Procaterol hydrochloride impurity preparation method
A technology for procaterol hydrochloride and impurities is applied in the field of preparation of procaterol hydrochloride impurities, which can solve the problems of difficulty in obtaining and high cost, and achieve the effects of improving selectivity, reducing synthesis cost and saving preparation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
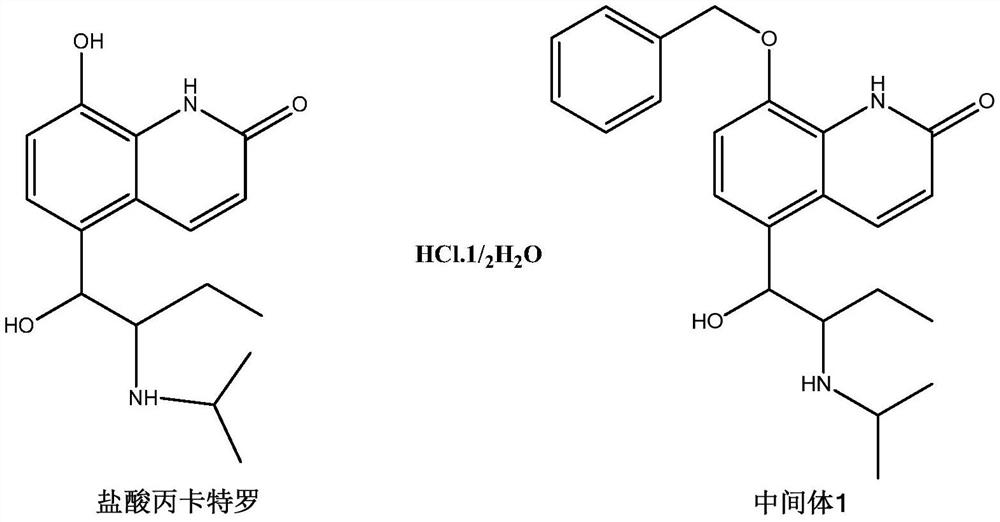
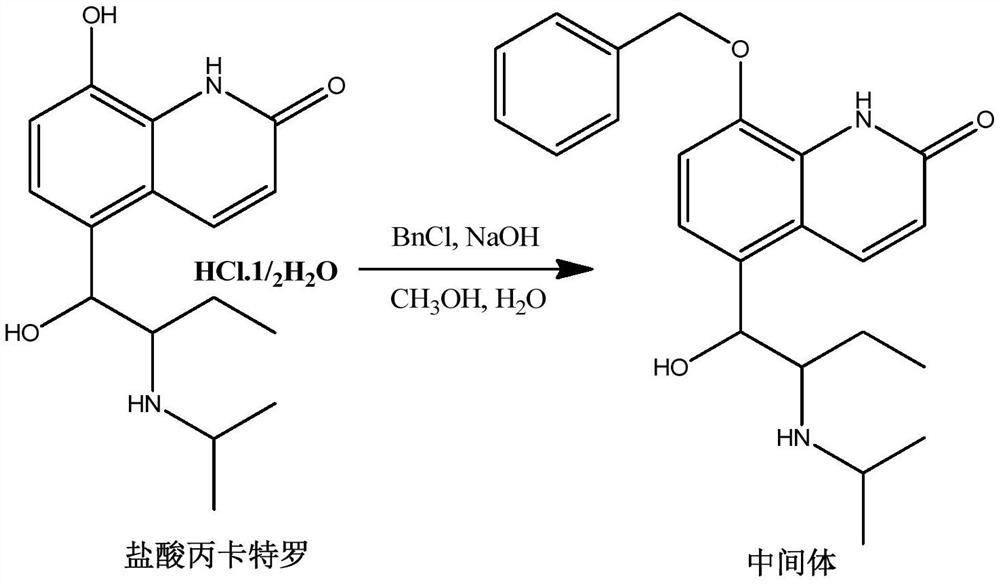
[0029] The preparation of embodiment 1 intermediate 1
[0030]
[0031] Sodium hydroxide (1.3g, 32.1mmol), 16ml of water, and 30ml of methanol were added to a 100ml single-necked flask, cooled to 0°C, procaterol hydrochloride (5.0g, 17.2mmol) was added, and then benzyl chloride (2.51g, 19.8mmol), heated to reflux to 80°C, after 4 hours of reaction, the reaction was complete, the reaction was stopped, and the temperature was lowered. Methanol was removed by rotary evaporation under reduced pressure, 150 ml of water was added, extracted twice with dichloromethane, dried and concentrated to obtain 4.70 g of a light yellow solid with a yield of 80.8%.
Embodiment 2
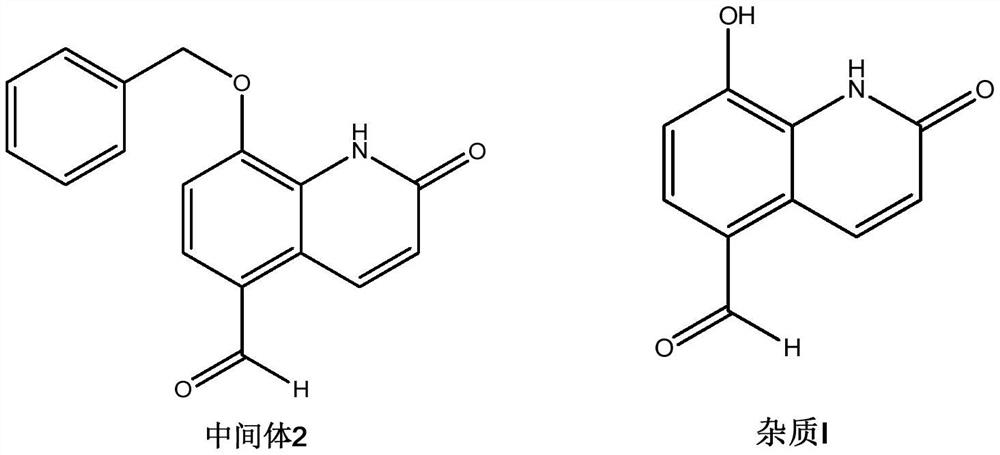
[0032] The preparation of embodiment 2 intermediate 2
[0033]
[0034] Intermediate 1 (4.0g, 10.5mmol) was added to 40ml of DMF, heated to 60°C, 150ml of sodium periodate aqueous solution (concentration: 0.1mol / L) was quickly added at one time, and reacted at 60°C for 15 minutes. After the reaction was complete, the reaction solution was poured into 400 ml of ice water, and a solid was precipitated, which was filtered and dried to obtain 2.4 g of a light yellow solid, with a yield of 82%.
Embodiment 3
[0035] The preparation of embodiment 3 Procaterol hydrochloride impurity
[0036]
[0037] Intermediate 2 (3.0g, 10.6mmol) and 90ml of dichloromethane were mixed and stirred, and the temperature was lowered to -5-0°C; boron trichloride (30ml, 30mmol) was slowly added dropwise, and reacted at -5-0°C for 2 hours until the reaction is complete. The reaction solution was poured into ice water, and a white solid was precipitated. The dichloromethane was removed by rotary evaporation, and the white solid was obtained by filtration, that is, Procaterol impurity I (1.95 g, 97.5%), and the HPLC purity was 99.65%.
[0038] 1H NMR (DMSO-d6, 400MHz): δ6.71(d, J=9.91Hz, 1H,=CH), 9.00(d, J=9.91Hz, 1H,=CH), 7.11(d, J=8.15Hz , 1H, ArH), 7.65 (d, J = 8.15 Hz, 1H, ArH), 10.03 (s, 1H, -CHO), 10.88 (s, 1H, -OH), 11.73 (s, 1H, -NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com