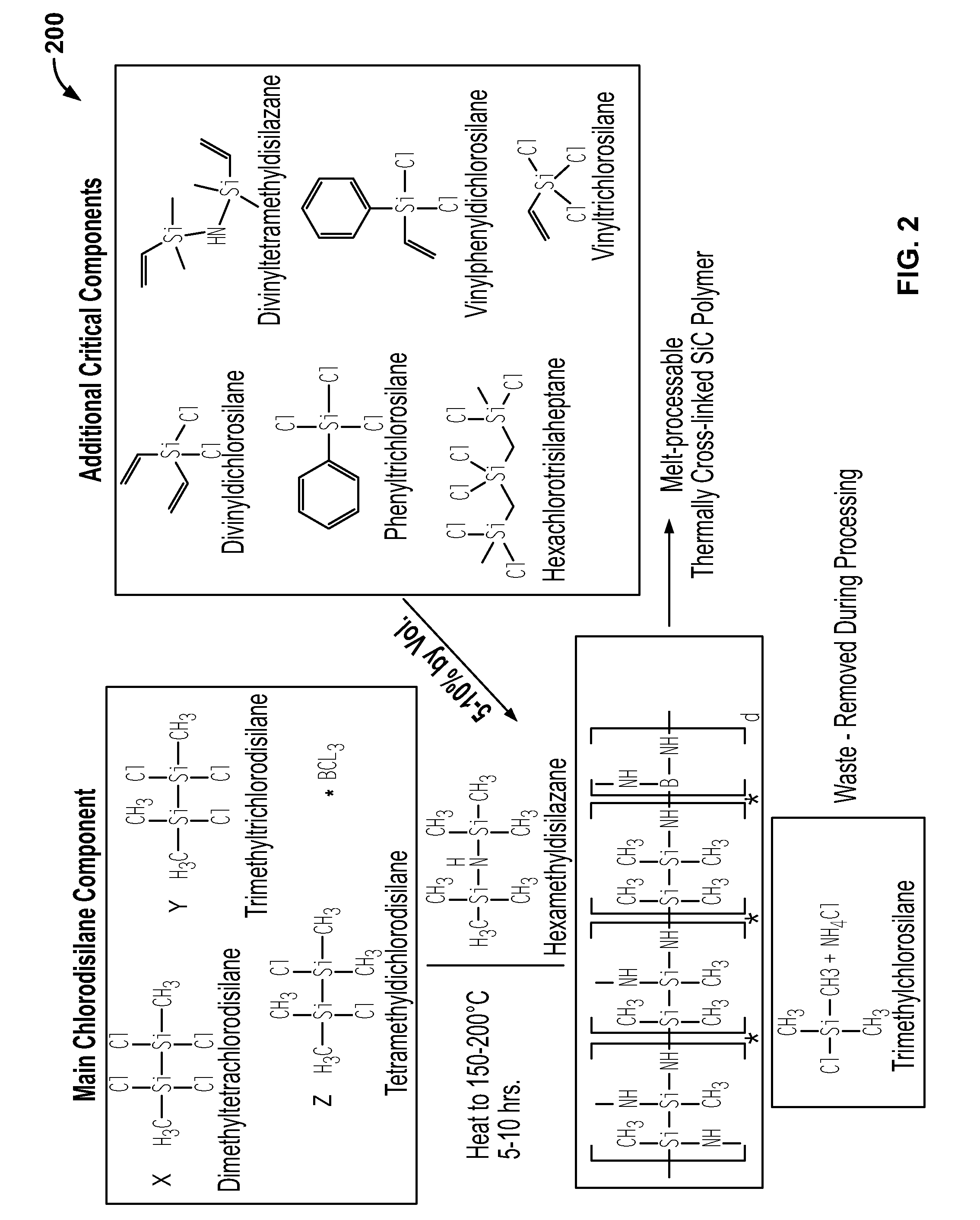
Stiochiometric silicon carbide fibers from thermo-chemically cured polysilazanes
ActiveUS20110212329A1Reduce manufacturing complexityReduce property variabilityNitrogen compoundsLayered productsPolymer sciencePolymer resin
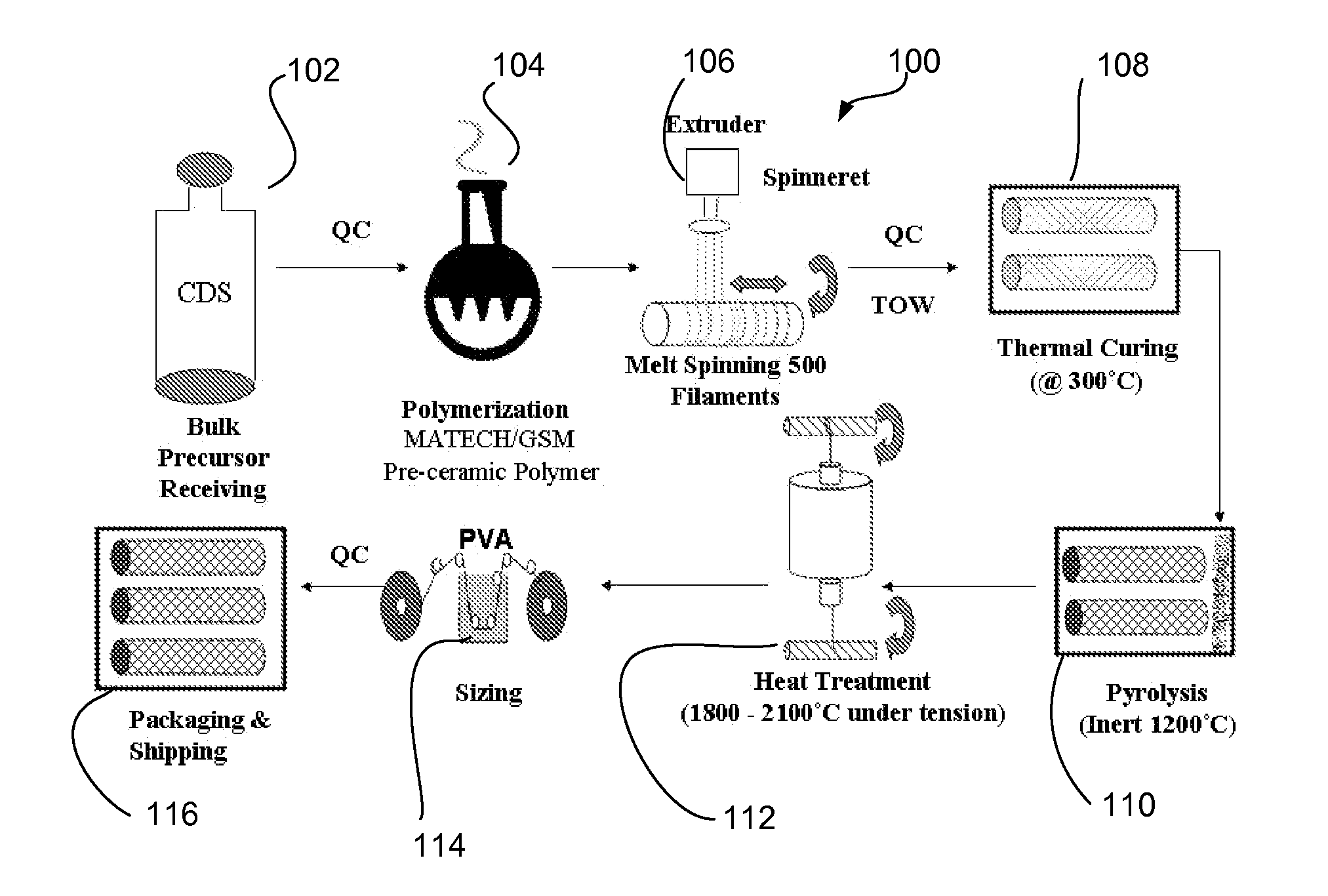
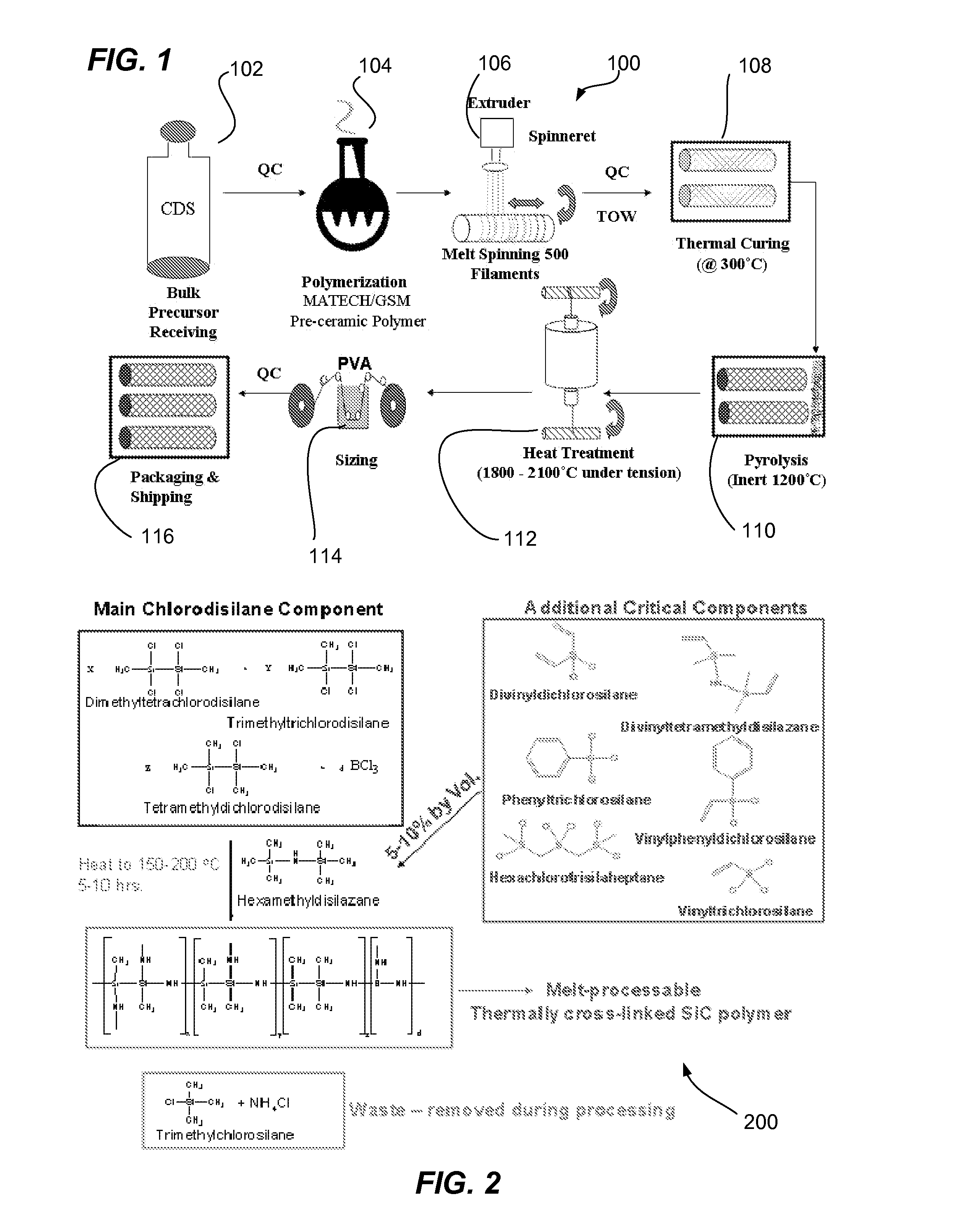
A novel polycrystalline stoichiometric fine SiC fiber substantially free of impurities is produced using a novel pre-ceramic polymer. The pre-ceramic polymer is prepared by reacting a mixture of chlorodisilane, boron trichloride, and a vinyl chlorodisilane with an excess of hexamethyldisilazane to form the pre-ceramic polymer resin, which may then be melt-spun, cured, pyrolyzed and heat-treated to obtain the finished SiC fiber. The manufacturing process for the production of the fine SiC ceramic fiber allows for flexibility with respect to cross-linking, in that low-cost thermal treatments may replace more complex methods, while obtaining fibers with improved materials properties as compared to currently available SiC fibers.
Owner:GENERAL ELECTRIC CO
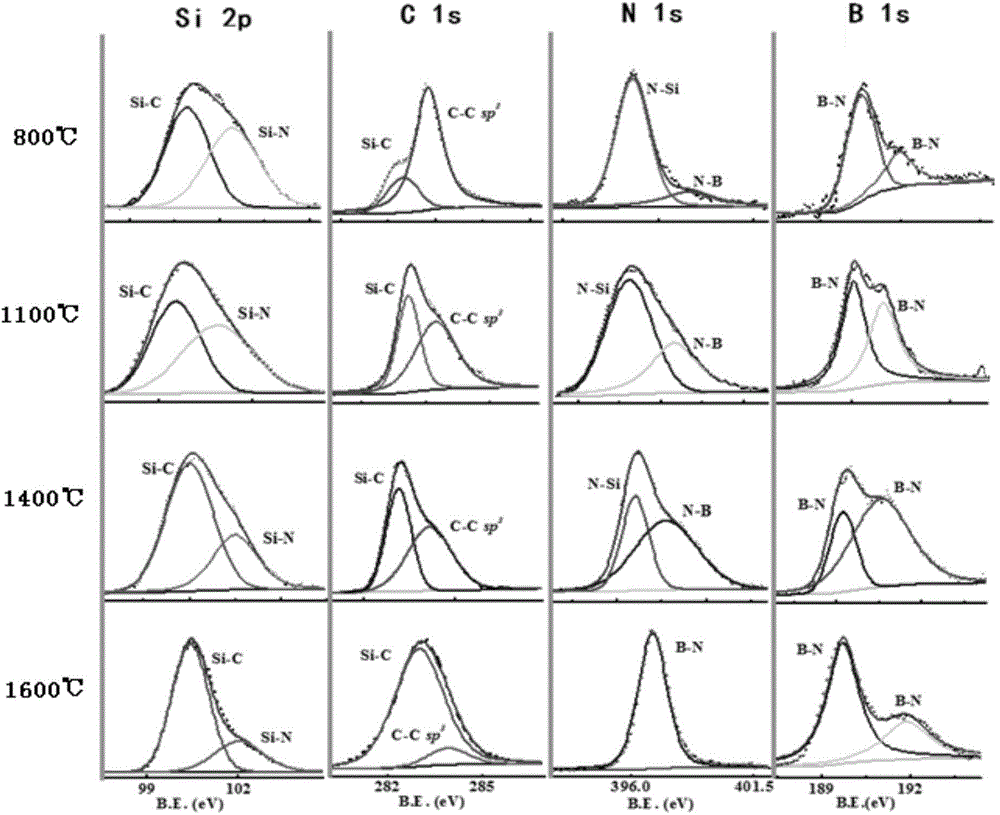
Preparation method for SiBN(C) ceramic fiber precursor
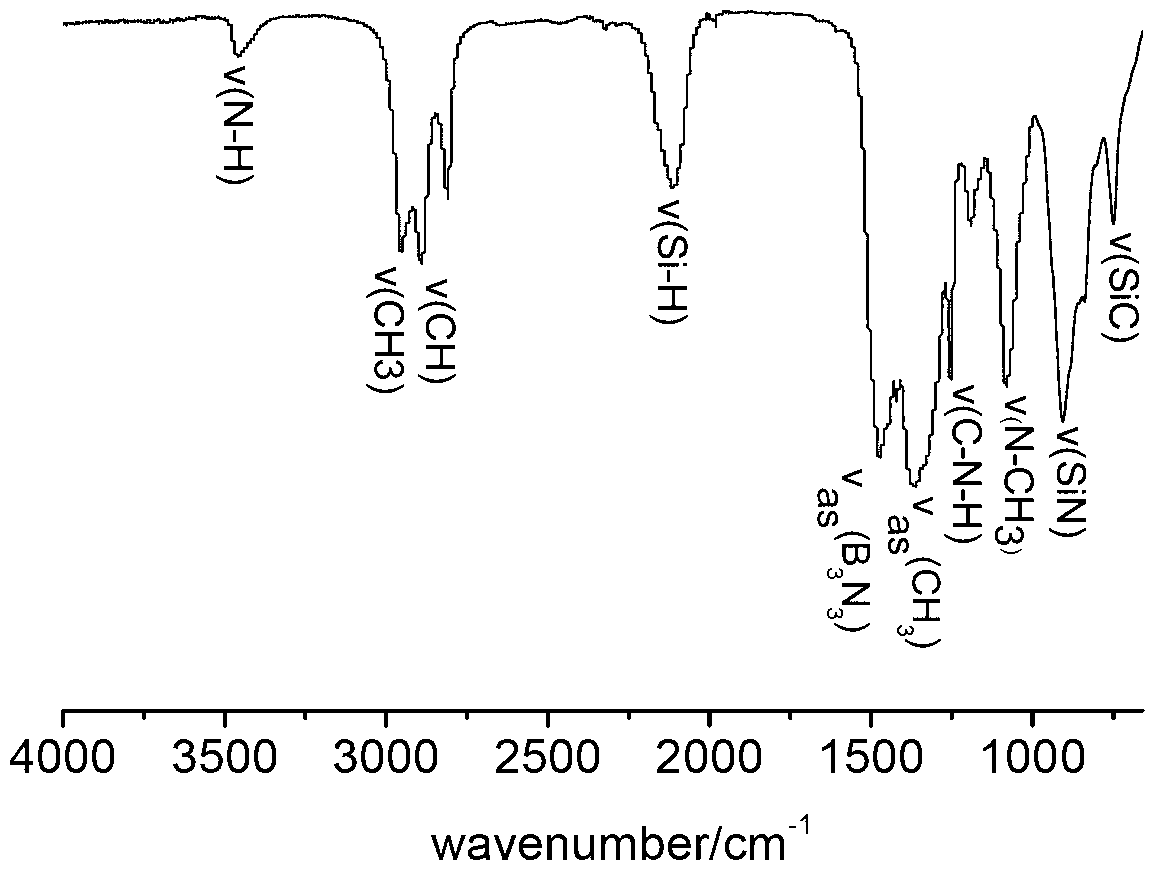
The invention relates to a preparation method for a SiBN(C) ceramic fiber precursor. The preparation method comprises the steps of synthesizing SiBN(C) ceramic fiber precursor molecules by using methyl dichlorosilane (MeHSiCl2), hexamethyl disilazane (HMDZ), boron trichloride (BCl3) and methylamine (CH3NH2) at a temperature ranging from-40 DEG C to-80 DEG C; removing toluene from an anhydrous toluene solution of the SiBN(C) ceramic fiber precursor molecules; heating to a temperature of 130-300 DEG C to obtain a prepolymer; then adding active group-terminated polydimethylsiloxane or active group-terminated long carbon chain molecules; keeping for 10-100 h at the temperature of 130-300 DEG C; defoaming and melt-spinning. Aiming at the problems of large friability, low strength and the like of the SiBN(C) ceramic fiber precursor, the SiBN(C) ceramic fiber precursor with excellent flexibility is prepared by employing a copolymerization method, thereby providing a foundation for continuous and integrated preparation of SiBN(C) ceramic fibers.
Owner:DONGHUA UNIV
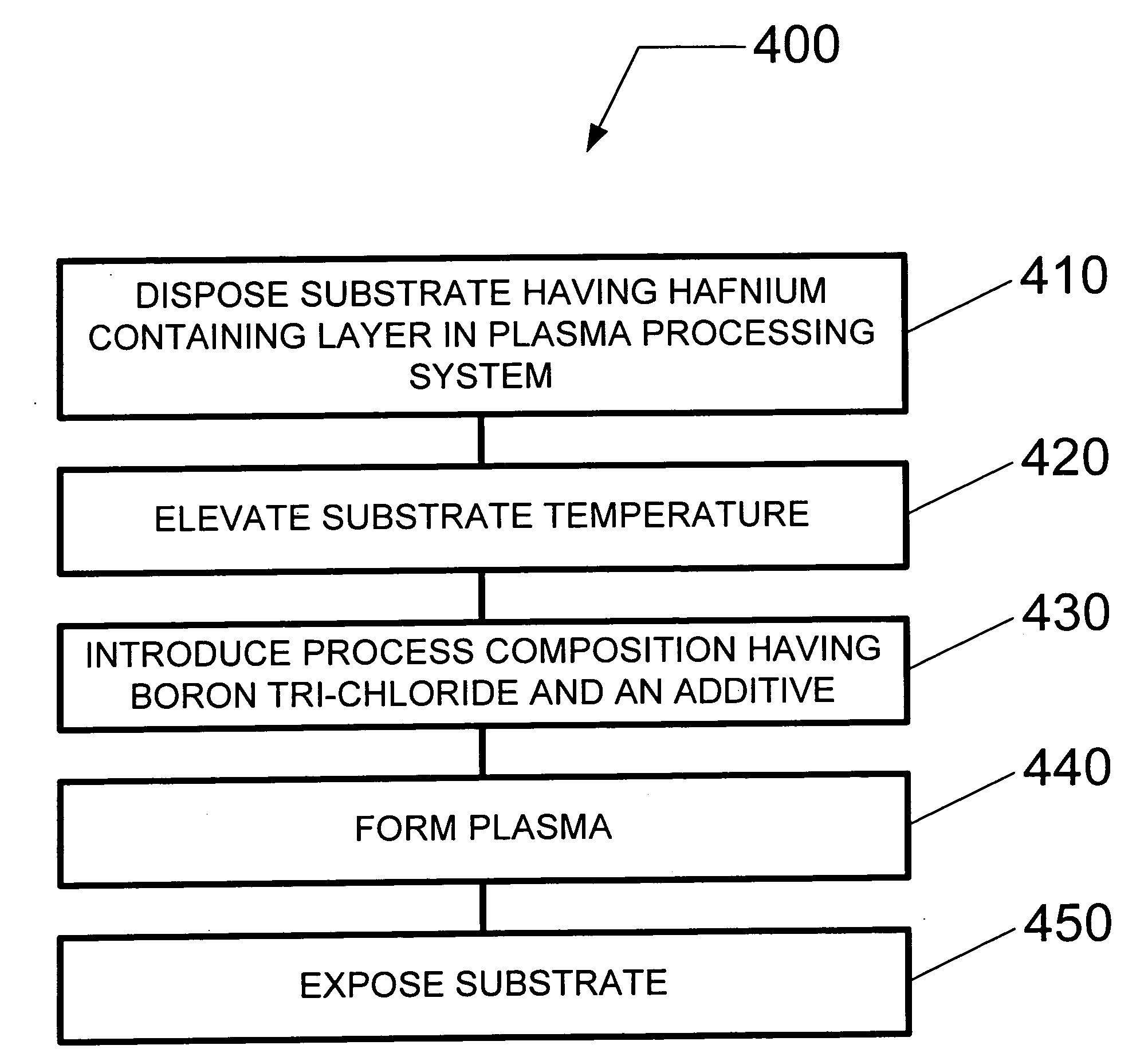
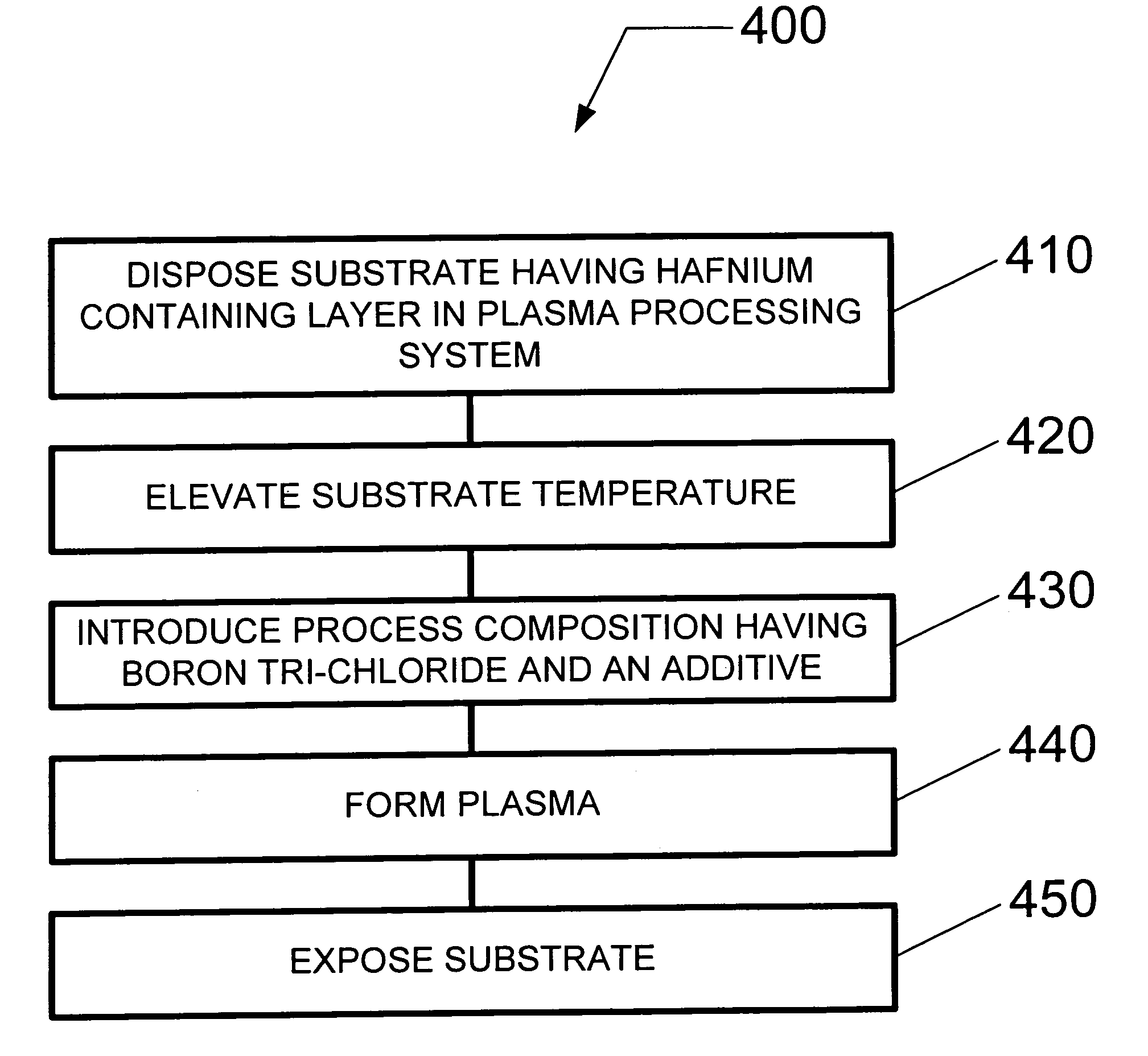
Method and system for dry etching a hafnium containing material
ActiveUS20080064220A1Electric discharge tubesDecorative surface effectsProcess chemistryBoron trichloride
A method and system for etching a hafnium containing material using a boron tri-chloride (BCl3) based process chemistry is described. A substrate having a hafnium containing layer, such as a layer of hafnium dioxide (HfO2) is subjected a dry etching process comprising BCl3 and an additive gas including: an oxygen-containing gas, such as O2; or a nitrogen-containing gas, such as N2; or a hydrocarbon gas (CxHy), such as CH4; or a combination of two or more thereof.
Owner:TOKYO ELECTRON LTD
Preparation method for SiBNC fiber/SiBNC composite material
The invention relates to a preparation method for a SiBNC fiber / SiBNC composite material. The preparation method for the SiBNC fiber / SiBNC composite material comprises the following steps: (1) preparing a precursor polymer by polymerizing a micromolecular monomer boron trichloride, methyl hydrogen dichlorosilane and hexamethyldisilazane; (2) pretreating the surface of the SiBNC fiber and performing pre-crosslinking with the precursor polymer; (3) performing thermoforming to obtain a prefabricated product; (4) performing infusible treatment on the prefabricated product; and (5) performing ceramic treatment at high temperature. The SiBNC fiber / SiBNC composite material prepared by the method has high density, uniform components, low porosity, high-temperature resistance and excellent mechanical performance.
Owner:DONGHUA UNIV
Precursor formulation of a silicon carbide material
A precursor formulation of a silicon carbide material that includes a ceramic material and a boron-11 compound. The ceramic material may include silicon and carbon and, optionally, oxygen, nitrogen, titanium, zirconium, aluminum, or mixtures thereof. The boron-11 compound may be a boron-11 isotope of boron oxide, boron hydride, boron hydroxide, boron carbide, boron nitride, boron trichloride, boron trifluoride, boron metal, or mixtures thereof. A material for use in a nuclear reactor component is also disclosed, as are such components, as well as a method of producing the material.
Owner:COI CERAMICS
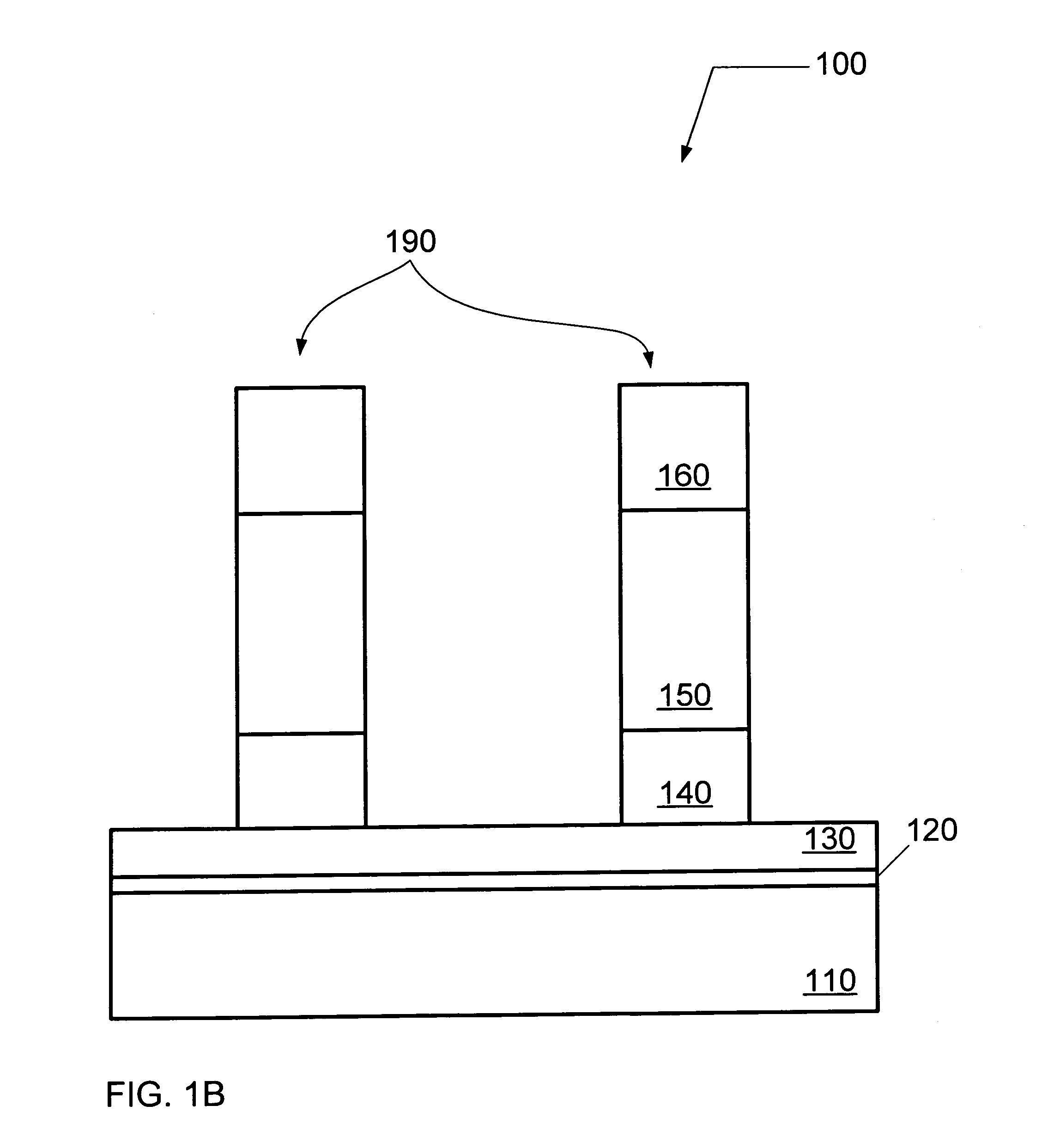
Manufacturing method for semiconductor device
InactiveUS7172931B2High selectivityFavorable uniform characteristicTransistorSolid-state devicesBoron trichlorideOxygen
It is an object of the present invention to enhance a selection ratio in an etching process, and provide a method for manufacturing a semiconductor device that has favorable uniform characteristic with high yield. In a method for manufacturing a semiconductor device according to the present invention, a semiconductor layer is formed, a gate insulating film is formed on the semiconductor film, a first conductive layer is formed on the gate insulating film, a second conductive layer is formed on the first conductive layer, the first conductive layer and the second conductive layer are etched to form a first conductive-layer pattern, the second conductive layer in the first conductive-layer pattern is selectively etched with plasma of boron trichloride, chlorine, and oxygen to form a second conductive-layer pattern, and a first impurity region and a second impurity region are formed in the semiconductor layer.
Owner:SEMICON ENERGY LAB CO LTD
Preparation method of homogenized boron nitride coating
InactiveCN105296960AUniform thicknessThickness is easy to controlChemical vapor deposition coatingBoron trichlorideScanning tunneling microscope
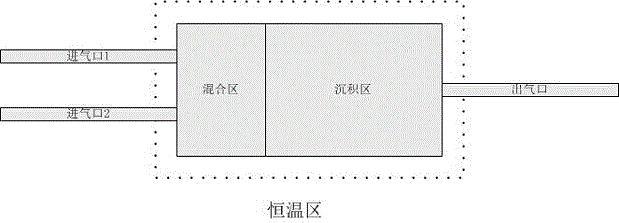
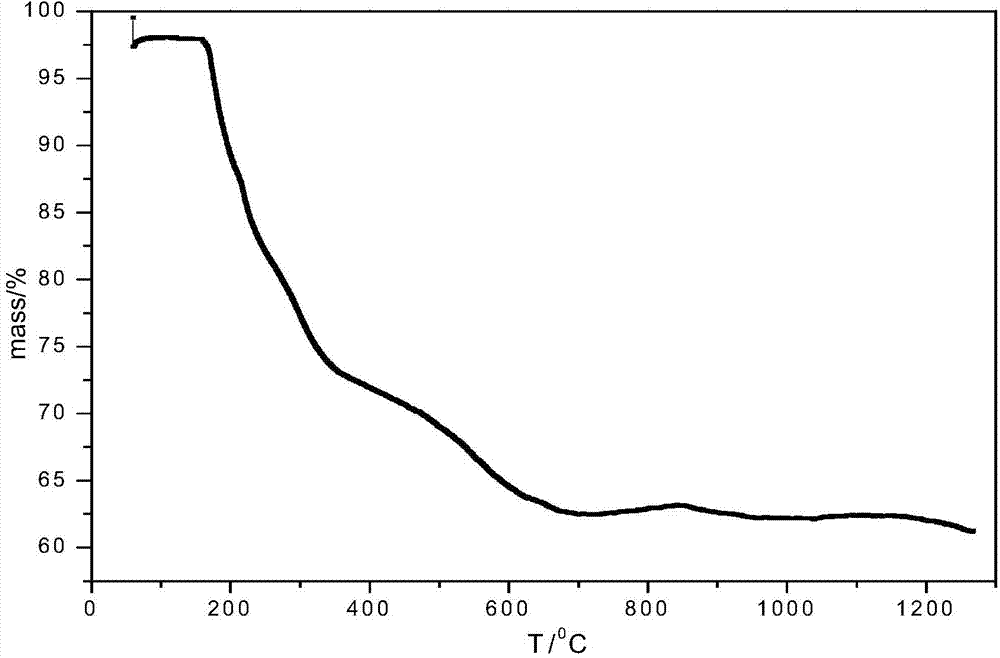
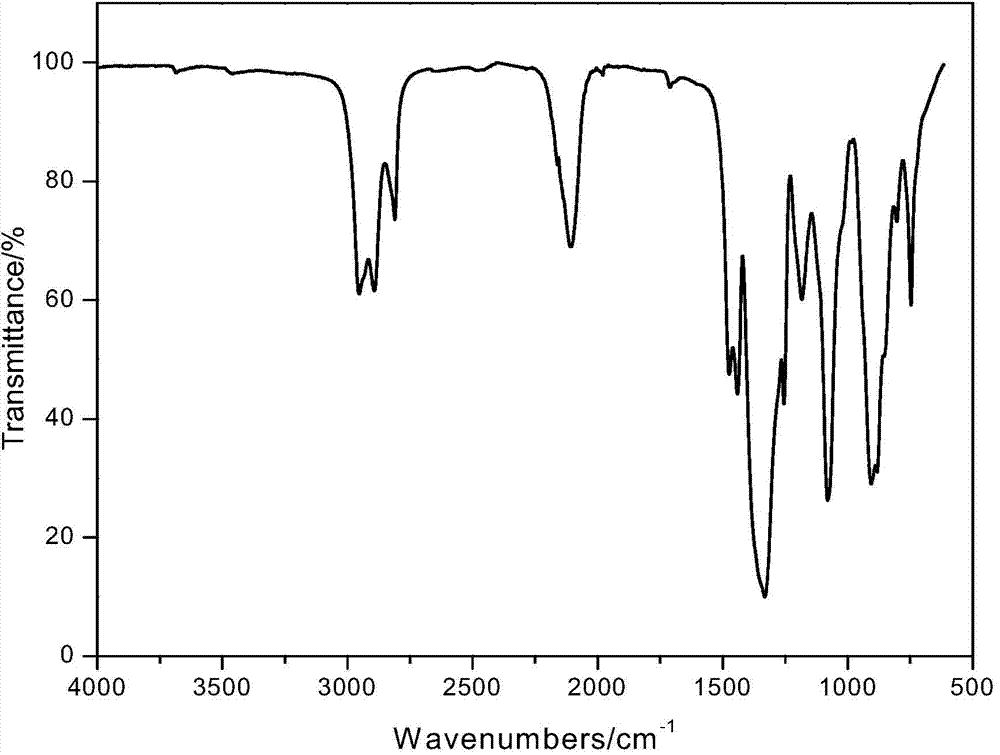
The invention relates to a preparation method of a homogenized boron nitride coating. According to the method, boron trichloride (BCl<3>) and ammonia gas (NH<3>) serve as reaction gases and are fed into a reactor, firstly the reaction gases are evenly mixed in a mixing region and then generate surface deposition in a deposition reaction region, finally an obtained sample is subjected to high-temperature heat treatment, and through a scanning tunneling microscope (SEM), Fourier transform infraRed (FT-IR) and X-ray diffraction (XRD) detection, the boron nitride coating which is even in thickness, singular in ingredient and high in degree of crystallinity is obtained. The method can be used for preparing a boron nitride interface in composite and for preparing boron nitride coatings on the surfaces of other samples and can be further used for researching the boron nitride vapor deposition process and mechanism. The method mainly solves the problem that gases are not evenly mixed in the two-component chemical gas phase sedimentary boron nitride process, so that the uniformity of the boron nitride coating is improved, and the coating thickness is better controlled.
Owner:SHANGHAI UNIV
Boron-carbon-nitrogen ceramic fiber and preparation method thereof
The invention relates to a boron-carbon-nitrogen ceramic fiber and a preparation method thereof. The boron-carbon-nitrogen ceramic fiber is of a continuous fiber, the chemical composition is of BxC3Ny, in the formula, x equals to 0.1-1.5, y equals to 0.1-1.5, the resistivity is 1.0 multiplied by 10<-2>-1.0 multiplied by10<3>omega.cm, and the tensile strength is 1.0-2.0GPa. The preparation method disclosed by the invention comprises the following steps of taking polyacrylonitrile precursors as raw materials, and performing circulating treatment with boron trichloride and ammonia so as to get the continuous boron-carbon-nitrogen ceramic fiber. The preparation method has the advantages of simple process, low cost and easy industrialized production; and the chemical composition and the resistivity of the boron-carbon-nitrogen ceramic fiber can be regulated, and the fiber can be applied to the field of functional composite materials with the functions of stealth, heat insulation and the like.
Owner:NAT UNIV OF DEFENSE TECH
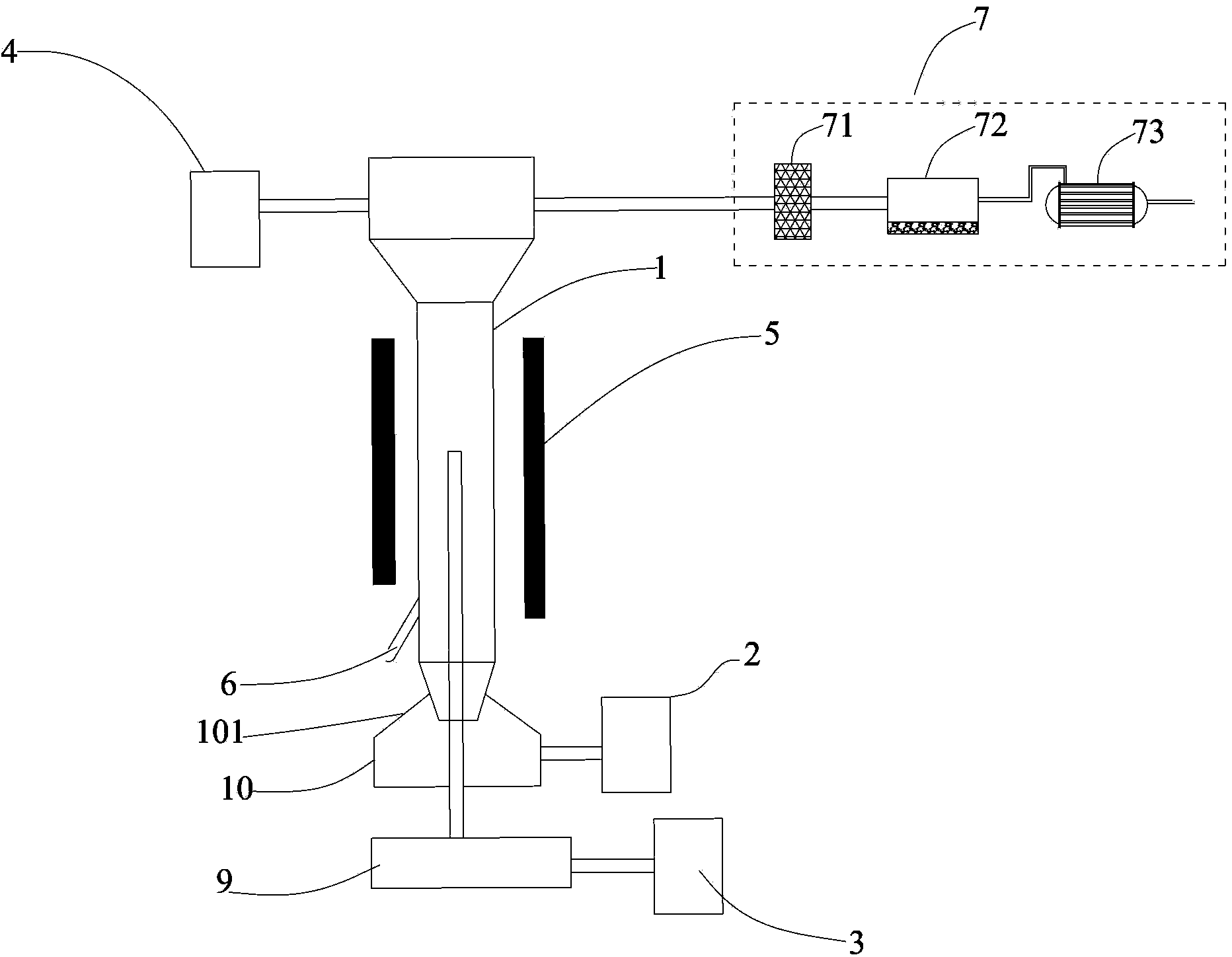
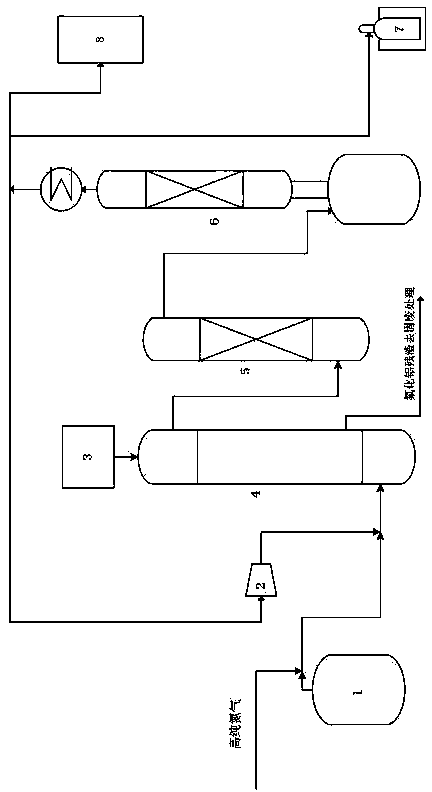
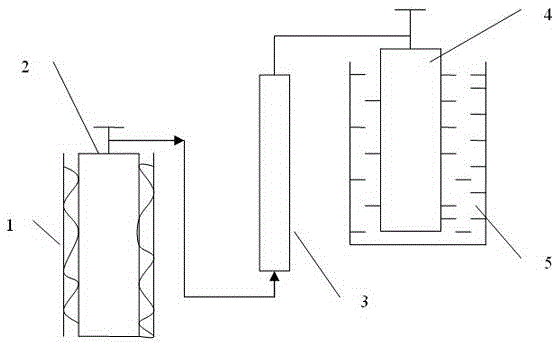
Sieve plate-free fluidized bed as well as preparation method of boron trichloride
ActiveCN103506056AReduce energy consumptionSolve the problem of easy clogging of sieve plate holesBoron halidesChemical/physical processesBorideAlkaline earth metal
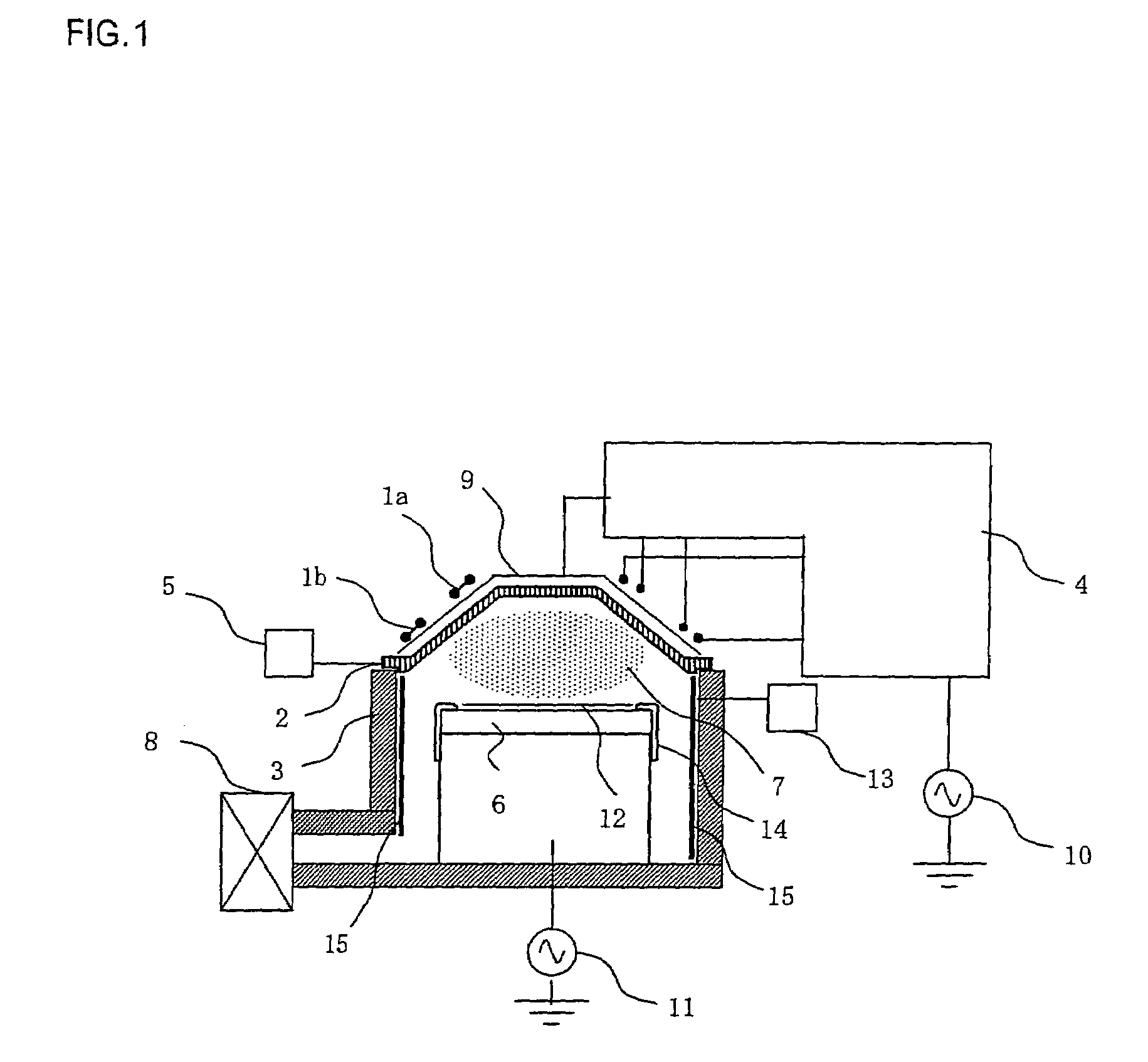
Disclosed are a fluidized bed without a sieve plate and a method for preparing boron trichloride using same. The method comprises: filling the bed body (1) of a fluidized bed without a sieve plate with a powder raw material, i.e. an alkaline earth metal boride, via a raw material supplying device (4); opening a carrier gas source (2), feeding the carrier gas into the bed body (1) of the fluidized bed without a sieve plate, so that the powder raw material i.e. the alkaline earth metal boride is in a fluidized state in the bed body (1) of the fluidized bed without a sieve plate; opening a heating device (5) to heat the bed body (1), feeding a reaction gas i.e. anhydrous hydrogen chloride when the temperature reaches the specified temperature; and after the reaction reaches the specified time, opening and starting a separating device (7), so as to obtain the necessary boron trichloride.
Owner:昆明先导新材料科技有限责任公司
Method of manufacturing a thin film magnetic head
InactiveUS20020053130A1Construction of head windingsElectrical transducersBoron trichlorideEngineering
A method of manufacturing a thin film magnetic head capable of improving a yield while making a pole width extremely minute with high precision is provided. A write gap layer and a bottom pole are selectively etched in a region other than a portion corresponding to a front end part through the RIE with the front end part having an extremely minute uniform width as a mask in an atmosphere of gas including at least chlorine out of chlorine and boron trichloride and at an ambient temperature within a range of 300° C. to 300° C. The width (pole width) of a pole portion can be made uniform with high precision along a length direction so that the yield of the thin film magnetic head can be improved.
Owner:TDK CORPARATION
Preparation method of high-purity boron trichloride-11
ActiveCN103950947AHigh purityMeet the requirements of the processBoron halogen compoundsAluminium chlorideVery large scale integrated circuits
The invention discloses a preparation method of high-purity boron trichloride-11, and belongs to the field of preparation methods of boron compounds. The preparation method comprises the steps such as pretreating raw materials, synthesizing boron trichloride-11 through reaction of aluminum chloride and boron trifluoride-11, filtering, preliminarily separating, rectifying and purifying, collecting the high-purity boron trichloride-11 product, treating tail gas, and the like. The high-purity boron trichloride-11 prepared by the preparation method disclosed by the invention is high in purity which can reach over 99.9999%, can satisfy requirements of manufacturing integrated circuit semiconductor apparatuses on a large scale, effectively improves interference resistance and radiation resistance of an integrated circuit, and can be used as the raw material for manufacturing raw materials such as a high-purity boron-11 isotope material, a special boron fiber material and light-guide fiber.
Owner:方治文
Method of manufacturing a thin film magnetic head
A method of manufacturing a thin film magnetic head capable of improving a yield while making a pole width extremely minute with high precision is provided. A write gap layer and a bottom pole are selectively etched in a region other than a portion corresponding to a front end part through the RIE with the front end part having an extremely minute uniform width as a mask in an atmosphere of gas including at least chlorine out of chlorine and boron trichloride and at an ambient temperature within a range of 30° C. to 300° C. The width (pole width) of a pole portion can be made uniform with high precision along a length direction so that the yield of the thin film magnetic head can be improved.
Owner:TDK CORPARATION
High-boron content silicon-boron-carbon-nitrogen precursor and preparation method thereof
InactiveCN109369918AAvoid over-crosslinkingIncreased boron contentViscous liquidElemental composition
The invention relates to a high-boron content silicon-boron-carbon-nitrogen precursor and a preparation method thereof. The preparation method comprises the following steps: uniformly mixing functional, bifunctional and trifunctional chlorosilane containing unsaturated groups and hexamethyl-disilazane at a low temperature according to a certain ratio, raising the temperature and completely reacting, dropping 1,3-diethyl cycloborazane under low temperature conditions according to a certain ratio, fully reacting the reactants, and finally performing reduced pressure distillation to obtain the solvent and by-products produced by the reaction, thereby obtaining faint yellow viscous liquid or a solid product, namely the SiBCN ceramic precursor. According to the synthetic method disclosed by theinvention, a novel Polyborosilicate resin is prepared by adopting a 'double boron source', the boron content of the prepared SiBCN ceramic can reach 20%, and the problems that the boron content is low and improvement of high temperature resistance and oxidation resistance of the ceramic is not obvious can be solved. The elementary composition regulation range of the precursor disclosed by the invention is wide, and resins which are suitable to serve as a high-temperature-resistant coating, a fiber reinforcement or a ceramic-based composite material matrix and have different elementary compositions can be prepared.
Owner:AEROSPACE RES INST OF MATERIAL & PROCESSING TECH +1
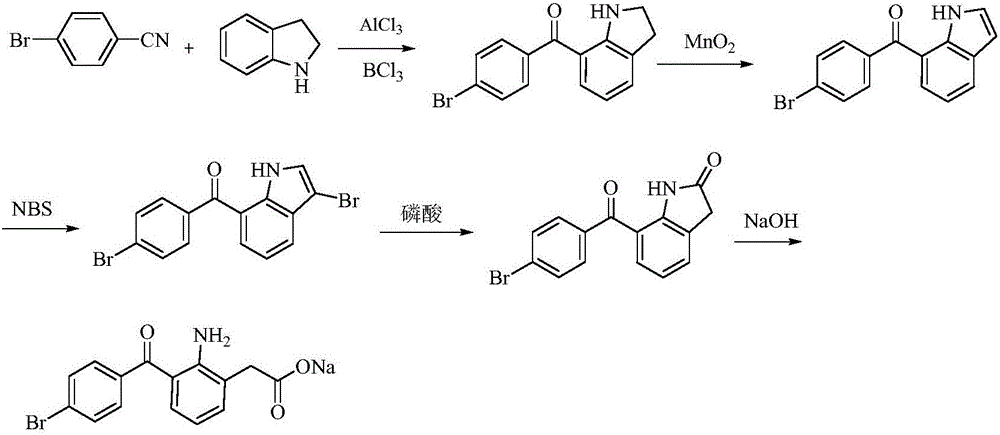
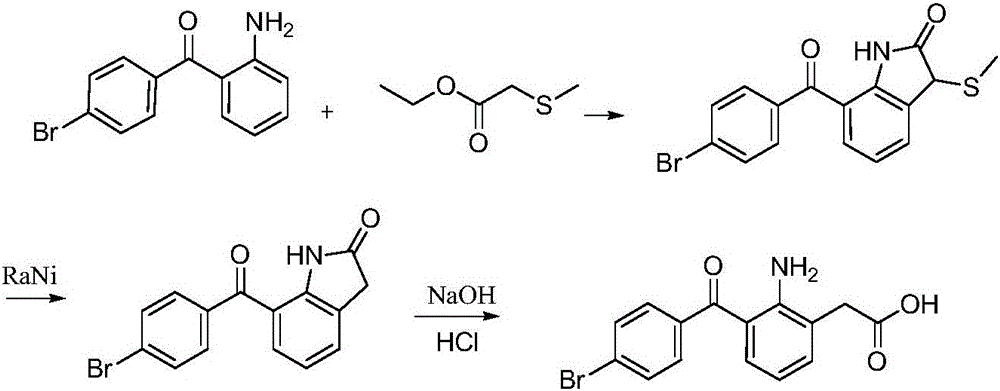
Bromfenac sodium preparation method
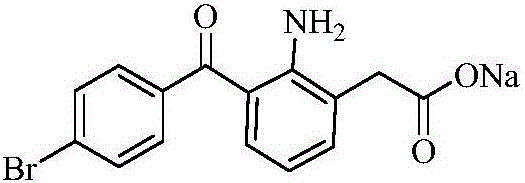
ActiveCN106397235AHigh purityShort synthetic routeOrganic compound preparationAmino-carboxyl compound preparationBoron trichlorideBromfenac
The invention relates to a bromfenac sodium preparation method. The preparation method comprises specific steps as follows: indole reacts under the action of DMSO (dimethylsulfoxide) to produce 3-bromoindole; 3-bromoindole is added to 2-methoxyethanol, acid is added for hydrolysis, and 2-indolinone is obtained; boron trichloride is added to methylbenzene, a methylbenzene mixed solution of p-bromobenzonitrile and 2-indolinone is added dropwise, aluminum chloride is then added, acid is added, a reaction is performed, and 7-(4-bromobenzoyl)-1,3-dihydro-indol-2-one is obtained; hydrolysis is performed with an alkaline solution, acid is added for neutralization, and bromfenac is obtained; ethanol is added to bromfenac, bromfenac and a sodium hydroxide solution form salt, the salt is cooled and subjected to recrystallization, and bromfenac sodium is obtained. Compared with the prior art, the synthetic route is short, high-purity bromfenac sodium can be prepared, the quality meets the latest standards of pharmacopoeia, industrial production is facilitated, and the method can provide powerful guarantee for industrial production of bromfenac sodium and intermediates of bromfenac sodium.
Owner:山东辰欣佛都药业股份有限公司
Preparation method of modified active carbon
ActiveCN105233790AOther chemical processesWater/sewage treatment by sorptionActivated carbonBoron trichloride
The invention discloses a preparation method of modified active carbon. The preparation method comprises the following steps: at first, adding active carbon into a mixture of ethylene glycol dimethacrylate and a boron trichloride-trimethyl amine complex, heating to carry out reactions so as to obtain a product (A), then adding the product (A) into oxalic acid, heating to a temperature of 50 to 60 DEG C under a vacuum condition, maintaining the temperature for 60 to 100 minutes, filtering to obtain a product (B); and finally reacting the product (B) with benzyl triethyl ammonium chloride to obtain the modified active carbon. The technology of the provided preparation method is simple, and the prepared modified active carbon has a good performance on adsorbing heavy metal ions.
Owner:江苏富汇通环保科技有限公司
Method and system for dry etching a hafnium containing material
ActiveUS8183161B2Electric discharge tubesDecorative surface effectsProcess chemistryBoron trichloride
A method and system for etching a hafnium containing material using a boron tri-chloride (BCl3) based process chemistry is described. A substrate having a hafnium containing layer, such as a layer of hafnium dioxide (HfO2) is subjected a dry etching process comprising BCl3 and an additive gas including: an oxygen-containing gas, such as O2; or a nitrogen-containing gas, such as N2; or a hydrocarbon gas (CxHy), such as CH4; or a combination of two or more thereof.
Owner:TOKYO ELECTRON LTD
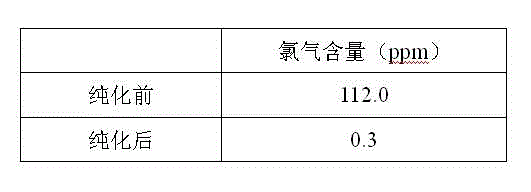
Purification method for boron trichloride
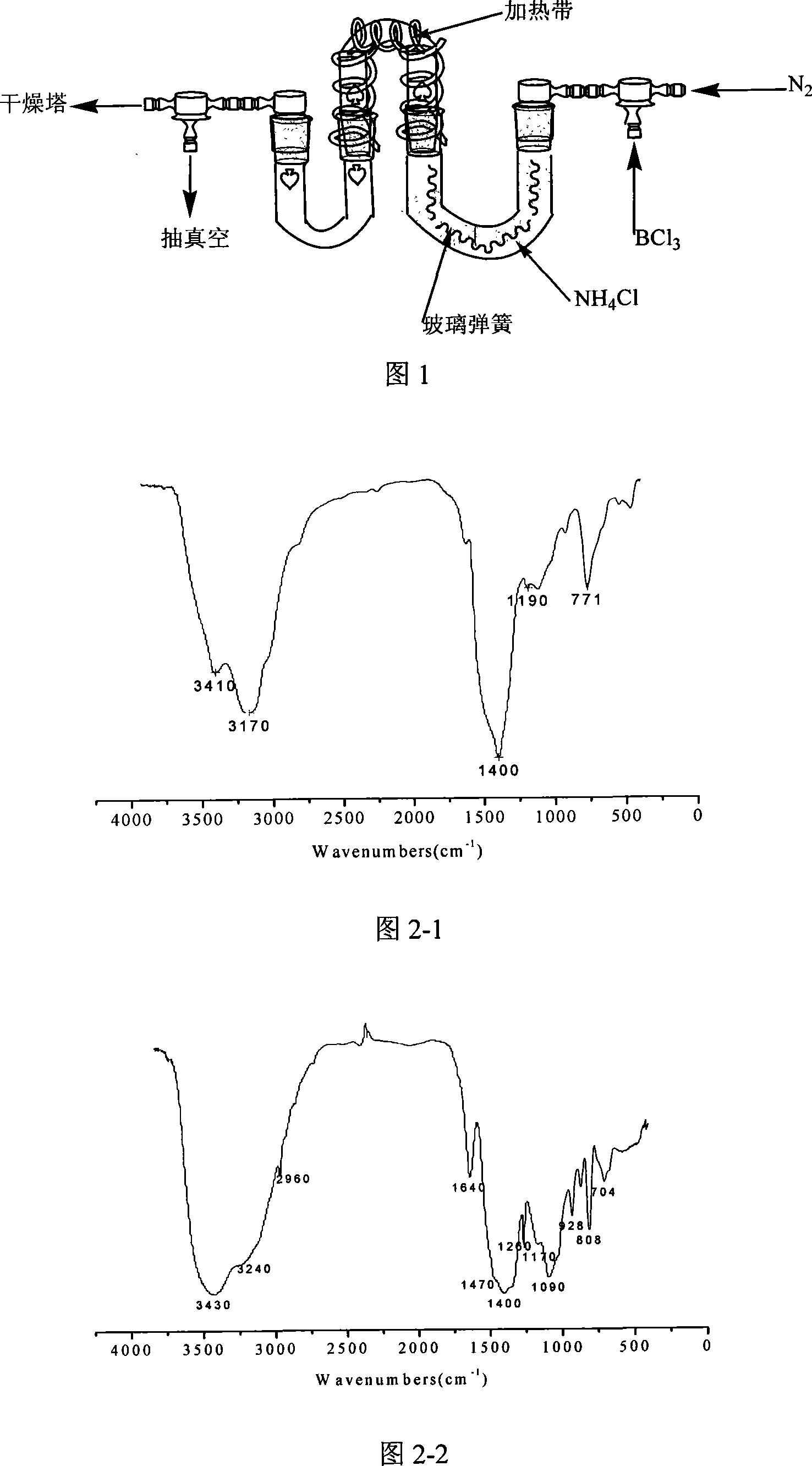
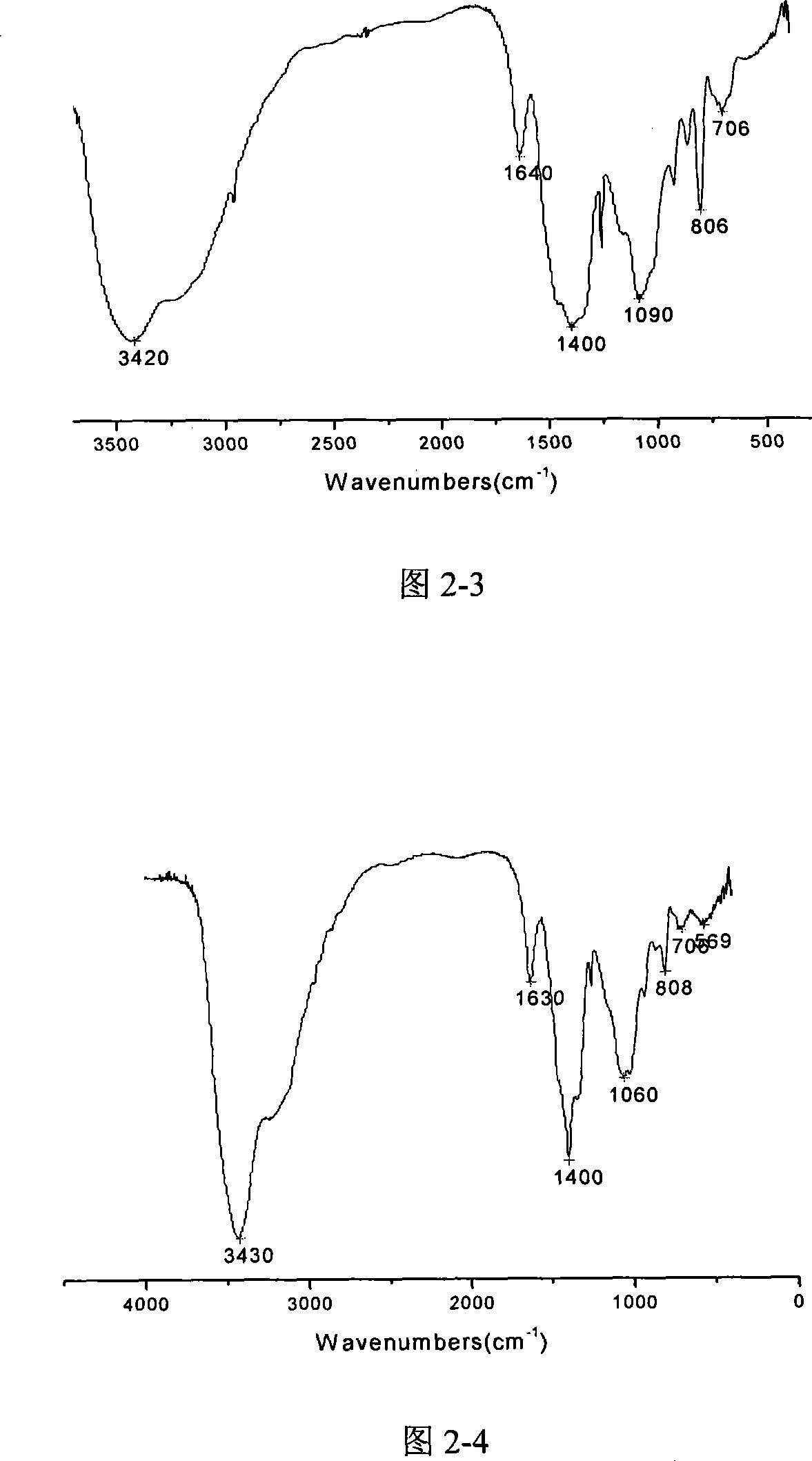
InactiveCN104098105AChlorine impurity levels are reducedBoron halogen compoundsReaction layerPurification methods
The invention belongs to the technical field of chemical engineering and relates to a purification method for boron trichlorid. The purification method comprises the following steps: a heating tape at the temperature of 35 DEG C is adopted to heat a boron trichloride raw material steel bottle and the temperature is controlled at 25 DEG C- 35 DEG C; boron trichloride raw materials with chlorine gas content greater than 1 ppm are heated and fully vaporized and the gas flow rate of the boron trichloride is controlled at 10 L / min; micro-scale chlorine gas contained in the boron trichloride gas and boron carbide chlorine gas react with boron carbide at high-temperature through a boron carbide reaction layer which is 5 cm in diameter, 50 cm in height and 850 DEG C in temperature; after the reaction, the gas is cooled by a cryogenic box at the temperature of -20 DEG C and collected into product bottles. The purification method has the benefits that as the high-temperature boron carbide reaction layer is adopted by the technology, the raw materials and the boron carbide are subjected to deep contact reaction to enable the chlorine gas impurity content in the boron trichloride is reduced to less than 1 ppm.
Owner:大连科利德半导体材料股份有限公司
Manufacturing method for semiconductor device
InactiveUS20040171242A1Good choiceFavorable uniform characteristicTransistorSolid-state devicesBoron trichlorideEngineering
It is an object of the present invention to enhance a selection ratio in an etching process, and provide a method for manufacturing a semiconductor device that has favorable uniform characteristics with high yield. In a method for manufacturing a semiconductor device according to the present invention, a semiconductor layer is formed, a gate insulating film is formed on the semiconductor film, a first conductive layer is formed on the gate insulating film, a second conductive layer is formed on the first conductive layer, the first conductive layer and the second conductive layer are etched to form a first conductive-layer pattern, the second conductive layer in the first conductive-layer pattern is selectively etched with plasma of boron trichloride, chlorine, and oxygen to form a second conductive-layer pattern, and a first impurity region and a second impurity region are formed in the semiconductor layer.
Owner:SEMICON ENERGY LAB CO LTD
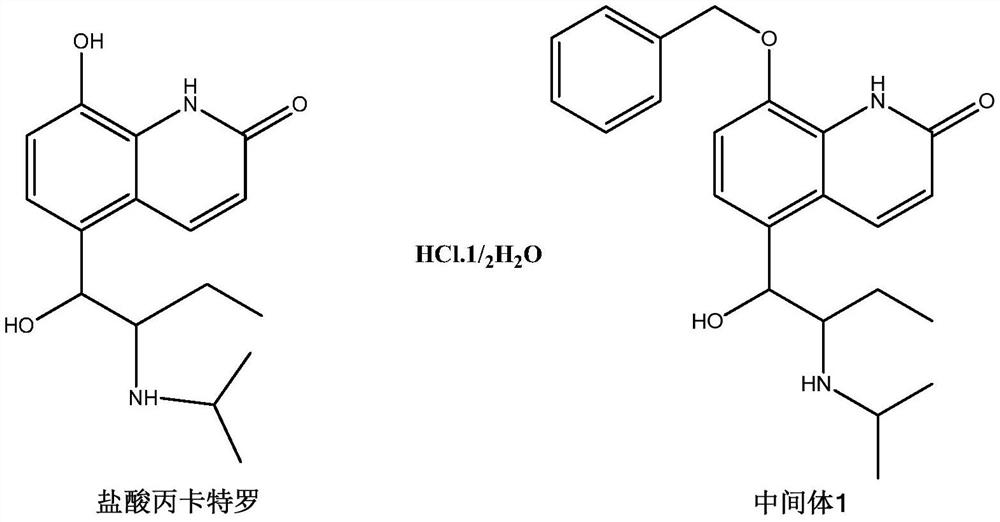
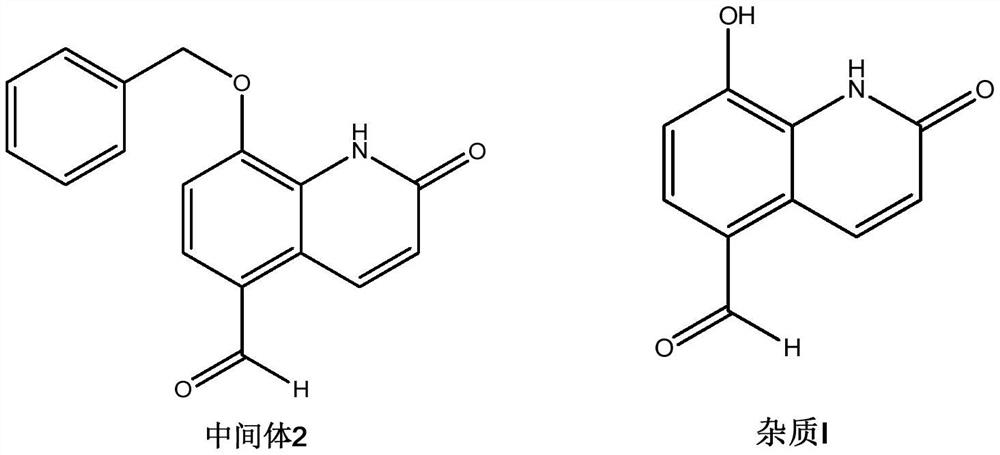
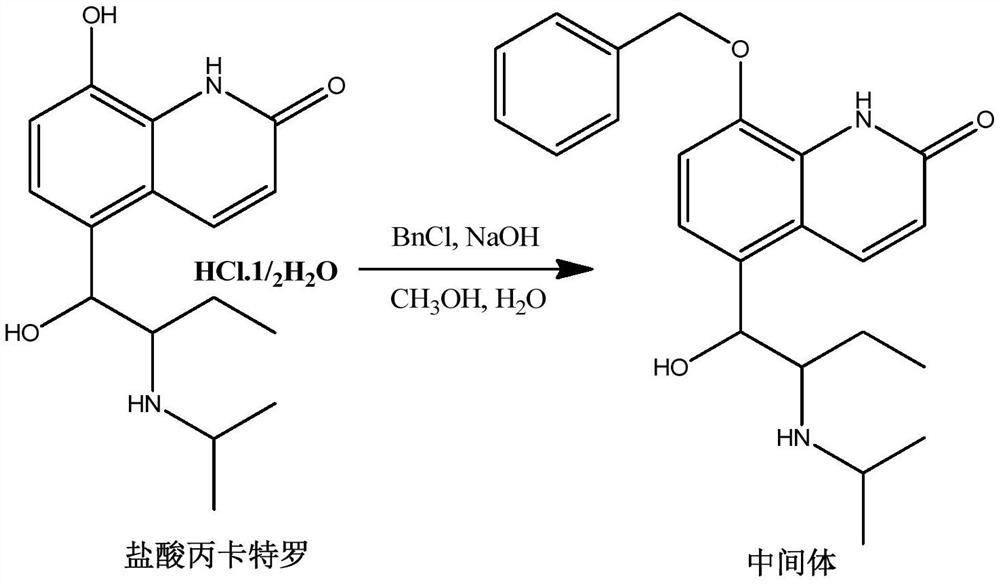
Procaterol hydrochloride impurity preparation method
InactiveCN112645875ALower synthesis costSimple preparation stepsOrganic chemistryIce waterOrganic solvent
The invention discloses a procaterol hydrochloride impurity preparation method, which comprises: 1, adding procaterol hydrochloride to an organic solvent under an alkaline condition, adding an appropriate amount of benzyl halide, carrying out heating reflux until the reaction is complete, and carrying out post-treatment to obtain an intermediate 1; step 2, adding the intermediate 1 into a reaction bottle, then adding an organic solvent, raising the temperature, adding an oxidant aqueous solution, pouring into ice water after heating reaction is completed, and separating out a solid to obtain an intermediate 2; and step 3, adding the intermediate 2 into a reaction bottle, then adding an organic solvent, cooling to a low temperature, slowly adding boron trichloride, keeping the low temperature until the reaction is complete, pouring into ice water, separating out solids, and filtering to obtain the procaterol impurity. Procaterol hydrochloride which is easy to obtain is used as an initial raw material to prepare the procaterol hydrochloride impurity, the synthesis cost of the procaterol hydrochloride impurity is reduced, the preparation steps of the procaterol hydrochloride impurity are simplified, and the selectivity is improved, so that the preparation time is saved, and the preparation efficiency is improved. The total yield of the reaction is 50-70%.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Stiochiometric silicon carbide fibers from thermo-chemically cured polysilazanes
ActiveUS20120237765A1Reduce manufacturing complexityReduce property variabilityNitrogen compoundsLayered productsPolymer sciencePolymer resin
A novel polycrystalline stoichiometric fine SiC fiber substantially free of impurities is produced using a novel pre-ceramic polymer. The pre-ceramic polymer is prepared by reacting a mixture of chlorodisilane, boron trichloride, and a vinyl chlorodisilane with an excess of hexamethyldisilazane to form the pre-ceramic polymer resin, which may then be melt-spun, cured, pyrolyzed and heat-treated to obtain the finished SiC fiber. The manufacturing process for the production of the fine SiC ceramic fiber allows for flexibility with respect to cross-linking, in that low-cost thermal treatments may replace more complex methods, while obtaining fibers with improved materials properties as compared to currently available SiC fibers.
Owner:GENERAL ELECTRIC CO
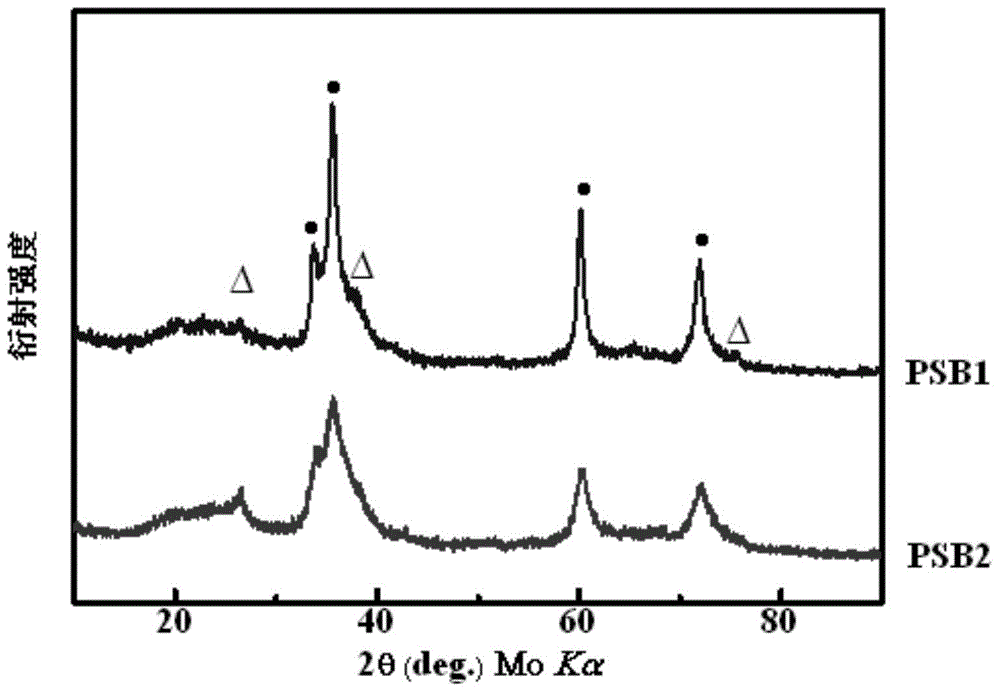
Method for preparing SiNCB ceramic material
The invention discloses a method for preparing a SiNCB ceramic material, relates to a method for preparing a ceramic material, and aims to solve the problems that a SiNCB ceramic material prepared by using a conventional method has high possibility of crystallization and instable at high temperature. The method comprises the following steps: I, putting a nitrogen source, a silicon source, an acid-binding agent and a solvent into a reactor to react at the room temperature under the protection of an inert gas; II, performing vacuum extraction filtration on the reaction product, mixing with borane tetrahydrofuran, and putting into a reactor to react; III, adding a boron trichloride n-hexane into the reactor to react, and heating to be the room temperature; IV, performing extraction filtration on the product, and performing vacuum rotary evaporation to obtain a ceramic precursor; V, putting the ceramic precursor into a tubular furnace, performing pyrolysis, and cooling to be the room temperature along the furnace to obtain the SiNCB ceramic material. The SiNCB ceramic material is excellent in oxidation resistance at 1450 DEG C and is not decomposed or crystallized when being used for a long time. The method can be applied to the field of silicon-based ceramic materials.
Owner:HARBIN INST OF TECH
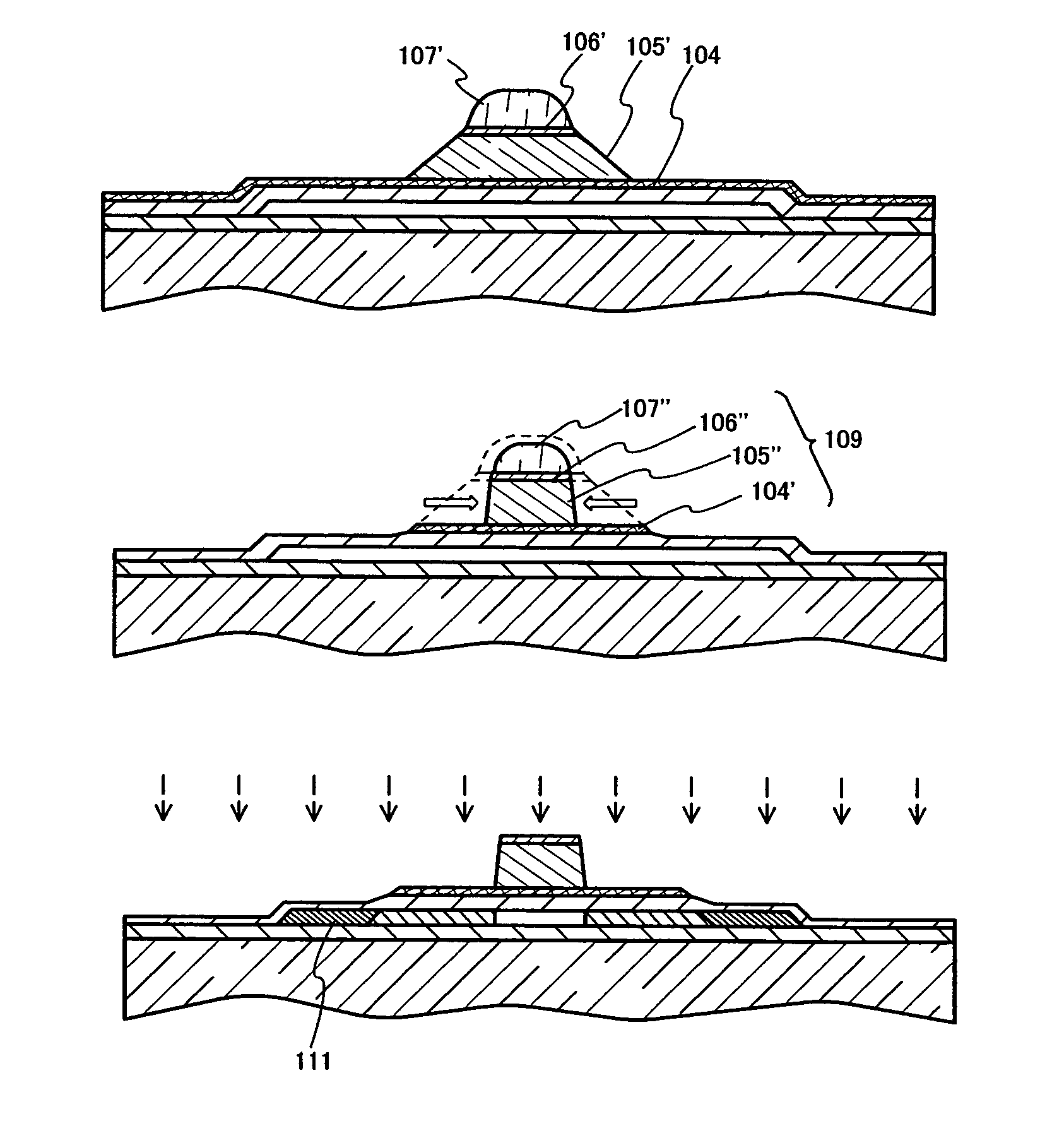
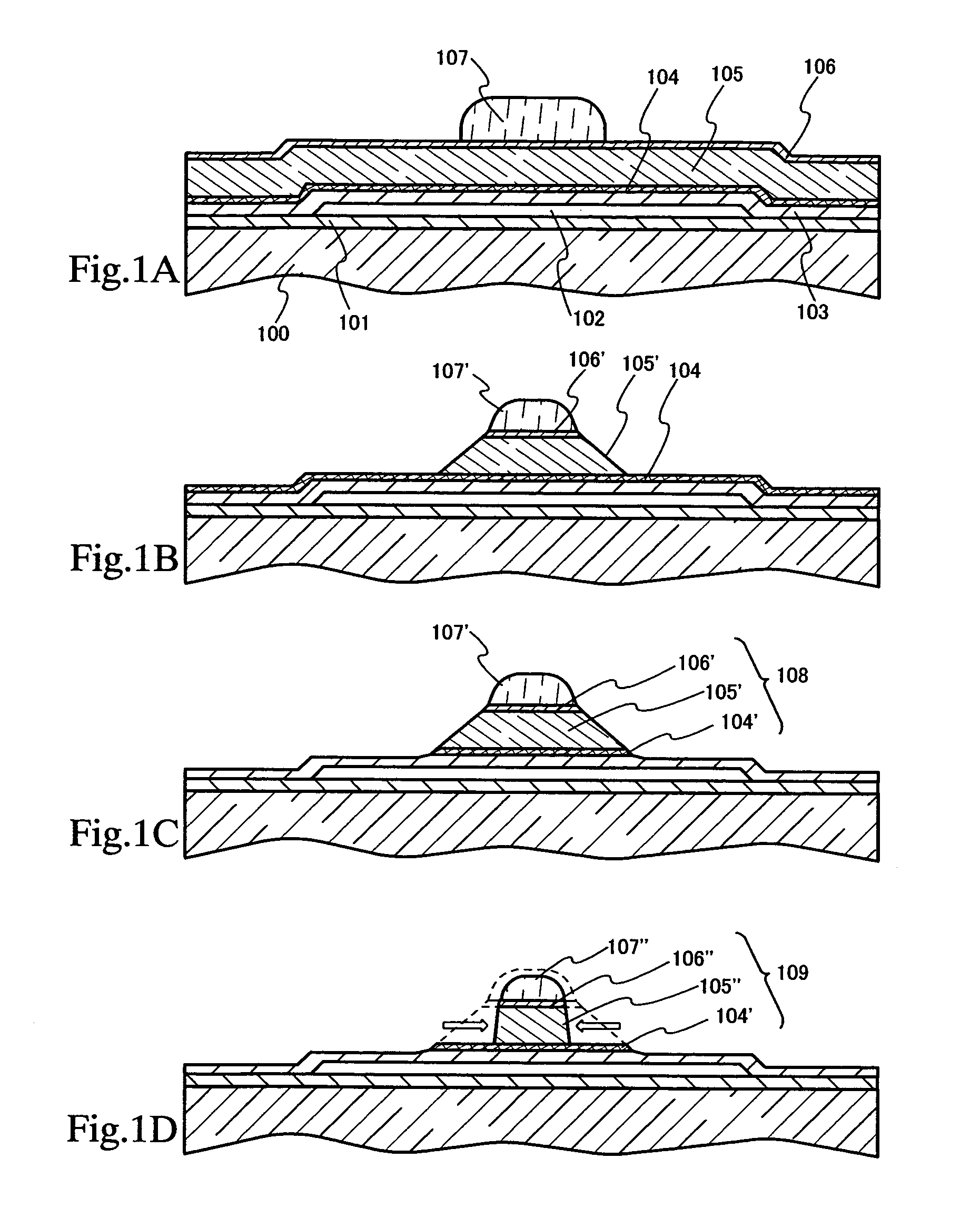
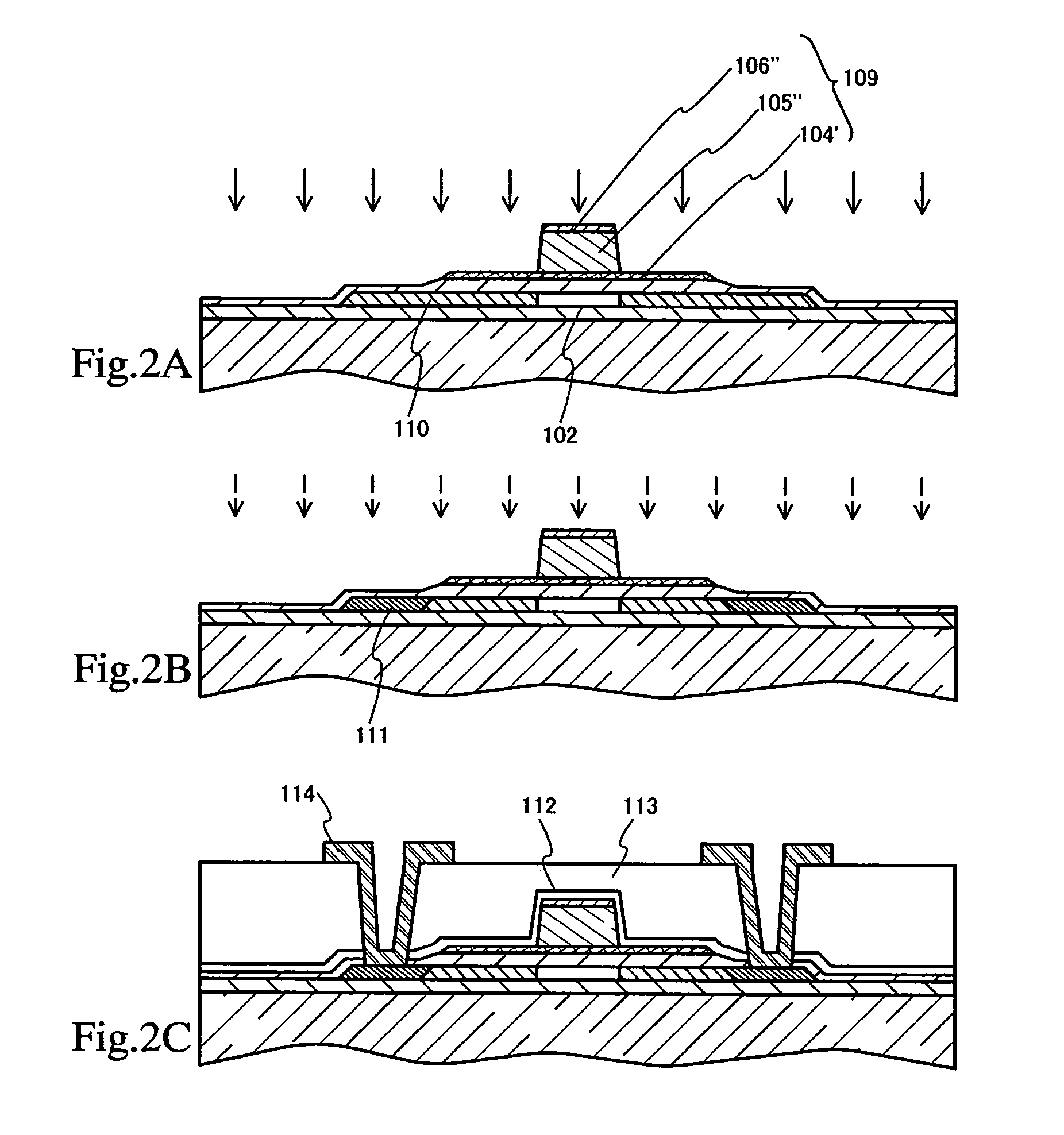
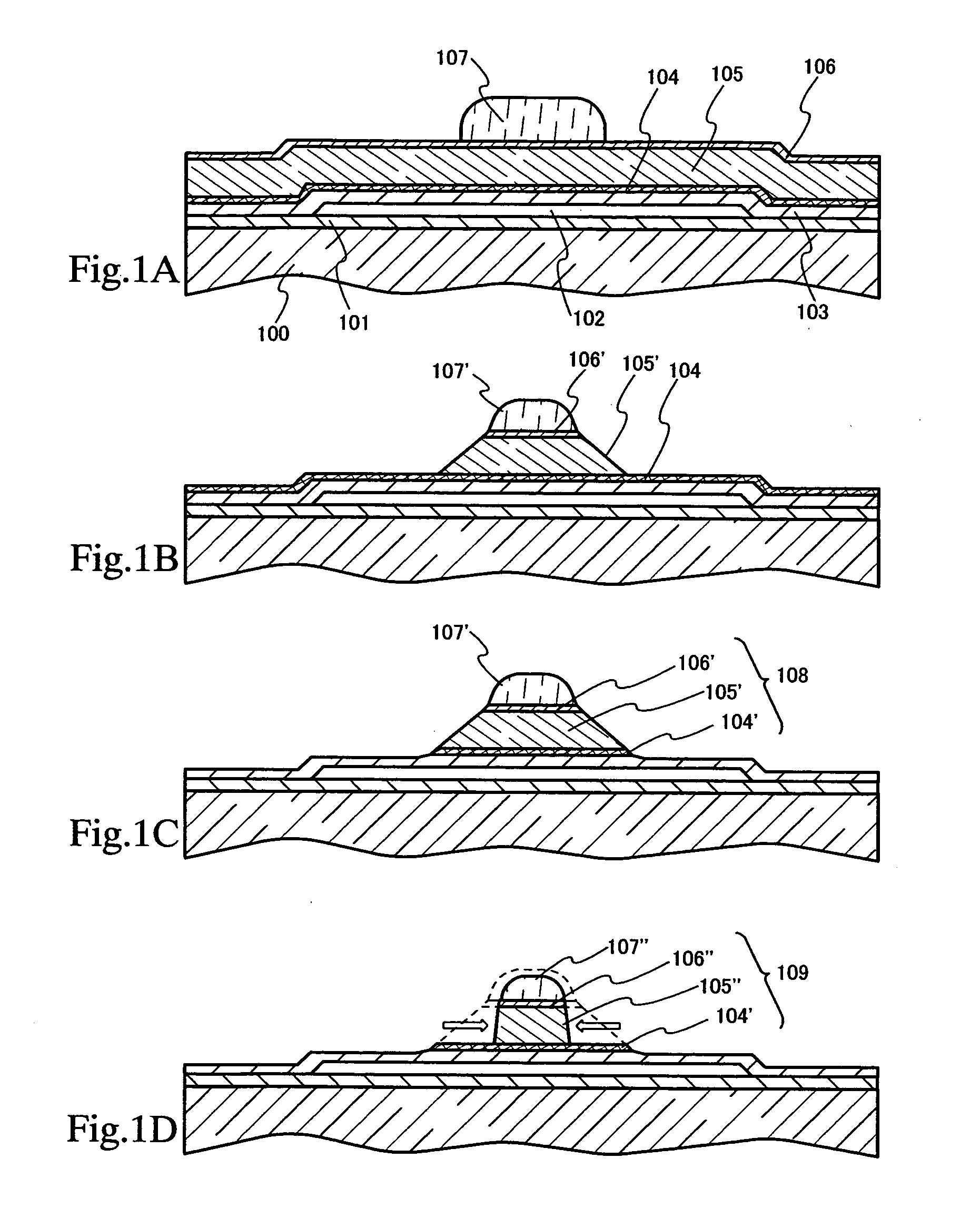
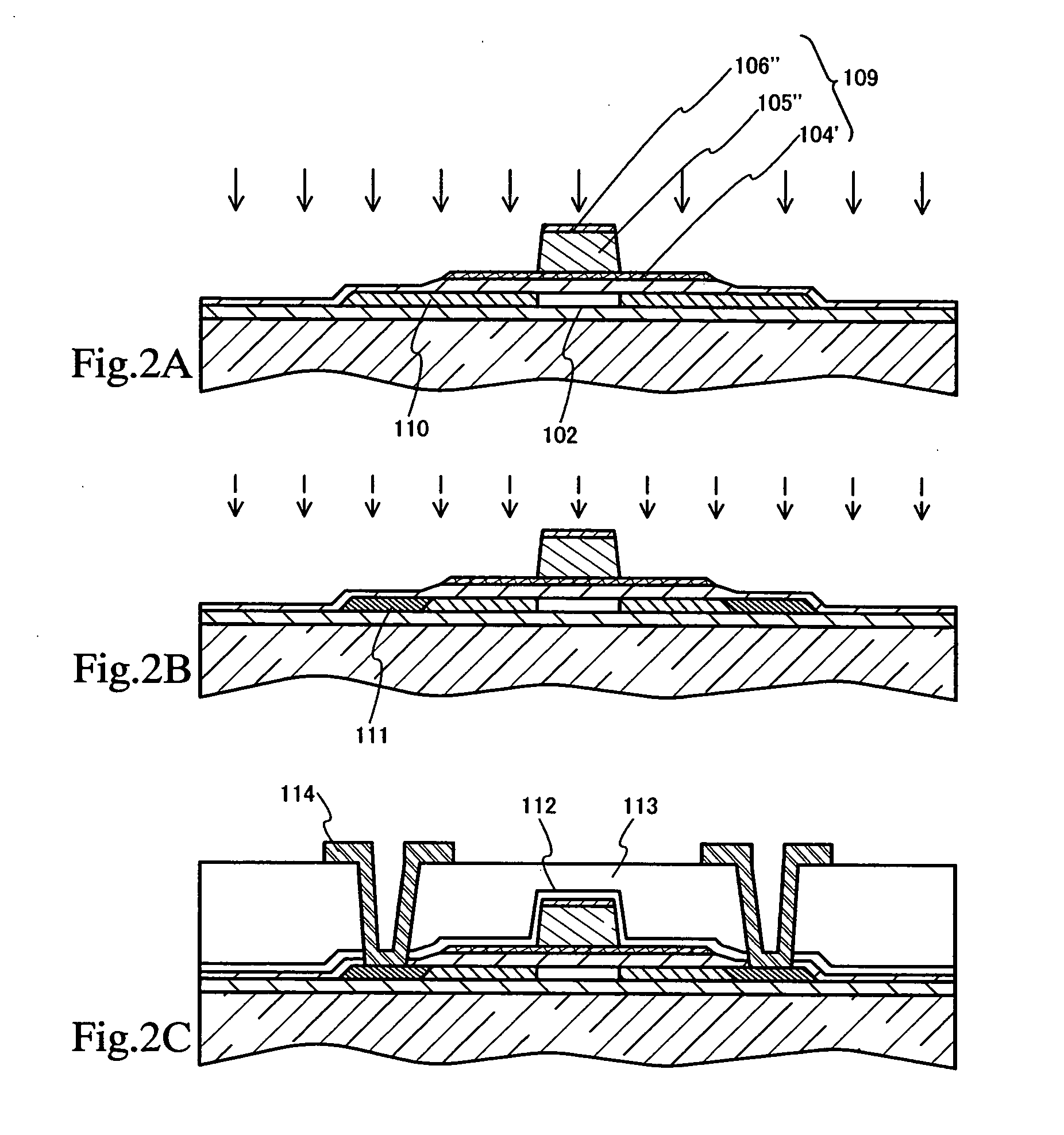
Method of cleaning etching apparatus
ActiveUS7662235B2Clean interiorMaintain repeatabilityHollow article cleaningElectrostatic cleaningBoron trichlorideCleaning methods
To provide a cleaning method for an etching apparatus for a metal film that efficiently removes an etching residue deposited in an etching process chamber, assures the reproducibility of the etching performance, and keeps the etching process chamber in a low-dust-emission condition.Each time one workpiece with a metal film is etched (S1), the interior of the vacuum chamber is cleaned by replacing the workpiece with a dummy substrate (S2), performing a first step of plasma processing using oxygen (O2) and carbon tetrafluoride (CF4) to remove a carbon-based deposit pile (S3), and performing a second step of plasma processing using boron trichloride (BCl3) and chlorine (Cl2) to remove a residue that could not be removed by the first step and an etching residue of the metal film (S4).
Owner:HITACHI HIGH-TECH CORP
Method for preparation of SiBN(C) ceramic fiber precursor by one-pot technique
ActiveCN103938296ASimple processEasy to operateMelt spinning methodsConjugated synthetic polymer artificial filamentsSynthesis methodsBoron trichloride
The invention relates to a method for preparation of an SiBN(C) ceramic fiber precursor by a one-pot technique. The method includes: at a temperature ranging from -30DEG C to -10DEG C, synthesizing an anhydrous n-hexane solution of an SiBN(C) ceramic fiber precursor molecule from dichloromethylsilane, boron trichloride, methylamine and a solvent anhydrous n-hexane; under 70-120DEG C, removing n-hexane from the precursor molecule solution, then adding active group terminated polydimethyl siloxane, raising the temperature to 140-300DEG C, conducting heat preservation for 5-150h to obtain an SiBN ceramic fiber precursor polymer, and carrying out defoaming and melt spinning, thus obtaining the SiBN(C) ceramic fiber precursor. Compared with the existing synthesis methods, the method has the advantages of simple process and convenient operation, and preparation of the precursor can be completed by one-step reaction. The method provided by the invention can effectively solve the brittleness problem of the SiBN(C) ceramic fiber precursor, thus laying the foundation for continuous preparation and cracking of the SiBN(C) ceramic fiber precursor.
Owner:DONGHUA UNIV
SiBCN metal-free ceramic wave-absorbing material obtained from cracking conversion of benzene ring-containing polymer and preparation method thereof
InactiveCN109251038AEvenly dispersedImprove absorbing performanceOther chemical processesGraphite carbonBoron trichloride
The invention discloses a SiBCN metal-free ceramic wave-absorbing material obtained from cracking conversion of benzene ring-containing polymer and a preparation method thereof, wherein the preparation method includes synthesis of benzene ring-containing hyperbranched polyborosilazane and preparation of the SiBCN ceramic wave-absorbing material. The hyperbranched polyborosilazane is synthesized bymethyl dichlorodiphenylsilazane, dichlorosilazane, boron trichloride and hexamethyldisiliazide, then the hyperbranched polyborosilazane is crosslinked and tabletted into a blank body, and the blank body is cracked into the ceramic wave-absorbing material. Starting from the design of the structure of a ceramic polymer precursor, synthesis of a high ceramic yield polyborosilazane precursor and construction of a crosslinking topology system are performed, and the benzene ring is directly introduced into the structure of the polyborosilazane precursor, so that sp2 hybrid carbon atoms in the benzene ring directly form graphite carbon, carbon nanotubes and other structures in situ in cracking conversion process, the high temperature wave absorbent is uniformly dispersed in the structure of an amorphous low-loss carrier, and the metal-free ceramic based composite material with good wave absorbing properties is obtained.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
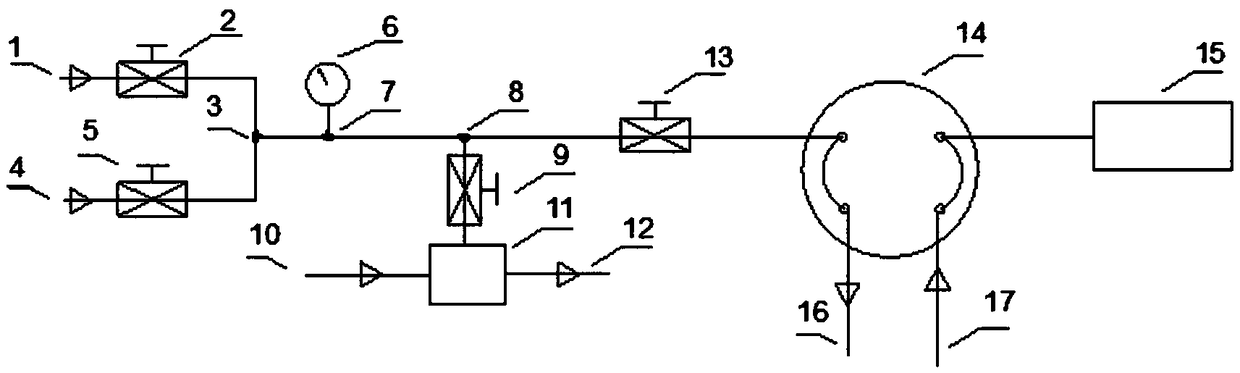
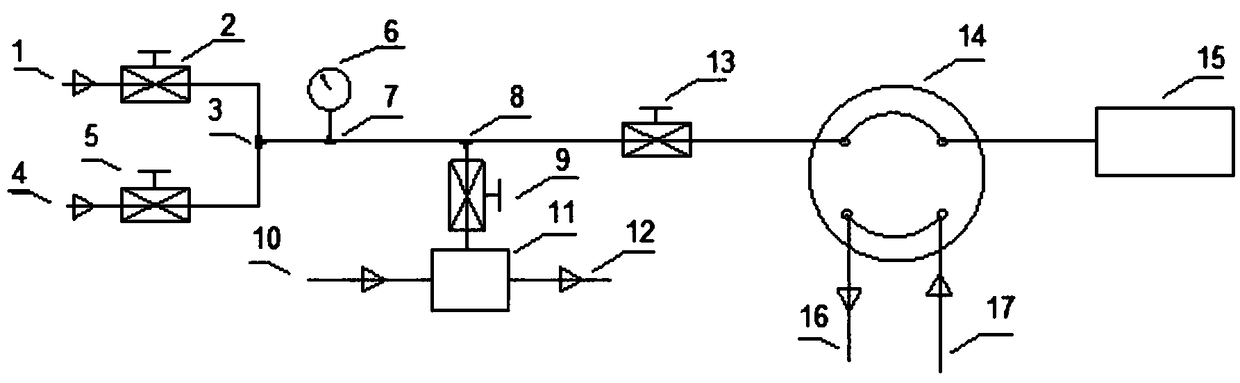
Chromatography sampling system for gas boron-trichloride analysis for electronic industry
PendingCN108896689AAvoid decomposition clogging problemsComponent separationFour-way valveBoron trichloride
The invention discloses a chromatography sampling system for gas boron-trichloride analysis for electronic industry. The chromatography sampling system comprises a helium replacement system, a venturivacuum pump system and a chromatography pre-processing system; the helium replacement system comprises a helium inlet, a switch valve a, a switch valve b and a four-way valve; the venturi vacuum pumpsystem comprises a venturi vacuum pump, a nitrogen inlet and a waste-gas outlet, both the nitrogen inlet and the waste-gas outlet are in air circuit connection with the venturi vacuum pump, and the venturi vacuum pump is in air circuit connection with a three-way valve b through a switch valve d. According to the chromatography sampling system for gas boron-trichloride analysis for electronic industry, as for the problem that boron trichloride is decomposed in water, hyper-pure helium is used for replacement, a sampling pipe is blown and swept, the system is vacuumized, and moisture of a sampling system can be reduced to 0.1 ppm or below through several times of blowing sweeping-vacuum replacement; as for the problem that the boron trichloride cannot enter a vacuum pump, a venturi vacuumpump is adopted, nitrogen serves as blowing sweeping air, and the decomposition and blocking problems of the boron trichloride can be solved.
Owner:朗析仪器(上海)有限公司
Method for producing boron-nitrogen ceramic fibre fore-runner body
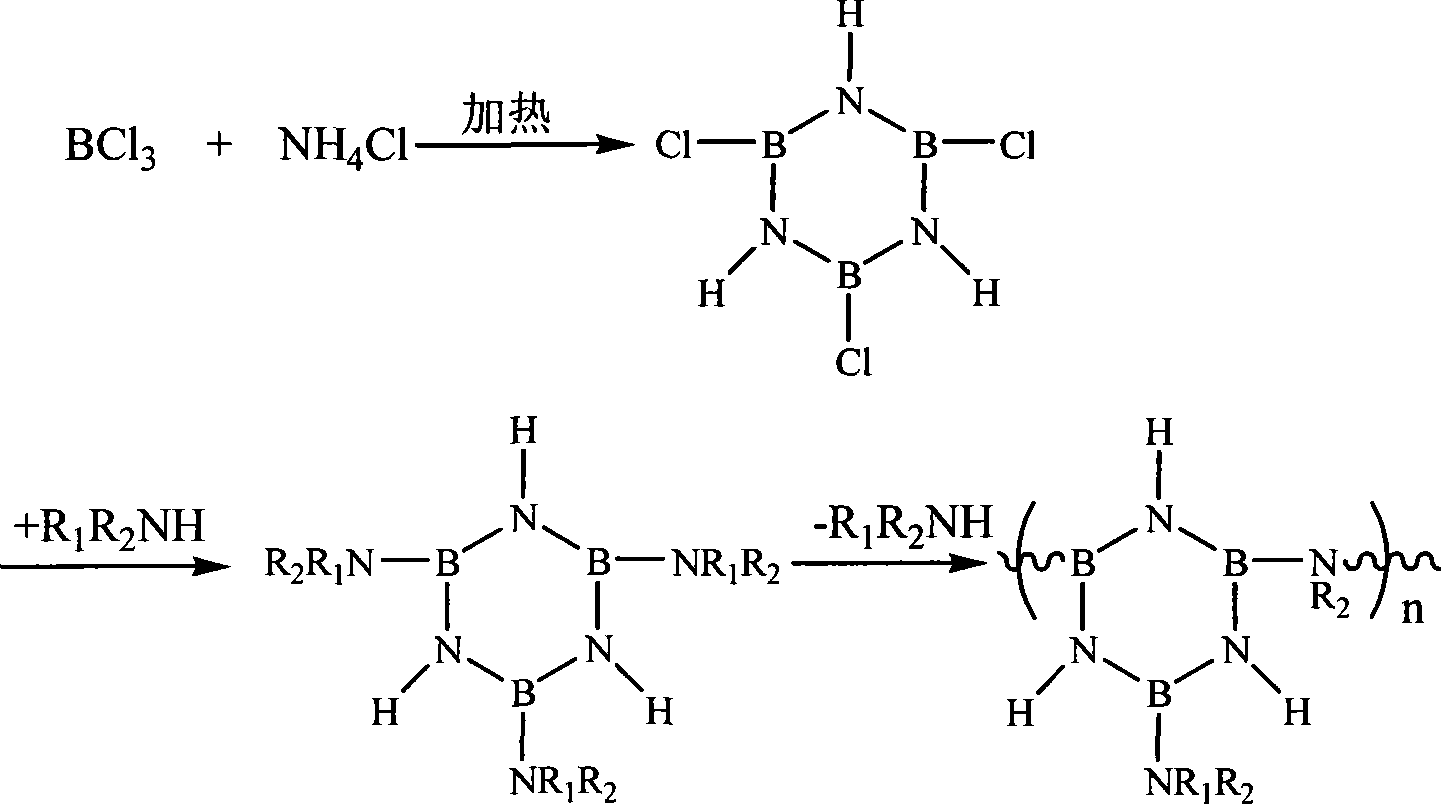
The invention relates to a preparation method of a boron nitrogen ceramic fiber precursor. The method synthesizes a trichloroborazine crystal through a high-temperature reaction of boron trichloride and ammonium chloride, and the product is mixed with an aliphatic compound containing an amino or imine group. The alkylamine is reacted at low temperature, then raised to room temperature for reaction, and then heated to 130°C to 250°C for reaction to obtain a precursor polymer. The precursor has good spinnability, and the pyrolysis product contains very little carbon element, which can make the final boron nitrogen fiber have good wave permeability.
Owner:DONGHUA UNIV
Method for preparing borosilazane ceramic fiber precursor
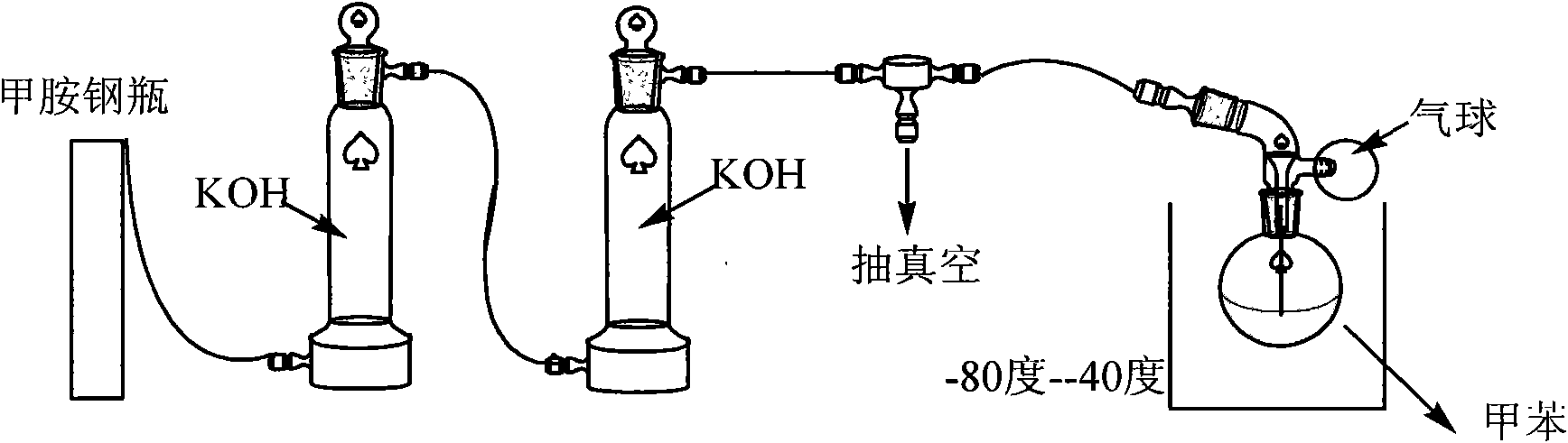
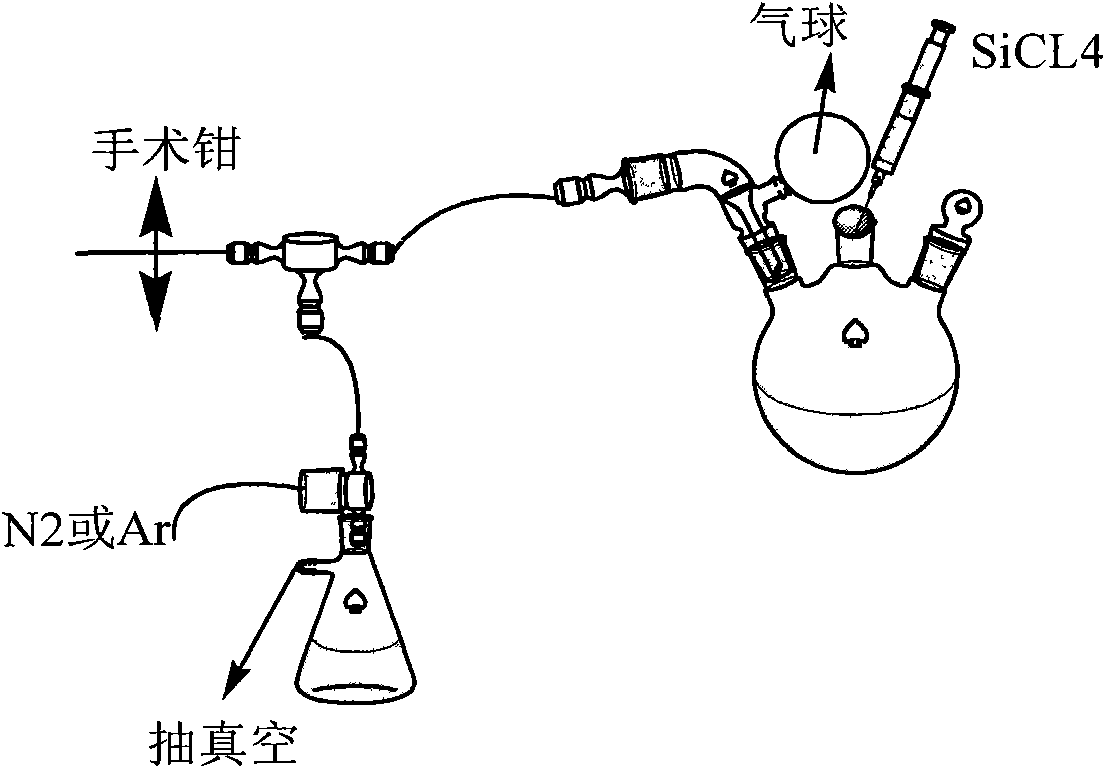
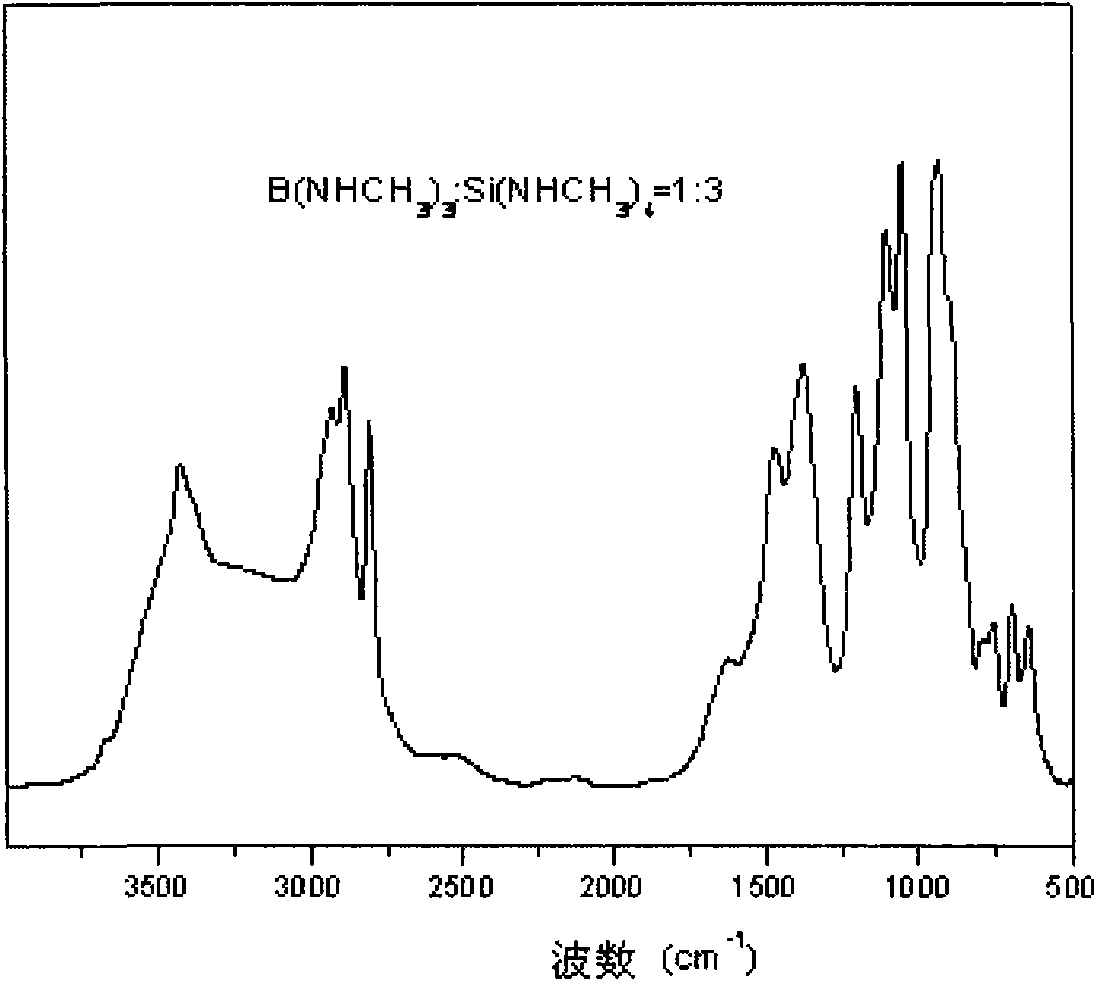
The invention relates to a method for preparing a borosilazane ceramic fiber precursor, which comprises the following steps of: reacting boron trichloride (BC13) with methylamine (CH3NH2) at -80DEG C for 12 to18 hours, reacting at -40 DEG C for 12 to 18 hours to synthesize B(NHCH3)3 micromolecules, and synthesizing Si(NHCH3)4 micromolecules by silicon tetrachloride (SiCl4) and the methylamine (CH3NH2); and removing excessive methylamine from the two type of micromolecules, filtering, mixing, condensing at the temperature of between 135 and 165 DEG C to obtain the precursor with good filament picking effect. The method has the advantages of low cost of raw materials, pure product, and high synthetic yield; and the prepared ceramic fiber precursor is heated to 1,700 DEG C in the ammonia atmosphere, the obtained product has the carbon content of less than 0.05 percent through element analysis, and has good wave permeability and good application prospect.
Owner:DONGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com