Method for producing boron-nitrogen ceramic fibre fore-runner body
A ceramic fiber and precursor technology, applied in the field of boron-nitrogen ceramic fiber, can solve problems such as poor mechanical properties, fiber damage, and fiber difficulties, and achieve the effects of good mechanical properties, favorable fiber formation, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
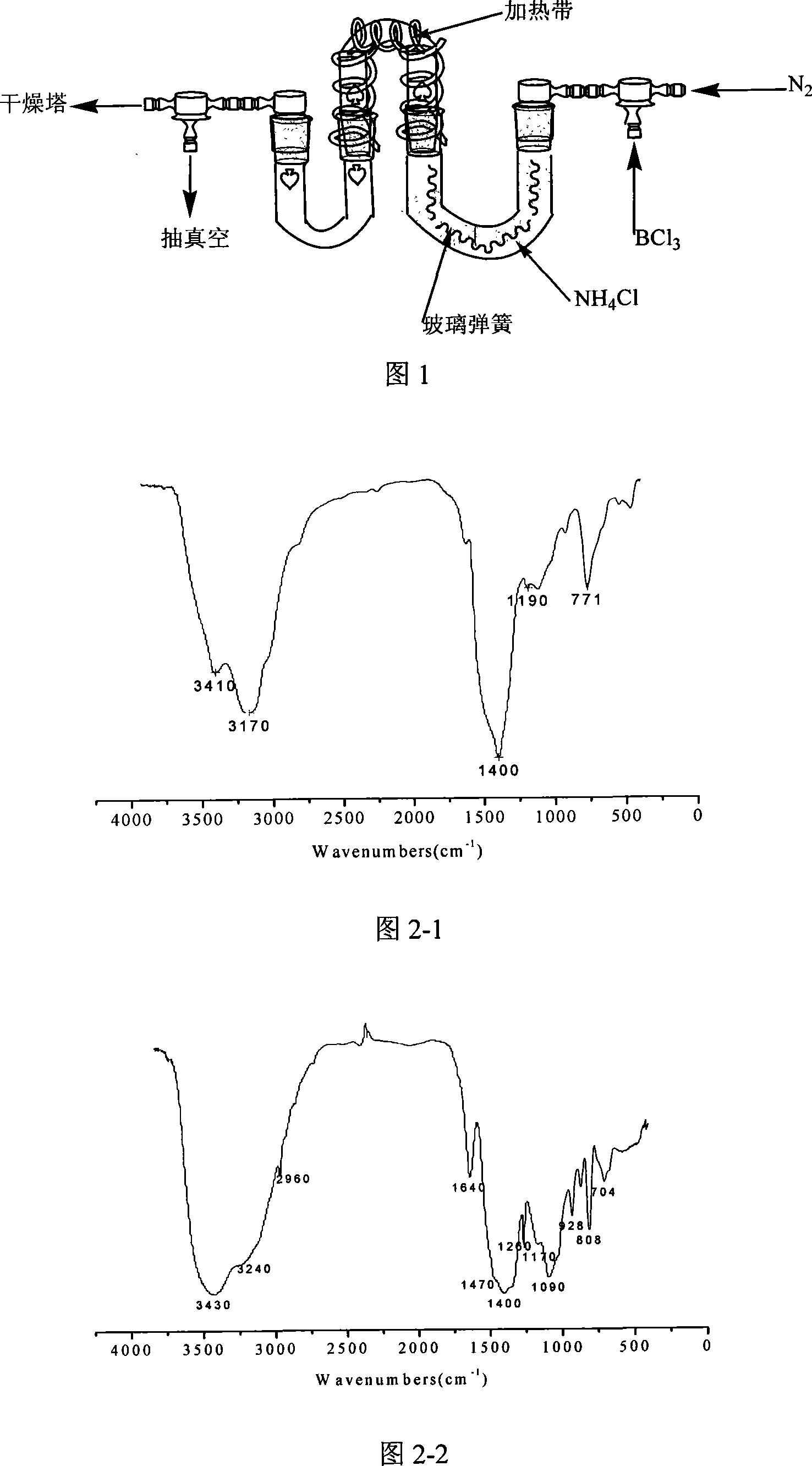
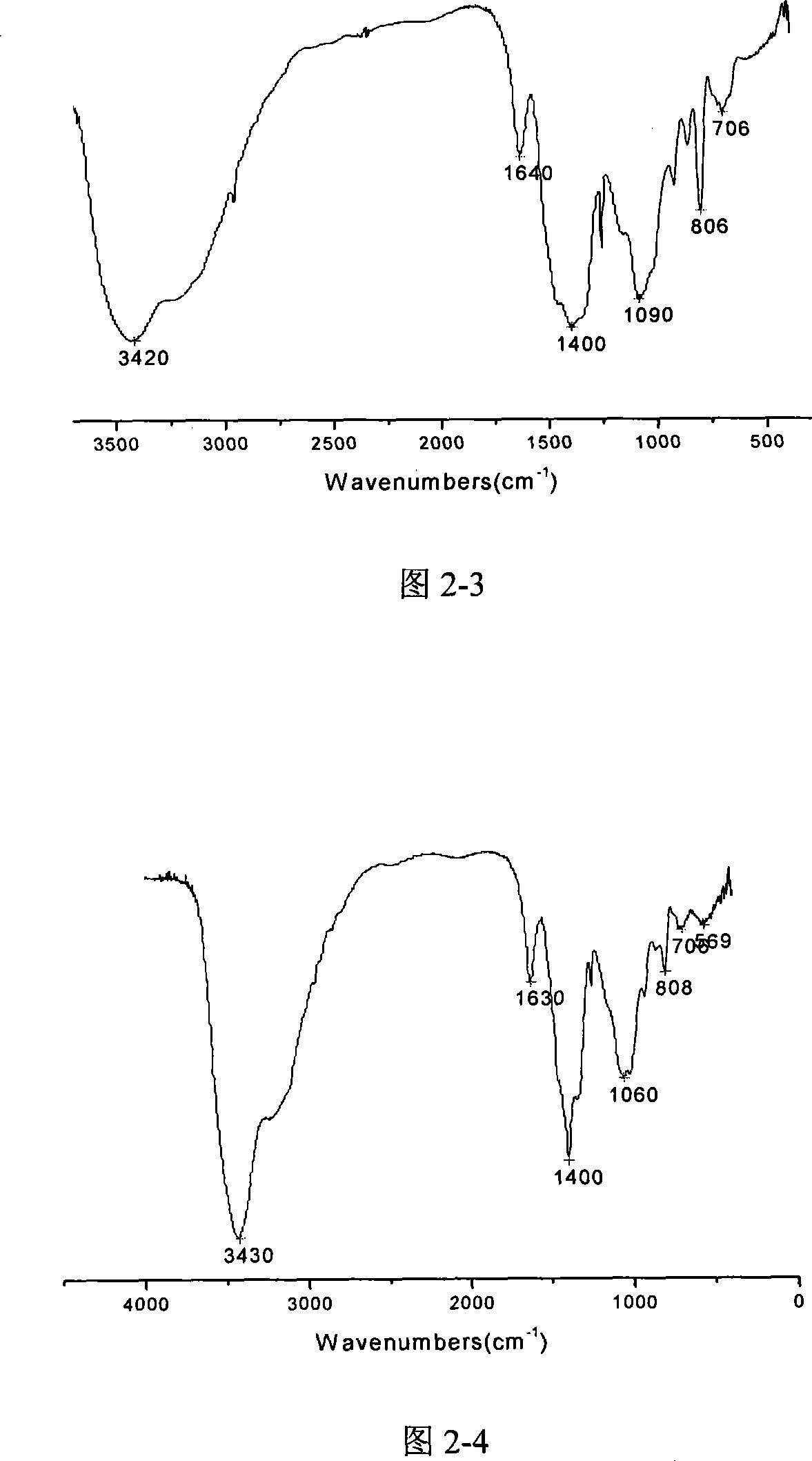
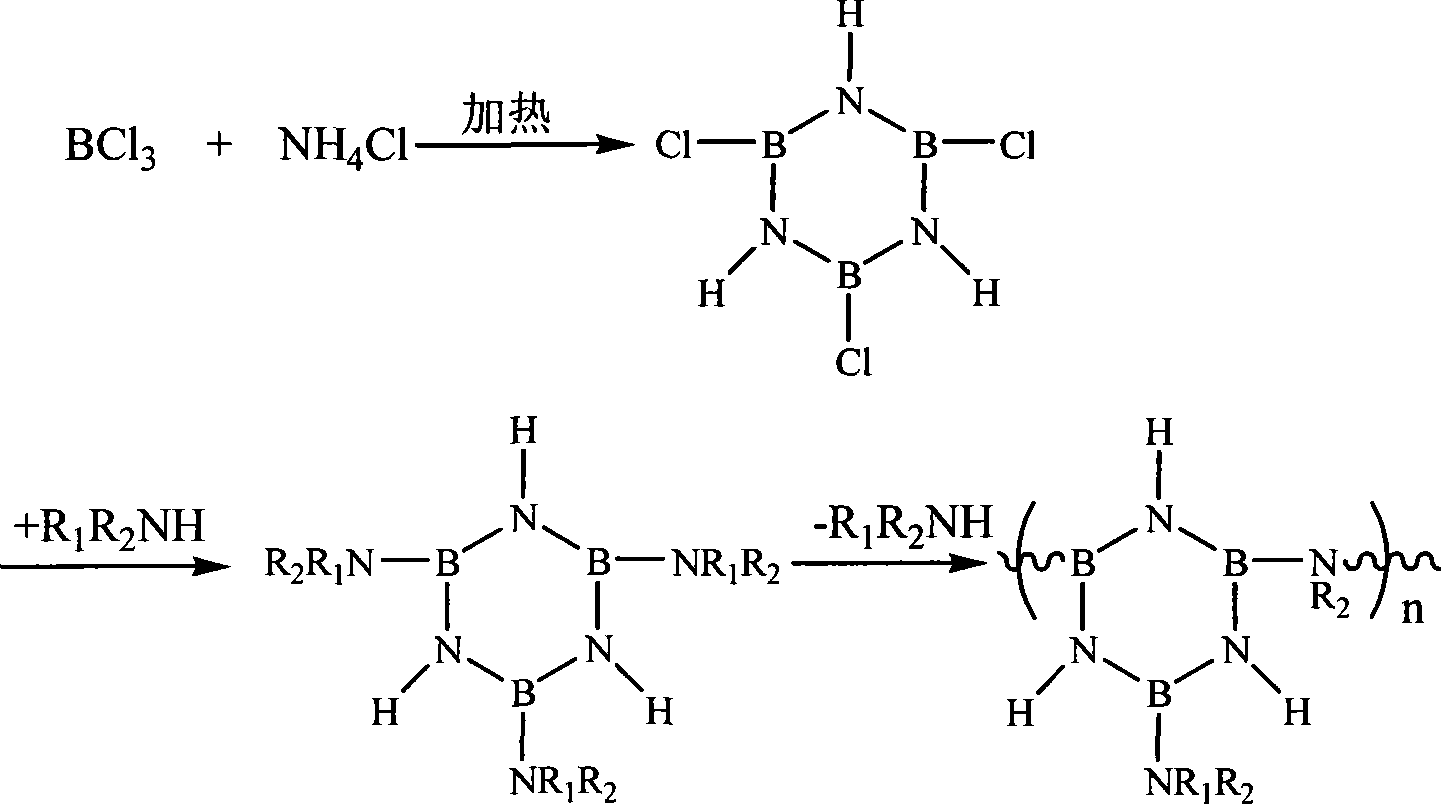
[0033] Put the ammonium chloride in a vacuum oven and dry it at 110°C for 12 hours to remove the moisture in it. After cooling, crush it to below 200 mesh and put it into a U-shaped tube. Connect the device according to Figure 1 and let dry nitrogen into it. , Heat the U-shaped tube filled with ammonium chloride to 165°C to 175°C and keep it for 2 hours to remove the water vapor in the device. After 2 hours, boron trichloride gas was introduced, and white needle-like crystals appeared immediately in another U-shaped tube, which was cyclic trichloroborazane (TCB). Figure 2-1 is the infrared spectrum of cyclic trichloroborazine.
Embodiment 2
[0035] 7.7g of TCB (41.9mmol) was dissolved in anhydrous toluene and placed in a 250ml three-necked flask at -78°C, and an excessive amount of 35ml of anhydrous methylamine (338.7mmol) was slowly added dropwise to the flask with strong magnetic stirring Anhydrous toluene solution (the volume ratio of methylamine and toluene is 1:1), white flocculent methyl ammonium chloride precipitates immediately. After the methylamine was added dropwise, the reaction was stirred for 1 hour, the temperature was raised to -40° C. and stirred for 5 hours, then the temperature was raised to room temperature and stirred for 20 hours, then the stirring was stopped. At this time, there were many white flocculent precipitates in the solution, and the precipitates were removed by filtration in a glove box filled with nitrogen, and the precipitates were washed 3 times with 50 ml of anhydrous toluene solution. After the filtrate and the extract were mixed, part of the toluene was removed under high va...
Embodiment 3
[0037] 7.7g of TCB (41.9mmol) was dissolved in anhydrous toluene and placed in a 250ml three-necked flask at -78°C, and excessive 35ml of anhydrous dimethylamine (346.5mmol) was slowly added dropwise to the flask with strong magnetic stirring. ) in anhydrous toluene solution (the volume ratio of dimethylamine and toluene is 1:1), immediately there will be white flocculent dimethylammonium chloride precipitate. After the dimethylamine was added dropwise, the reaction was stirred for 1 hour, the temperature was raised to -40° C. and stirred for 5 hours, then the temperature was raised to room temperature and stirred for 20 hours, then the stirring was stopped. At this time, there were many white flocculent precipitates in the solution, and the precipitates were removed by filtration in a glove box filled with nitrogen, and the precipitates were washed 3 times with 50 ml of anhydrous toluene solution. After the filtrate and the extract were mixed, part of the toluene was removed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com