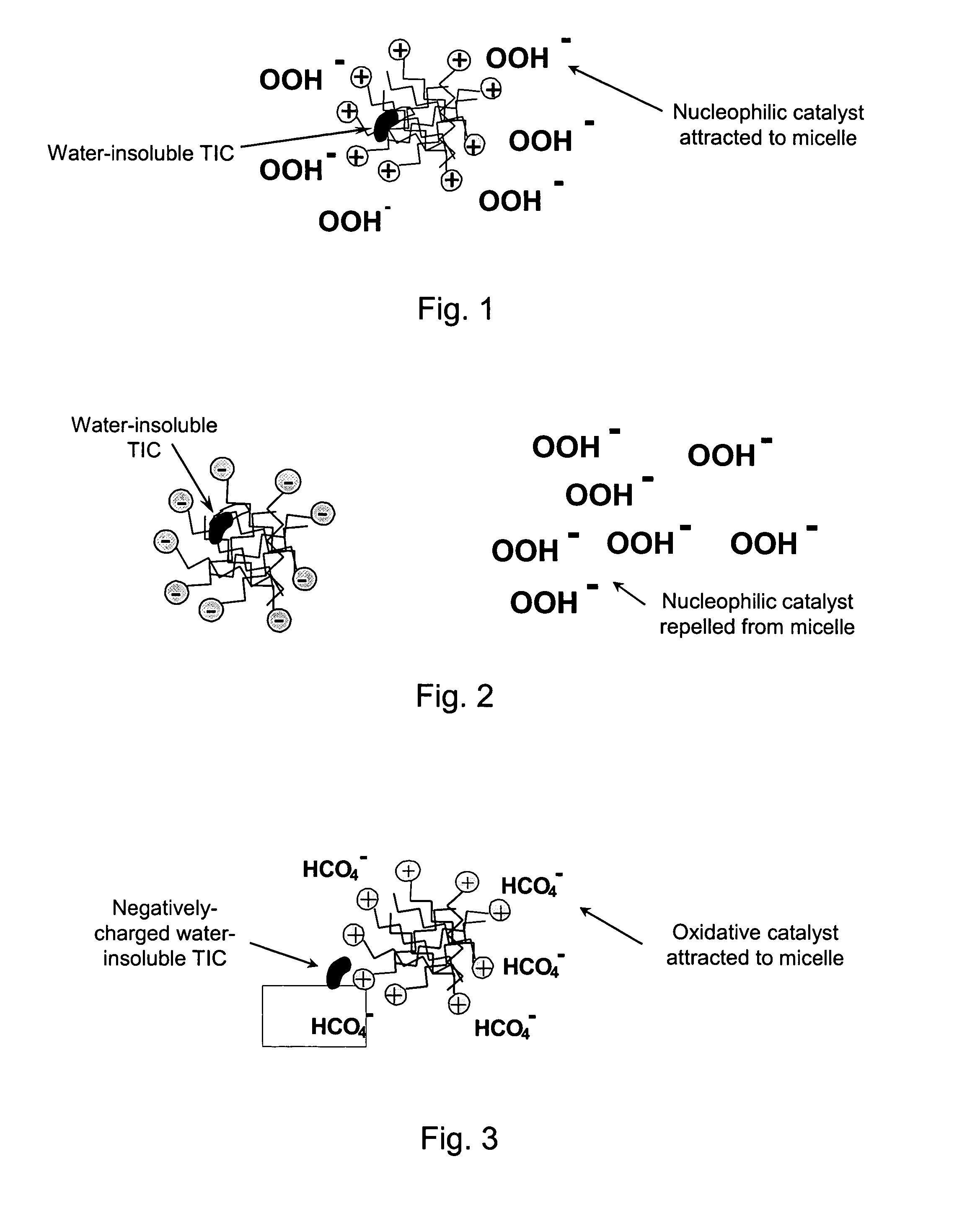
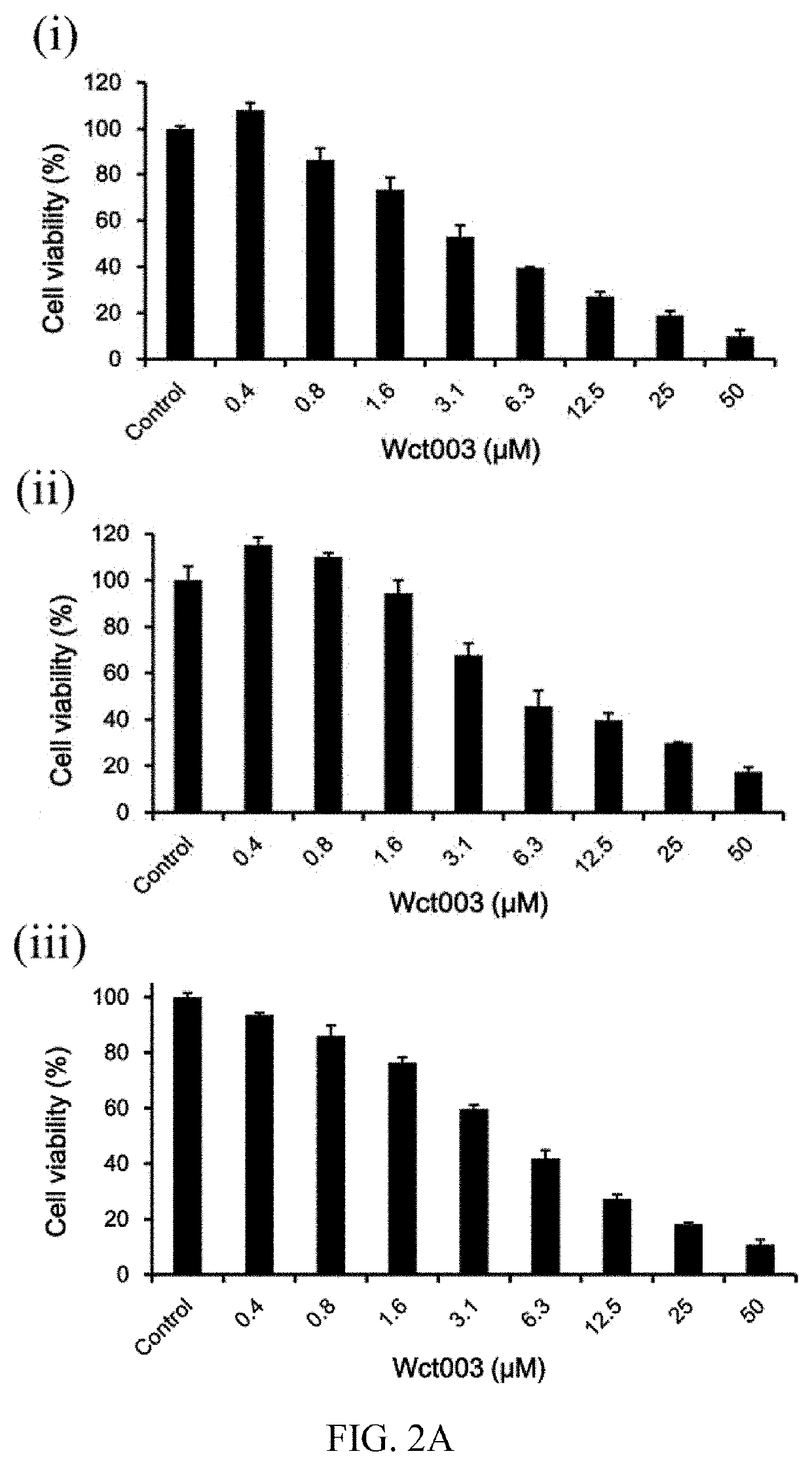
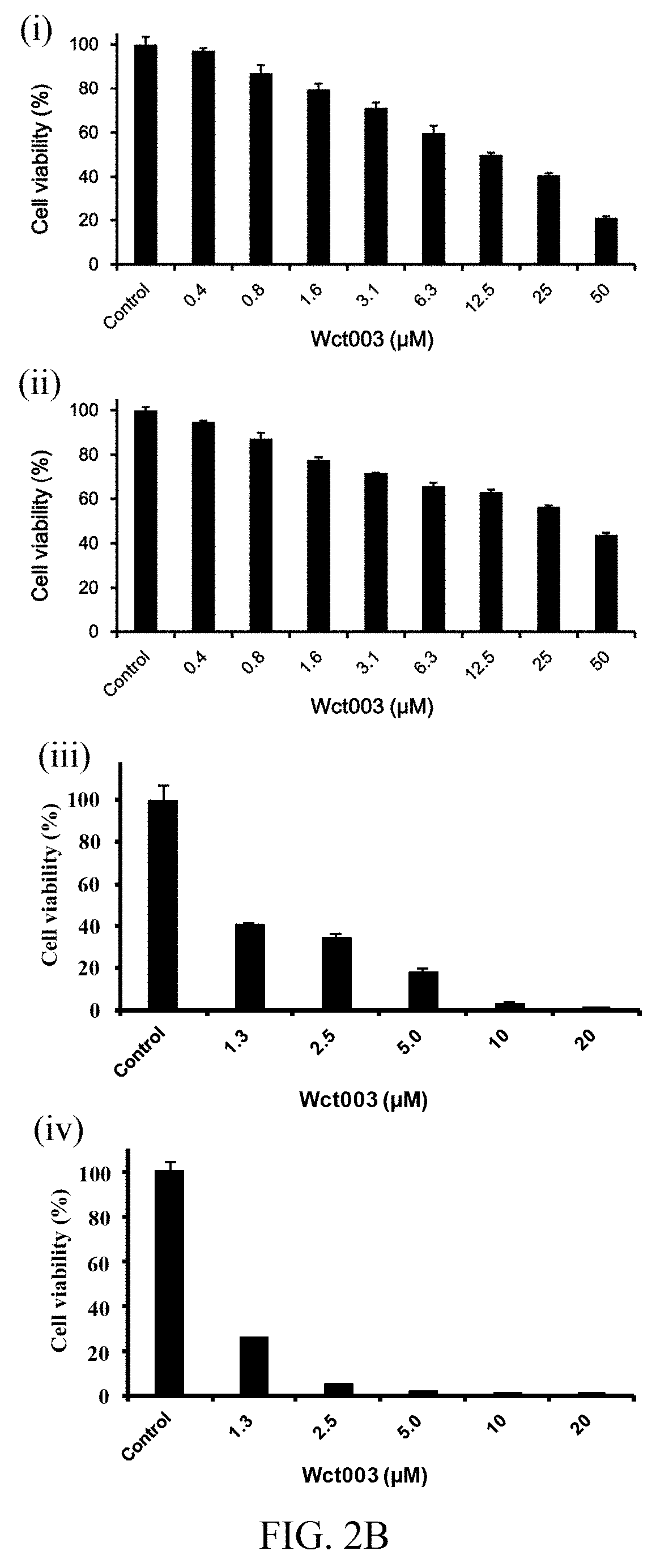
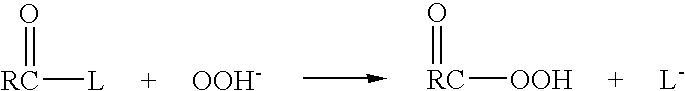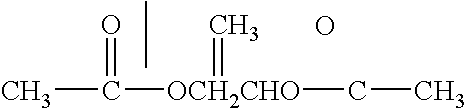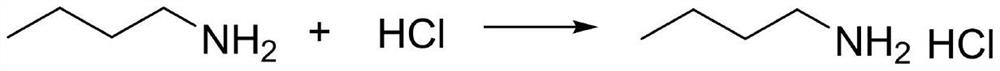Patents
Literature
39 results about "Butyl isocyanate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Occupational exposure to N-butyl isocyanate may occur through inhalation, and dermal contact in the workplace where this chemical is used or manufactured. Butyl isocyanate is an irritant to the eyes of workers in an occupational setting. It is a strong irritant to to the skin.
Reactive formulations for a neutralization of toxic industrial chemicals
InactiveUS7125497B1Efficiently neutralizedHydrogen peroxideLiquid degasificationBoron trichlorideMalathion
Decontamination formulations for neutralization of toxic industrial chemicals, and methods of making and using same. The formulations are effective for neutralizing malathion, hydrogen cyanide, sodium cyanide, butyl isocyanate, carbon disulfide, phosgene gas, capsaicin in commercial pepper spray, chlorine gas, anhydrous ammonia gas; and may be effective at neutralizing hydrogen sulfide, sulfur dioxide, formaldehyde, ethylene oxide, methyl bromide, boron trichloride, fluorine, tetraethyl pyrophosphate, phosphorous trichloride, arsine, and tungsten hexafluoride.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Method for preparing cetrorelix
InactiveCN104086632ADoes not affect yieldLow synthetic purityLuteinising hormone-releasing hormonePeptide preparation methodsPolymer scienceBackbone chain
The invention discloses a preparation method of cetrorelix. The method has the specific steps that: (A) AM resin is adopted as initial resin; according to cetrorelix main-chain peptide sequence, amino acids with N-terminal Fmoc protection and side-chain protection are coupled sequentially, wherein peptide sequence 6-site amino acid coupling adopts Fmoc-D-Orn(Dde)-OH; (B) when coupling is finished, Fmoc protecting group is removed, and an acetylation reaction is carried out on the N terminal; (C) a mixed solution with hydrazine hydrate and DMF with a volume ratio of 3:97 is used for removing D-ornithine side-chain Dde protecting group; tert-butyl isocyanate is added, such that D-ornithine side-chain in decapeptide resin is subjected to a modification reaction, and fully protected cetrorelix peptide resin is obtained; and (D) peptide resin is subjected to cracking, purification, desalination, and lyophilization, such that cetrorelix can be obtained. The content of a process impurity [D-Cit(Ac)<6>]-cetrorelix is less than 0.1%. The preparation process provided by the invention has the advantages of high product purity and low cost. The preparation method is suitable for large-scale cetrorelix production. With the process, the content of the impurity [D-Cit(Ac)<6>]-cetrorelix can be effectively controlled without influencing the yield of cetrorelix.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
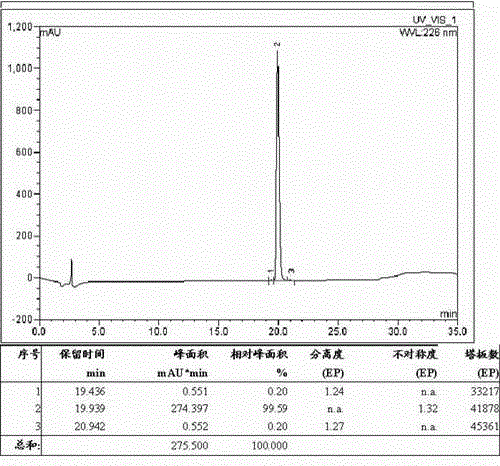
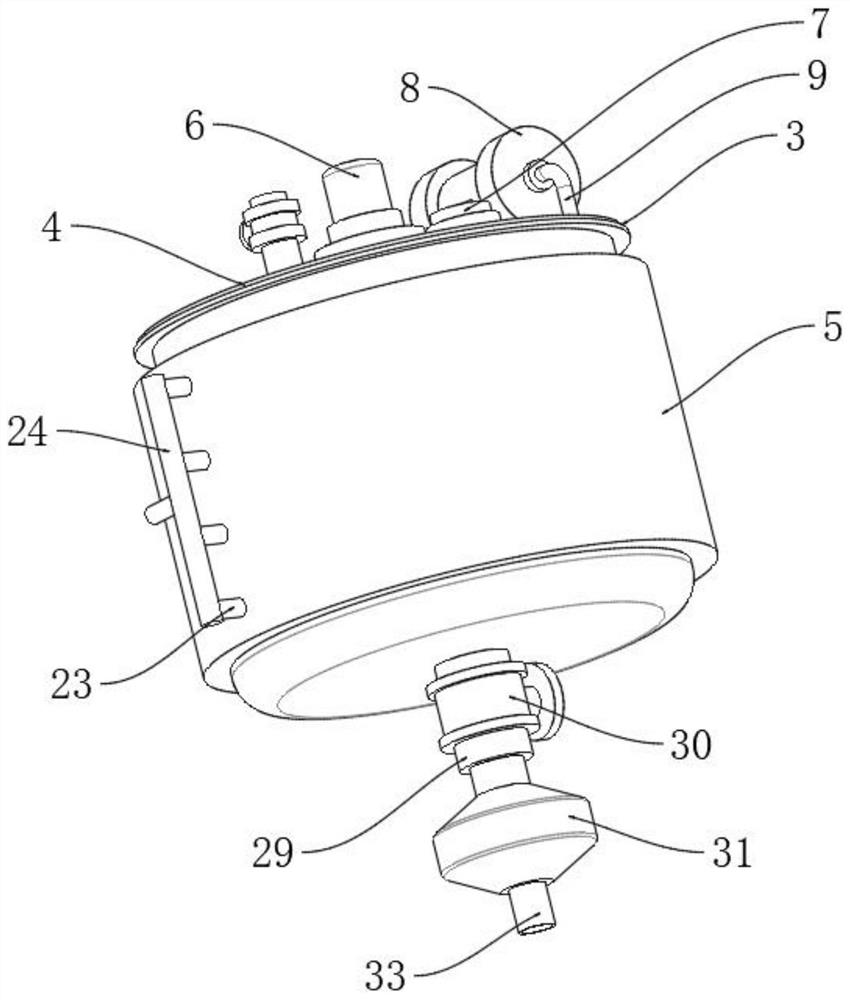
Process for preparing butyl isocyanate
ActiveCN1844091AReduce side effectsImprove conversion rateIsocyanic acid derivatives preparationOrganic compound preparationCarbonyl chlorideDistillation
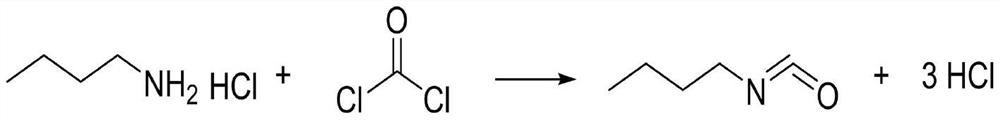
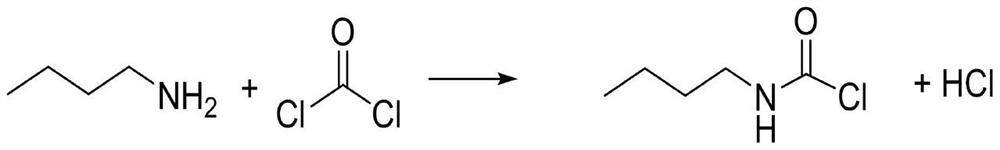
The invention discloses a Method for preparation of butylisocyanate, using n-butylamine as raw materal, dimethylbenzene as dissolvant, reacting with excess carbonyl chloride, getting the product which the purity is higher than 99% after two steps photo aerification reaction of low-temperature, high-temperature and distillation. Adopting two steps photo aerification reaction of low-temperature, high-temperature can reduce occurrence of secondary reaction effectly. In n-butylamine terms, the total yield of this invention is more than 95%.
Owner:JIANGSU ANPON ELECTROCHEM
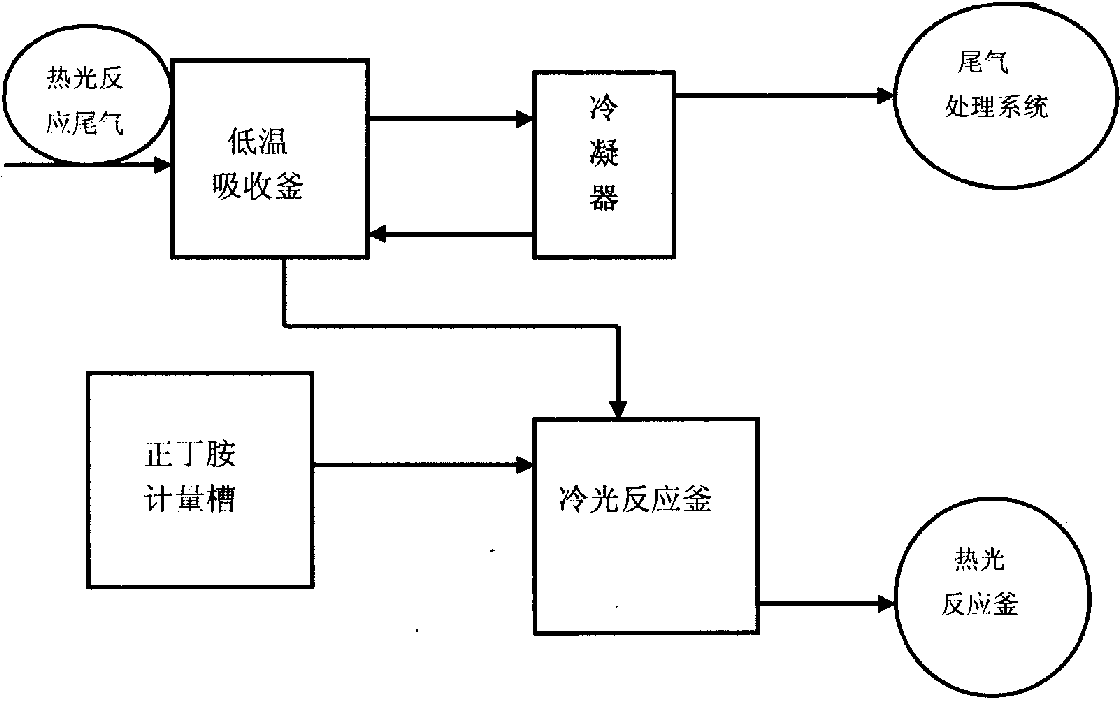
Recycling method for phosgene in tail gas generated in calorescence reaction for synthesizing normal-butyl isocyanate
ActiveCN103638688ATake advantage ofMaximize utilizationDispersed particle separationVapor condensationCalorescenceN-butylisocyanide
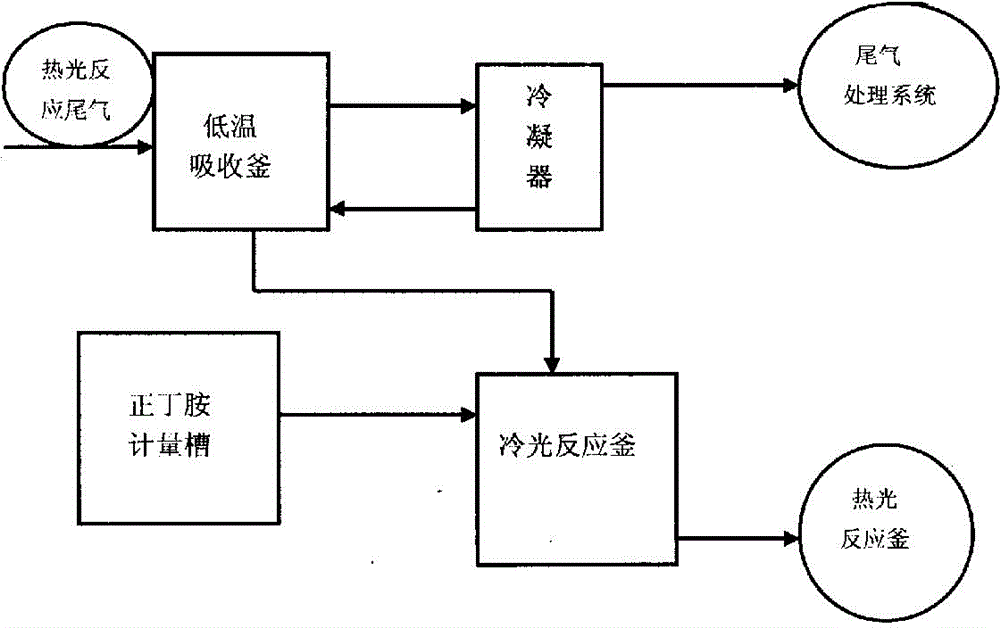
The invention relates to a recycling method for phosgene in tail gas generated in calorescence reaction for synthesizing normal-butyl isocyanate. The recycling method comprises the steps of continuously cooling and absorbing the tail gas generated in the calorescence reaction for synthesizing the normal-butyl isocyanate through a low temperature solvent, and condensing the tail gas through a condenser, wherein the non-condensing gas directly enters a tail gas processing system; preparing normal-butyl acyl chloride from the solvent containing the phosgene with certain quality concentration through cold light reaction, metering the phosgene-containing solvent, shifting the solvent into a cold light kettle, dropwise adding n-butylamine at -8 DEG C to -2 DEG C, after the dropwise adding, keeping the temperature for 1 hour so as to obtain the n-butylamine acyl chloride; and resolving the n-butylamine acyl chloride in a calorescence reaction kettle to obtain the normal-butyl isocyanate. The method provided by the invention has the advantages that the phosgene is utilized to the greatest extent, the load of the tail gas generated in the normal-butyl isocyanate calorescence reaction in a tail gas processing system and the alkali charge during tail gas processing are reduced, the production cost of the product is reduced, and the purpose of saving and recycling resources is achieved.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
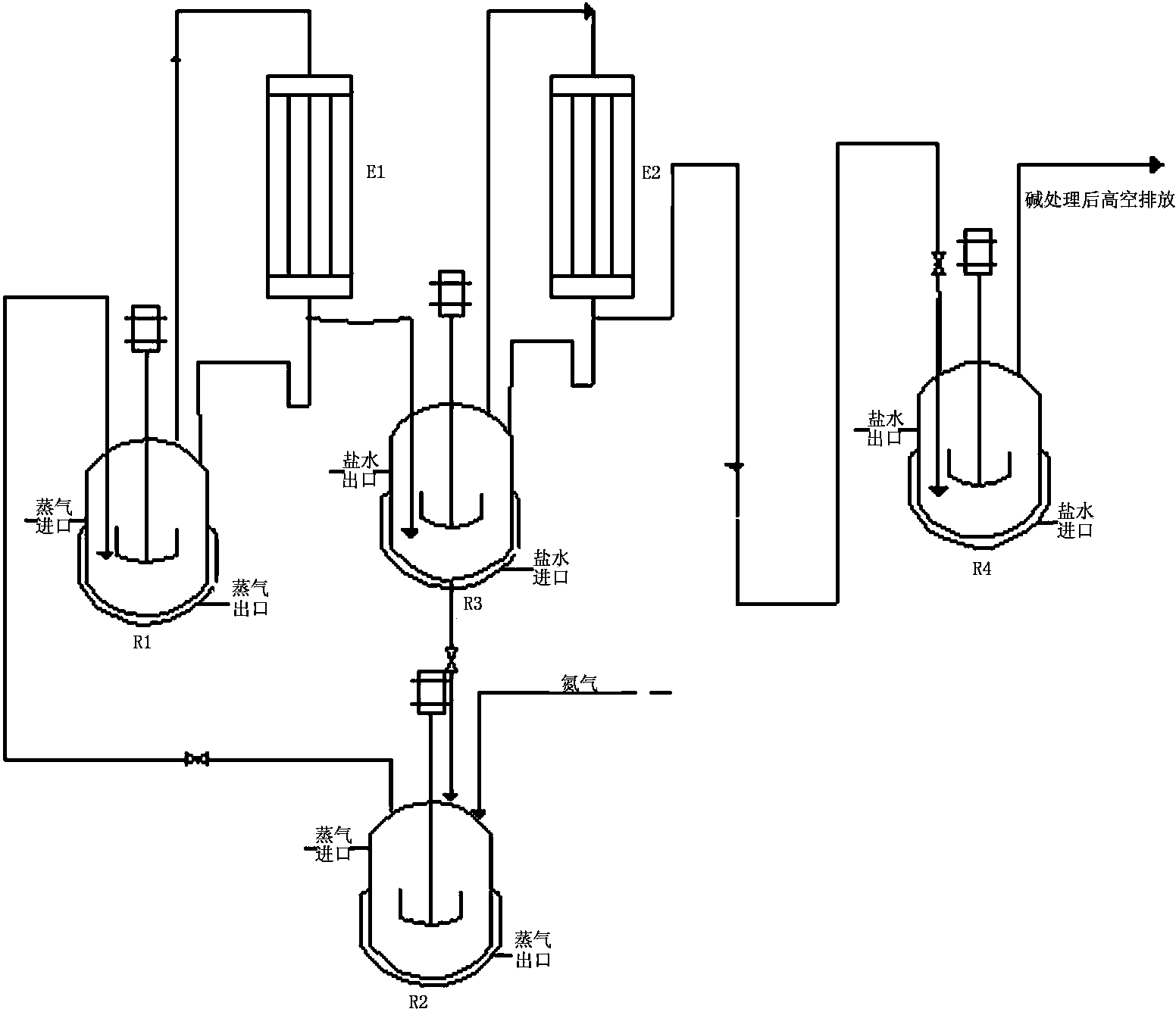
Method for applying esterification tail gas produced in synthesis of butyl isocyanate to salification
ActiveCN103848759AMaximize utilizationSolve the costIsocyanic acid derivatives preparationAmino preparation from aminesN-butylisocyanideEngineering
The invention relates to a method for applying esterification tail gas produced in synthesis of butyl isocyanate to salification. The method comprises the following steps: (1) bubbling tail gas produced in a butyl isocyanate esterification reaction by using a solvent at the temperature of between 5 DEG C below zero and 0 DEG C below zero for absorbing, and introducing remaining gas hydrogen chloride condensed by a condenser into a salification kettle to undergo a salification reaction with n-butylamine; (2) bubbling the solvent in the step (1) for absorbing, and adding a 50-60wt% phosgene-containing solvent into a phosgene removing kettle; (3) heating the phosgene-containing solvent in the phosgene removing kettle to remove phosgene, wherein the removed phosgene is applied to an esterification reaction in the preparation of the butyl isocyanate; (4) heating the solvent from which the phosgene is removed, and introducing N2 to remove the phosgene, wherein the solvent from which phosgene is removed by introducing N2 is directly taken as the solvent in the step (1); (5) applying the tail gas produced in the step (3) to the butyl isocyanate esterification reaction. The method has the advantages of low energy consumption in the entire process, low cost, small equipment investment and small amount of waste acid.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
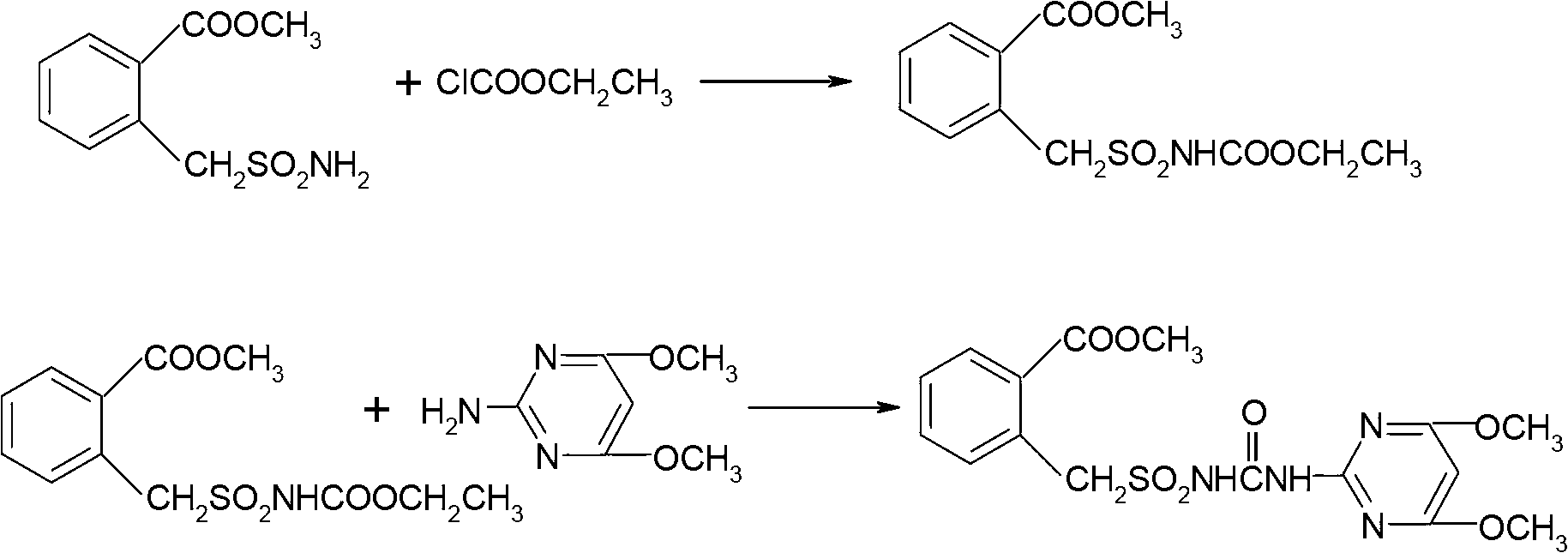
Method for preparing bensulfuron methyl
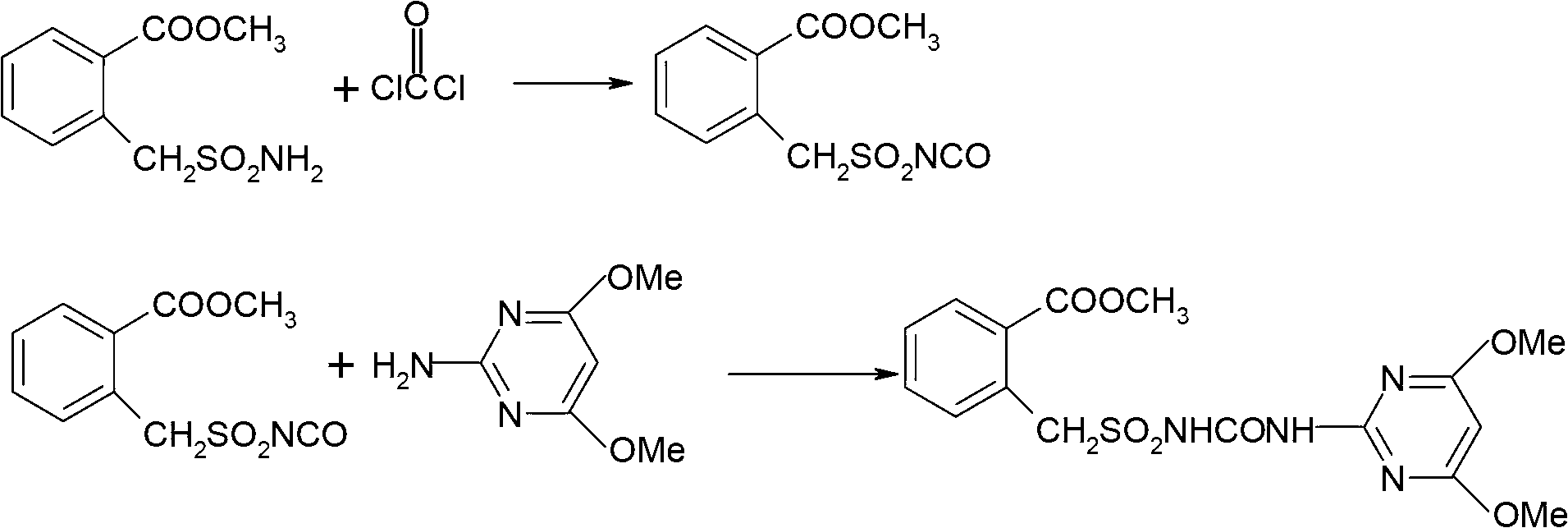
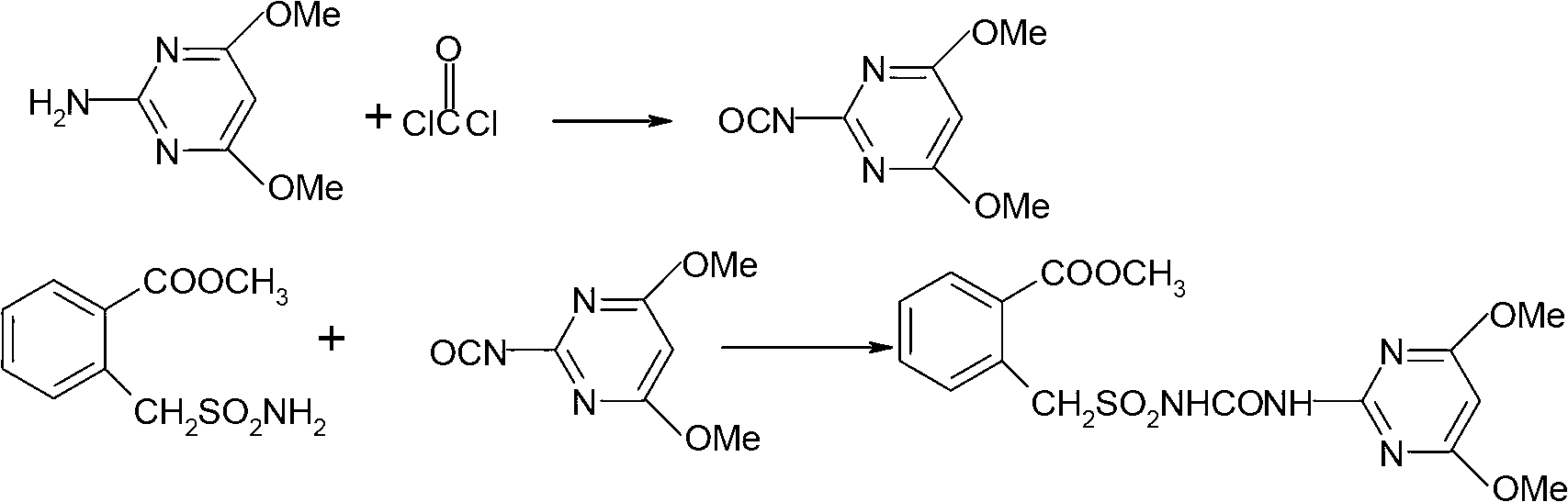
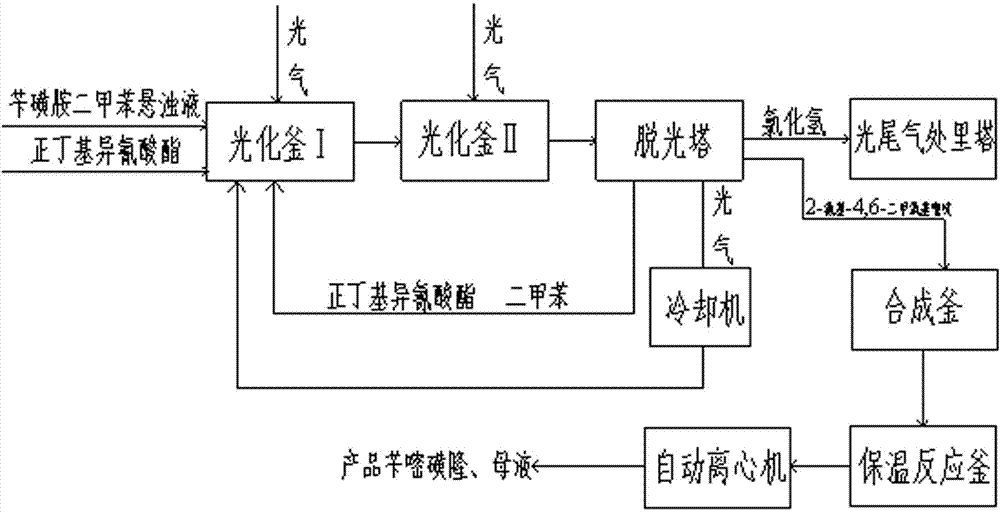
The invention discloses a method for preparing bensulfuron methyl. The method comprises the following steps of: mixing o-methyl formate benzyl sulfamide, bis(trichloromethyl) carbonate and normal-butyl isocyanate serving as a catalyst in dimethylbenzene, heating to the temperature of between 70 and 85 DEG C, heating to the temperature of between 110 and 130 DEG C slowly, adding trichloromethyl chloroformate slowly at the temperature of between 110 and 130 DEG C, performing esterification reaction at the temperature of between 110 and 130 DEG C, and removing a solvent and the catalyst after reaction; and adding o(methyl formate) benzyl sulfonyl isocyanate obtained by the esterification reaction into a condensation reaction solvent, stirring, cooling to the temperature of between 30 and 50 DEG C, continuing to add 2-amino-4,6-dimethoxy pyrimidine, performing condensation reaction at the temperature of between 50 and 90 DEG C, cooling, and drying to obtain the bensulfuron methyl. By the method, the bensulfuron methyl of which the content is more than 97 percent can be obtained, and the total yield is more than 90 percent.
Owner:JIANGSU TIANRONG GROUP
Method for synthesizing n-butyl isocyanate
InactiveCN105384659ALess side effectsNon-volatilePreparation from carboxylic acid nitrogen analoguesN-butylisocyanideEvaporation
The invention discloses a method for synthesizing n-butyl isocyanate. The method comprises the following steps: weighing n-valeric acid and thionyl chloride according to the mole ratio of 1: 1, putting n-valeric acid and thionyl chloride in a round-bottomed flask, enabling an opening of the round-bottomed flask to be connected to a reflux condensing tube, of which an upper opening is provided with a calcium chloride drying tube, carrying out magnetic stirring, heating the round-bottomed flask to the temperature of 60-70 DEG C in oil bath, and carrying out reaction for 4-5 hours, so as to obtain a n-valeryl chloride crude product; and dissolving the crude product and a catalyst in an anhydrous toluene solvent, heating the solution to the temperature of 60-80 DEG C in a three-necked flask, enabling the three-necked flask to be connected to a thermometer, a reflux condensing tube, of which an upper opening is provided with a calcium chloride drying tube, and a flask cork respectively, carrying out magnetic stirring for 5-8 minutes, then, slowly adding drying sodium azide, of which the mole is equal to that of n-valeric acid, carrying out reaction until no gas is produced, keeping the reaction for 10-15 minutes, then, filtering out insolubles, and carrying out rotary evaporation to remove toluene, thereby obtaining n-butyl isocyanate. The method has the advantages of high yield, mild reaction conditions, simplicity in operation, short reaction time and little environmental pollution.
Owner:ANHUI GUANGXIN AGROCHEM
Production method for n-butyl isocyanate
InactiveCN105294498AHigh purityHigh yieldIsocyanic acid derivatives preparationOrganic compound preparationN-butylisocyanideNitrogen
The invention provides a production method for n-butyl isocyanate. The production method comprises the following steps that a xylene solution is added into a reaction kettle, and n-butylamine steam is introduced into the xylene solution in the reaction kettle; phosgene is introduced into the xylene solution; the phosgene is continuously introduced into the reaction kettle; the temperature of the reaction kettle is lowered, and nitrogen is introduced into the reaction kettle for gas driving. According to the production technology, the purity of an n-butylamine intermediate of the n-butyl isocyanate can be increased, so that the yield can be increased by 10%-20%, wherein the purity of the n-butylamine is increased to 75.9%-80.5%; in addition, the efficiency is improved, the phenomenon that the production efficiency and purity of the n-butyl isocyanate are influenced due to the fact that the n-butylamine has too many impurities is avoided, and by-products are few.
Owner:ANHUI GUANGXIN AGROCHEM
Method for co-producing n-butyl isocyanate from chloroformic acid-2-ethylhexyl ester
InactiveCN112390729ARelieve stressHigh industrial valueChlorine/hydrogen-chlorideOrganic compound preparationEthyl groupChloroformic acid
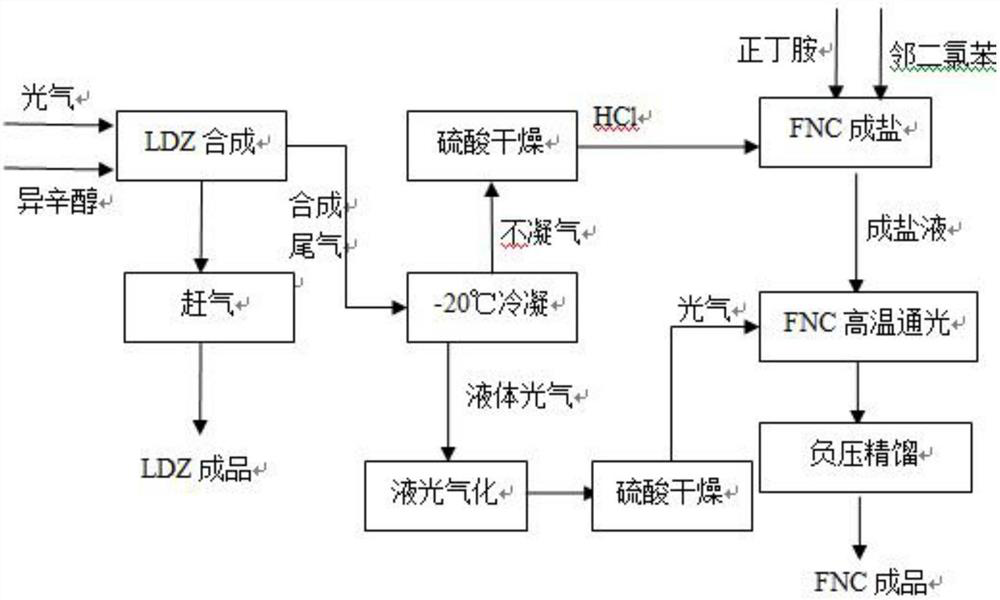
The invention discloses a method for co-producing n-butyl isocyanate from chloroformic acid-2-ethylhexyl ester, wherein the method comprises the steps: preparing LDZ from phosgene and isooctanol as raw materials, wherein the tail gas composition is byproduct hydrogen chloride and excessive phosgene; introducing inert gas into the LDZ synthetic liquid, and performing a gas driving process to obtaina finished product LDZ and synthetic tail gas; condensing the LDZ synthetic tail gas by a condenser to obtain condensate, and enabling the condensate to be liquid phosgene containing LDZ and uncondensed gas to be HCl, wherein HCl is used for n-butylamine salifying after passing through a concentrated sulfuric acid scrubbing device, and liquid phosgene is used as a phosgene source for FNC synthesis after being washed with sulfuric acid; and introducing HCl for gas washing into an o-dichlorobenzene solution containing n-butylamine, carrying out a salifying reaction until n-butylamine in o-dichlorobenzene is not detected and salifying is finished, introducing phosgene, carrying out FNC synthesis until the synthesis solution is clear, and carrying out light driving and rectification on the synthesis solution to obtain an FNC finished product. According to the co-production scheme provided by the invention, the discharge amount of tail gas is greatly reduced, the waste gas is reduced by 85% or more, and the economic effect is remarkable.
Owner:NINGXIA RUITAI TECH
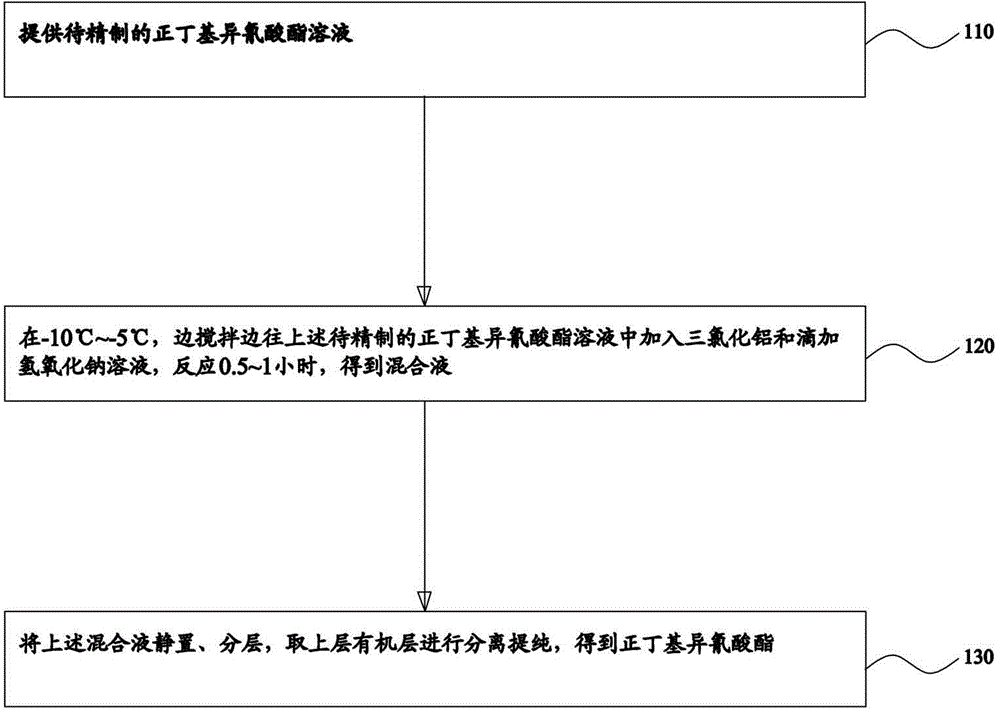
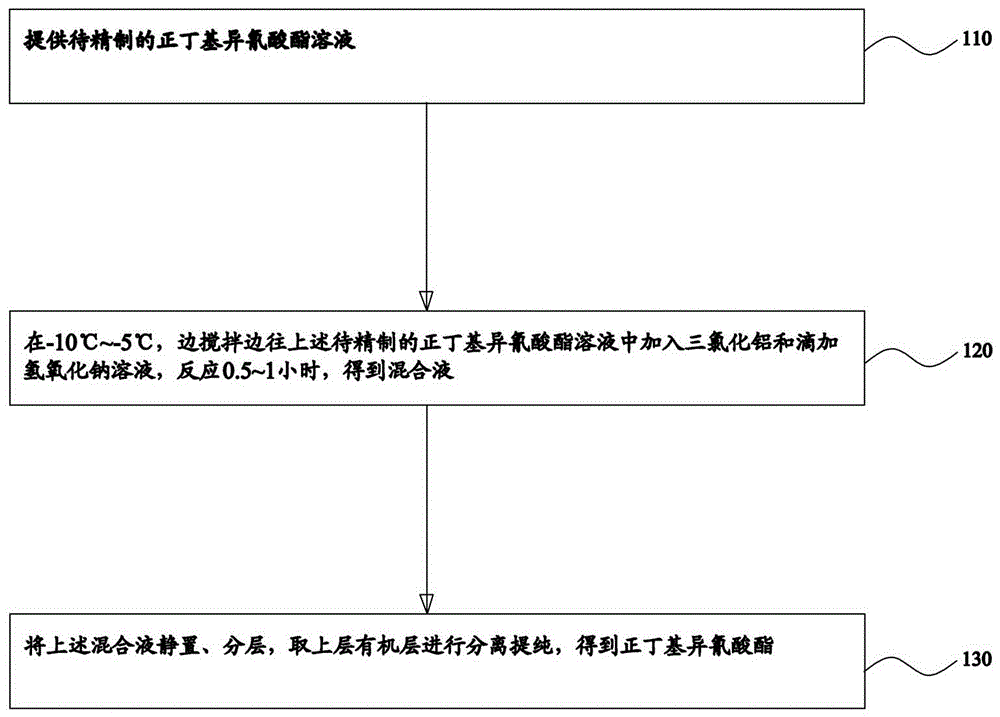
Refining method for normal-butyl isocyanate
ActiveCN104447411AImprove product qualityIsocyanic acid derivatives purification/separationN-butylisocyanideOrganic layer
The invention provides a refining method of normal-butyl isocyanate. The method comprises the following steps: providing normal-butyl isocyanate solution to be refined, adding alchlor and dropwise adding a sodium hydroxide solution to the normal-butyl isocyanate solution to be refined at minus 10 to minus 5 DEG C while stirring, ensuring that reaction is performed for 0.5 to 1 hour, so as to obtain mixed liquor, ensuring that the mixed liquor is subjected to standing and layering, and separating and purifying an organic layer in the upper layer, so as to obtain the normal-butyl isocyanate. According to the refining method for normal-butyl isocyanate, impurity acid and a poly (normal-butyl isocyanate) floccule in the normal-butyl isocyanate to be refined are neutralized by using by the sodium hydroxide under the action of alchlor, then standing and layering are carried out, and the organic phase in the upper layer is separated and purified to obtain the normal-butyl isocyanate; according to the method, the product quality is improved.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
Refining method for n-butyl isocyanate
InactiveCN105254535AHigh purityReduce generationOrganic compound preparationIsocyanic acid derivatives purification/separationFiltrationN-butylisocyanide
The invention provides a refining method for n-butyl isocyanate. The refining method comprises the steps that xylene solution is added into a reaction kettle, and the temperature in the reaction kettle is reduced; phosgene is led into the xylene solution; n-butylamine solution is dripped into the reaction kettle; phosgene continues to be led into the reaction kettle; nitrogen is led into the reaction kettle, where reaction is conducted, for gas expelling and filtration; sodium hydroxide solution is added to filtered solution; after the solution is filtered, xylene solution is added thereto to extract n-butyl isocyanate. By the adoption of the production process, the purity of the n-butyl isocyanate can be improved, a great improvement is achieved compared with an existing rectification process, and production of by-products is reduced since temperature and time are easy to control.
Owner:ANHUI GUANGXIN AGROCHEM
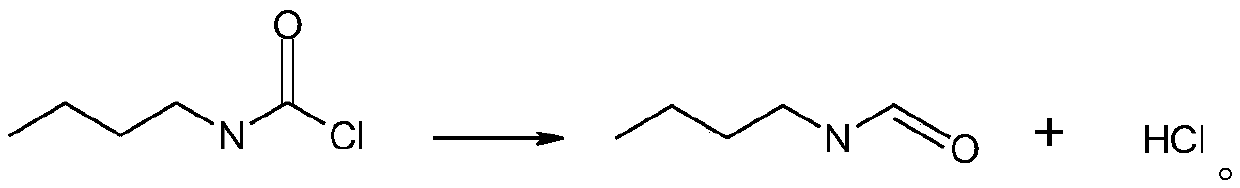
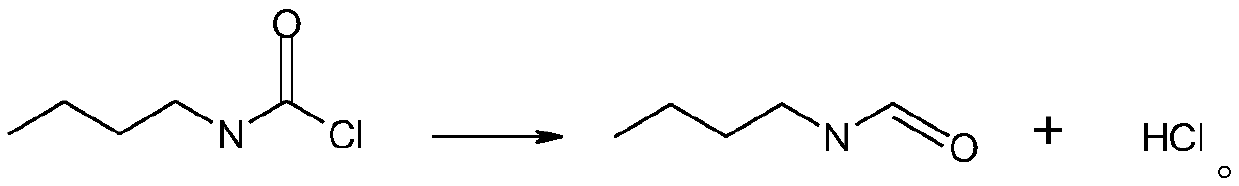
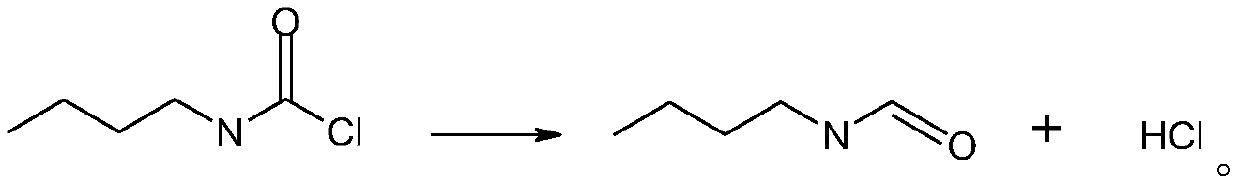
Synthetic method for n-butyl isocyanate
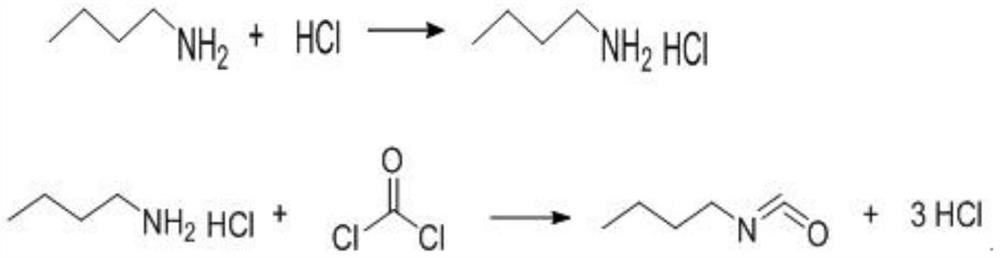
InactiveCN110218163AEliminate potential safety hazardsPromote safe productionPreparation from carbamatesChemical synthesisOrganic solvent
The invention discloses a synthetic method for n-butyl isocyanate. The method comprises the following steps: selecting n-butylcarbamyl chloride, performing a reaction in an organic solvent for 8-15 hunder catalysis of a catalyst at a temperature of 80-160 DEG C, and performing thermal decomposition to obtain the n-butyl isocyanate, wherein the reaction equation is shown in the description. According to the synthetic method for the n-butyl isocyanate provided by the invention, the n-butylcarbamyl chloride is directly subjected to the reaction in the inert solvent to obtain the n-butyl isocyanate, and the chemical synthesis method eliminates safety hazards from a process source, and is a preparation method having a reasonable process, safe production, a high reaction yield, low production costs and substantially no three waste (waste water, waste gas and industrial residue) for the n-butyl isocyanate; and the method has an advanced technological route, avoids highly toxic phosgene and diphosgene, and has simple and safe operation, a high reaction yield, low production costs, less three waste (waste water, waste gas and industrial residue), larger implementation values and social andeconomic benefits.
Owner:XINYI AGRI CHEM PLANT JIANGSU PROV
Tail gas treatment method for n-butyl isocyanate
ActiveCN105289249AThorough treatmentReduce pollutionDispersed particle separationN-butylisocyanideNitrogen
The invention provides a tail gas treatment method for n-butyl isocyanate. The tail gas treatment method comprises the following steps that a xylene solution is added into a reaction kettle, and the temperature in the reaction kettle is lowered; phosgene is introduced into the xylene solution; an n-butylamine solution is dropwise added into the reaction kettle; the phosgene is continuously introduced into the reaction kettle; nitrogen is introduced into the reacted reaction kettle for first-time gas driving, and mixed gas obtained after first-time gas driving is performed is introduced into a cooling kettle; nitrogen is introduced into the cooling kettle for second-time gas driving, gas obtained after second-time gas driving is performed is transferred into a spraying kettle, and cold water is sprayed into the spraying kettle; third-time gas driving is performed on the spraying kettle; filtering is performed, fourth-time gas driving is performed on the filtered materials, gas obtained after fourth-time gas driving is performed is introduced into a NaOH solution, and the balance gas is directly exhausted into air. According to the production technology, tail gas can be stepwise treated, the components in the tail gas can be stepwise decomposed, then the tail gas can be treated more thoroughly, and therefore the pollution to the environment is reduced.
Owner:ANHUI GUANGXIN AGROCHEM
Refining method of n-butyl isocyanate
ActiveCN104447411BImprove product qualityIsocyanic acid derivatives purification/separationPurification methodsN-butylisocyanide
The invention provides a refining method of normal-butyl isocyanate. The method comprises the following steps: providing normal-butyl isocyanate solution to be refined, adding alchlor and dropwise adding a sodium hydroxide solution to the normal-butyl isocyanate solution to be refined at minus 10 to minus 5 DEG C while stirring, ensuring that reaction is performed for 0.5 to 1 hour, so as to obtain mixed liquor, ensuring that the mixed liquor is subjected to standing and layering, and separating and purifying an organic layer in the upper layer, so as to obtain the normal-butyl isocyanate. According to the refining method for normal-butyl isocyanate, impurity acid and a poly (normal-butyl isocyanate) floccule in the normal-butyl isocyanate to be refined are neutralized by using by the sodium hydroxide under the action of alchlor, then standing and layering are carried out, and the organic phase in the upper layer is separated and purified to obtain the normal-butyl isocyanate; according to the method, the product quality is improved.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
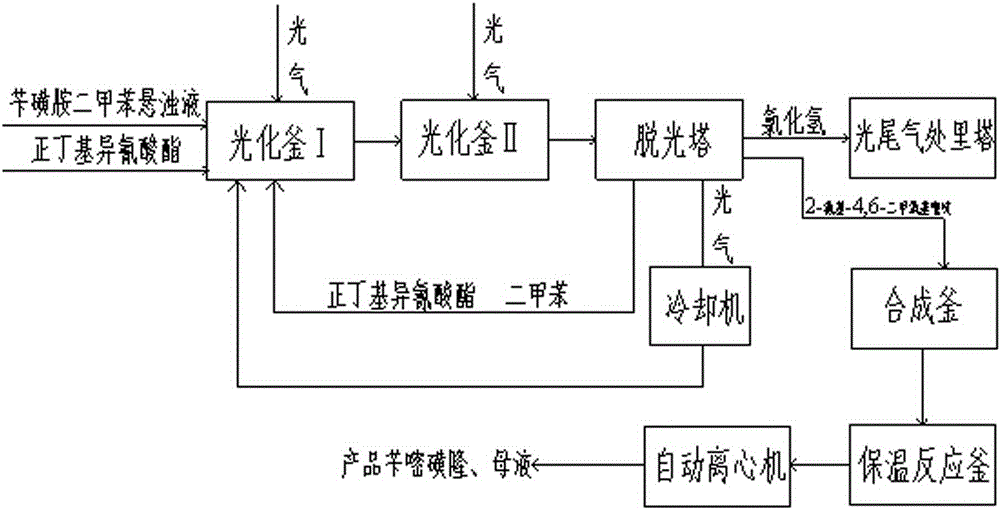
A kind of method for preparing bensulfuron-methyl
ActiveCN103483274BReduce decompositionImprove conversion rateOrganic chemistryChemical recyclingBensulfuron methylN-butylisocyanide
The invention discloses a method for preparing bensulfuron methyl. The method comprises the following steps: adding phosgene, benzylsulfamide xylene and normal-butyl isocyanate into a photochemical kettle I to synthesize o-methoxy carboxyl benzyl sulfamide; after the o-methoxy carboxyl benzyl sulfamide enters a photochemical kettle II, adding phosgene to synthesize o-methoxy carboxyl benzylsulfo isocyanate; enabling o-methoxy carboxyl benzylsulfo isocyanate to enter a stripping tower to strip normal-butyl isocyanate, dimethyl benzene and phosgene; adding o-methoxy carboxyl benzylsulfo isocyanate subjected to gas stripping and 2-amino-4,6-dimethoxy pyrimidine into a synthesis kettle; after the material enters an automatic centrifuger, performing centrifugal separation to obtain the product bensulfuron methyl. The method has the benefits that a catalyst is recycled in the photochemical process, so that the consumption is reduced, and the cost and the treatment cost are both lowered; the stripping tower is adopted to remove phosgene in a dimethyl benzene solution, so that the consumption of dimethyl benzene is reduced; when the method provided by the invention is adopted for synthesis, the synthesis yield and the product quality are improved and the synthesis yield can be up to 98%.
Owner:JIANGSU KUAIDA AGROCHEM
A kind of method of synthesizing cetrorelix
ActiveCN107778355BHigh purityHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsSide chainTrifluoroacetic acid
The invention relates to the field of medicine synthesis and discloses a method for synthesizing cetrorelix. The present invention adopts a brand-new amino resin as a carrier, and adopts protected D-Orn as the precursor of D-Cit, then removes the side chain protecting group and reacts with tert-butyl isocyanate to generate D-Cit (tBu), through the unique containing Hydrogen bromide trifluoroacetic acid solution to acid hydrolyze the cetrorelix peptide resin, and simultaneously remove the side chain protecting group tBu of the generated D-Cit(tBu) to the greatest extent, and the finally obtained cetrorelix has a higher The purity and total yield avoid the generation of toxic impurity [D-Cit(Ac)]-cetrorelix, the whole method is simple and easy to operate, the conditions are mild, and the product quality is high.
Owner:CHENGDU SHENGNUO BIOPHARM
Preparation method of toughening conducting material
The invention discloses a preparation method of a toughening conducting material. The preparation method comprises the following steps: weighing carbon fiber and carbon nano tubes into a hydrochloricacid solution with the concentration of 2 to 5 mol / L, treating the solution for 1 to 3 h at the temperature of 30 to 50 DEG C, then carrying out ultrasonic stirring treatment for 1 to 2 h, and filtering to obtain a filter cake; adding N-hydroxymethyl phthalimide and butyl isocyanate into the filter cake, adjusting the PH value to be 7.5 to 8.5, and continuously stirring for 30 to 40 min; adding apolypropylene / polyethylene mixture into the obtained substance, and mixing the solution for 40 to 60 min at the temperature of 70 to 80 DEG C, wherein the mass ratio of polypropylene to polyethylene is 1 to 3 to 1; then putting the mixture in a mold for hot press molding to obtain the toughening conducting material.
Owner:SUZHOU KEMAO ELECTRONICS MATERIALS TECH
A method for recovering and utilizing phosgene in the tail gas of thermo-optic reaction for synthesizing n-butyl isocyanate
ActiveCN103638688BTake advantage ofMaximize utilizationDispersed particle separationVapor condensationCalorescenceN-butylisocyanide
The invention relates to a recycling method for phosgene in tail gas generated in calorescence reaction for synthesizing normal-butyl isocyanate. The recycling method comprises the steps of continuously cooling and absorbing the tail gas generated in the calorescence reaction for synthesizing the normal-butyl isocyanate through a low temperature solvent, and condensing the tail gas through a condenser, wherein the non-condensing gas directly enters a tail gas processing system; preparing normal-butyl acyl chloride from the solvent containing the phosgene with certain quality concentration through cold light reaction, metering the phosgene-containing solvent, shifting the solvent into a cold light kettle, dropwise adding n-butylamine at -8 DEG C to -2 DEG C, after the dropwise adding, keeping the temperature for 1 hour so as to obtain the n-butylamine acyl chloride; and resolving the n-butylamine acyl chloride in a calorescence reaction kettle to obtain the normal-butyl isocyanate. The method provided by the invention has the advantages that the phosgene is utilized to the greatest extent, the load of the tail gas generated in the normal-butyl isocyanate calorescence reaction in a tail gas processing system and the alkali charge during tail gas processing are reduced, the production cost of the product is reduced, and the purpose of saving and recycling resources is achieved.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
Preparation method of amicarbazone
PendingCN113845487AHigh selectivityMild reaction conditionsOrganic chemistryChemical reactionOrganic synthesis
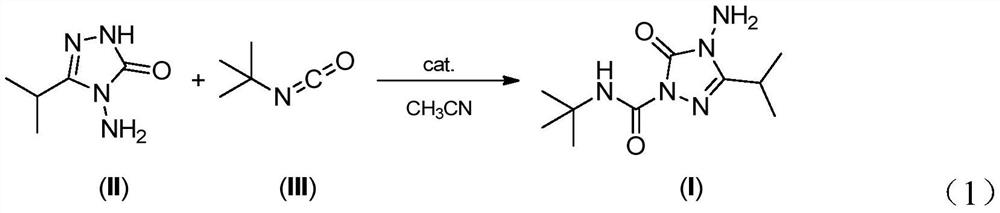
The invention belongs to the field of organic synthesis, and discloses a preparation method of amicarbazone, and amicarbazone has a structure as shown in a formula (I) in the specification. The method comprises the following steps: carrying out chemical reaction on 3-isopropyl-4-amino-1, 2, 4-triazoline-5-ketone and tert-butyl isocyanate in an acetonitrile medium under the catalytic action of lithium hydroxide, and converting the 3-isopropyl-4-amino-1, 2, 4-triazoline-5-ketone and the tert-butyl isocyanate into the target product amicarbazone with high selectivity and high yield. The method has the advantages of mild reaction conditions, strong reaction operability and no strict requirements on the charging sequence, and is especially suitable for industrial mass production.
Owner:北京怡力生物科技有限公司 +1
Synthesis method of benomyl
The invention relates to a method for synthesizing benomyl, which comprises the following steps: 1) putting a certain amount of solvent and carbendazim into a reaction vessel at normal temperature, and stirring for 10-20 minutes, wherein the solvent is dichloroethane; 2) at normal temperature Put a certain amount of n-butyl isocyanate into the reaction vessel, and stir for 10-20 minutes after adding; 3) Raise the temperature, and keep it at 45°C~65°C for 2-4h; 4) Freeze, crystallize and filter the above-mentioned reacted materials , drying to obtain benomyl. The benomyl produced by the benomyl production process of the present invention has a white appearance, a content of more than 95.5%, high safety and low cost.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
Synthesis process and synthesis equipment for mixture of buprofezin intermediate tert-butyl thiocyanate and tert-butyl isocyanate
PendingCN112694419AImprove stabilityControl contentIsocyanic acid derivatives preparationOrganic compound preparationCombinatorial chemistryButyl isocyanate
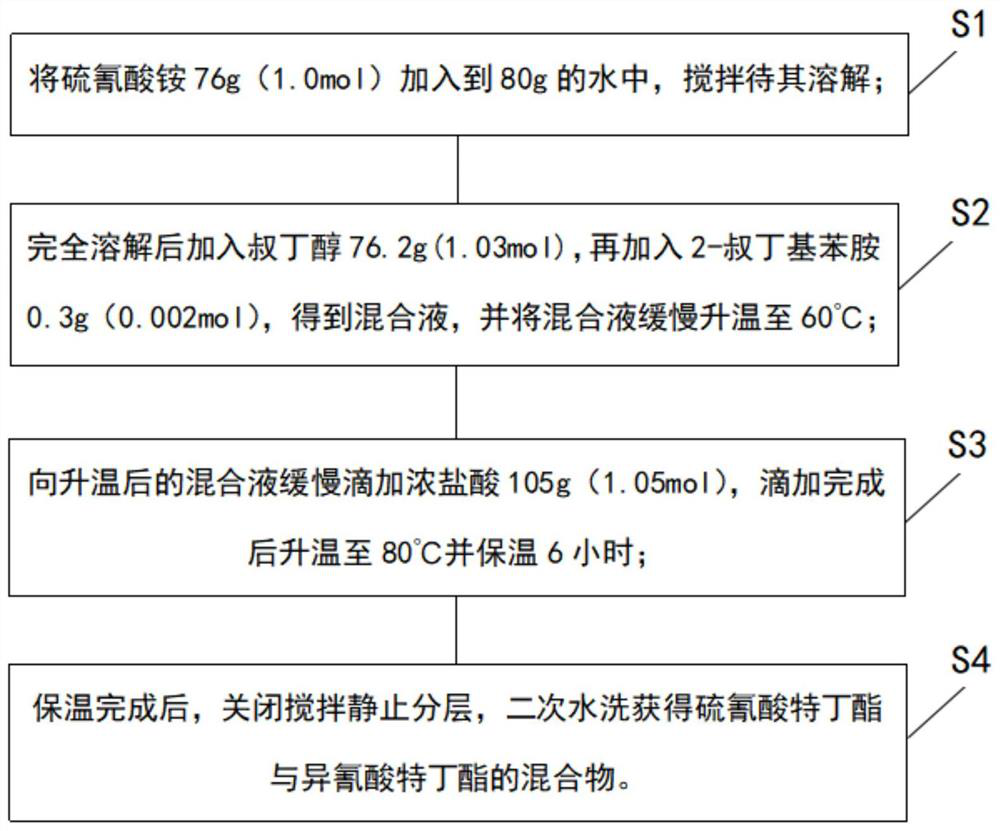
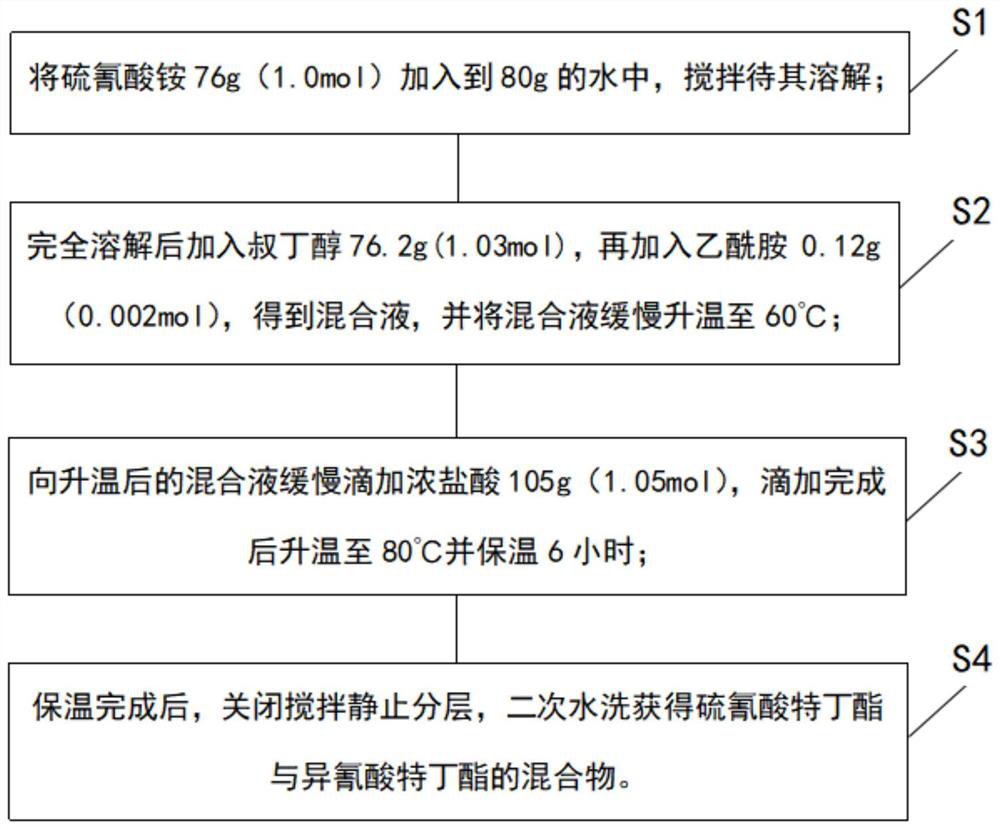
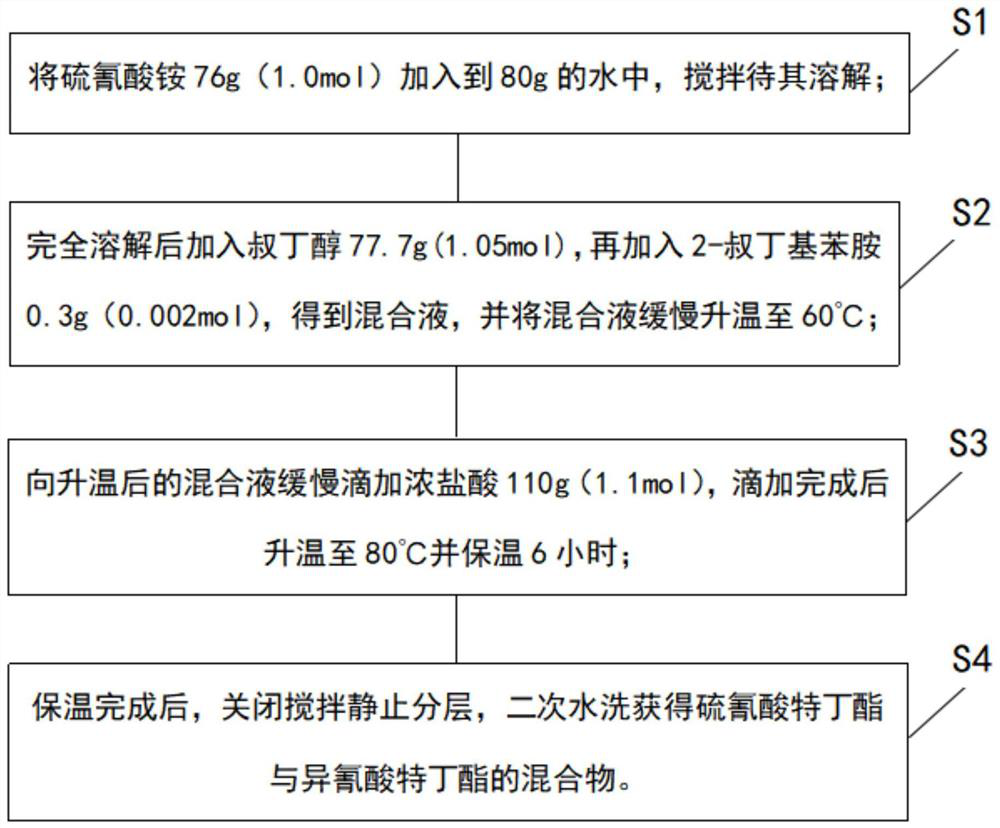
The invention discloses a synthesis process and synthesis equipment for a mixture of buprofezin intermediate tert-butyl thiocyanate and tert-butyl isocyanate. The process comprises the following steps of: adding ammonium thiocyanate into water, adding tert-butyl alcohol at normal temperature, performing heating to 60-75DEG C, adding an ammonium thiocyanate stabilizer, slowly adding concentrated hydrochloric acid dropwise, controlling the dropwise adding time at 2-6h, performing heating to 75-90DEG C, and keeping the temperature for 2-4h, and controlling the molar mass ratio of ammonium thiocyanate to tert-butyl alcohol to concentrated hydrochloric acid at 1:1-1.1:1-1.2; and carrying out water separation on the reaction product, and carrying out secondary water washing to obtain a mixture (mixed ester for short) of tert-butyl thiocyanate and tert-butyl isocyanate. The method has the beneficial effects that: the process is simple, the product quality is relatively high, and the content of carbon disulfide in the mixed ester is greatly reduced.
Owner:JIANGSU ANPON ELECTROCHEM
Method for synthesizing n-butyl isocyanate by gas phase method
ActiveCN114044744AShort processReduce labor intensityIsocyanic acid derivatives preparationOrganic compound preparationPtru catalystGas phase
The invention discloses a method for synthesizing n-butyl isocyanate by adopting a vapor phase method. The method comprises the following steps: firstly, preheating a raw material n-butylamine; preheating the raw material phosgene; introducing the preheated gaseous n-butylamine and preheated phosgene into a fixed bed reactor containing a catalyst to react, and condensing the materials, removing phosgene and rectifying the reacted material to prepare an FNC finished product. According to the method, the n-butyl isocyanate is continuously synthesized by adopting a gas-phase solvent-free method, the reaction time is greatly shortened, the process route is short, and the method is suitable for industrial production, wherein the FNC is catalytically synthesized by adopting a fixed bed, the reaction is carried out under a pressurized condition, and the reaction conversion rate and selectivity are relatively high; in addition, the FNC is synthesized by a solvent-free gas-phase one-step method, the post-treatment process is simple, the procedures of desolventizing, recovering the solvent and the like are not needed, the energy consumption is greatly reduced, and the cost is remarkably reduced.
Owner:NINGXIA RUITAI TECH +1
Exhaust gas treatment process for n-butyl isocyanate intermediate
InactiveCN105384643ARaise the ratioReduce generationOrganic compound preparationAmino compound preparationN-butylisocyanideN-Butanol
The invention provides an exhaust gas treatment process for an n-butyl isocyanate intermediate. The process comprises the following steps: fully filling a reaction vessel with ammonia gas; introducing n-butanol vapor into the reaction vessel, and meanwhile, adding a catalyst cobalt oxide into the reaction vessel; heating up the reaction vessel; carrying out first-time gas purging on a reacted material with nitrogen gas, and collecting gas with a first-stage collecting tank; cooling the gas in the first-stage collecting tank; introducing nitrogen gas into the cooled first-stage collecting tank for second-time gas purging; introducing nitrogen gas into a third-stage reaction vessel for third-time gas purging, and transferring liquid in the reaction vessel into a rectifying still for rectification; and transferring the gas subjected to third-time gas purging into a cooling tank, and adding hydrochloric acid into the cooling tank. According to the process, by adopting the production process, side reactions are further reduced, and the reaction efficiency is increased; and meanwhile, exhaust gases are further treated more thoroughly, and formed nontoxic gases are discharged into air and cannot cause pollution to the air.
Owner:ANHUI GUANGXIN AGROCHEM
Method for synthesizing aryl nitrile compound from tert-butyl isonitrile
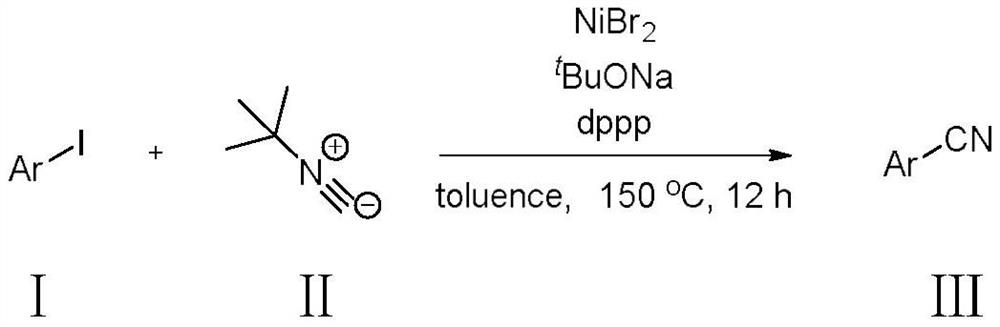
PendingCN114213204ARich reservesLow pricePreparation by cyanide reactionCyano group formation/introductionT-butyl isocyanideTert butyl
The invention relates to a synthesis method of an organic compound, in particular to a method for synthesizing a nitrile substance under the action of nickel by using tert-butyl isocyanate as a cyanation reagent, and the method is characterized in that an iodobenzene compound as shown in a formula I and tert-butyl isocyanide as shown in a formula II are used as raw materials to obtain a nitrile compound as shown in a formula III. The nickel bromide is rich in reserve and low in price, the reaction cost is reduced, the toxicity of the used tert-butyl isocyanide is low, the production difficulty is reduced, and the life safety of production personnel is protected. The method provided by the invention can effectively synthesize various aryl nitrile compounds.
Owner:ZHEJIANG UNIV OF TECH
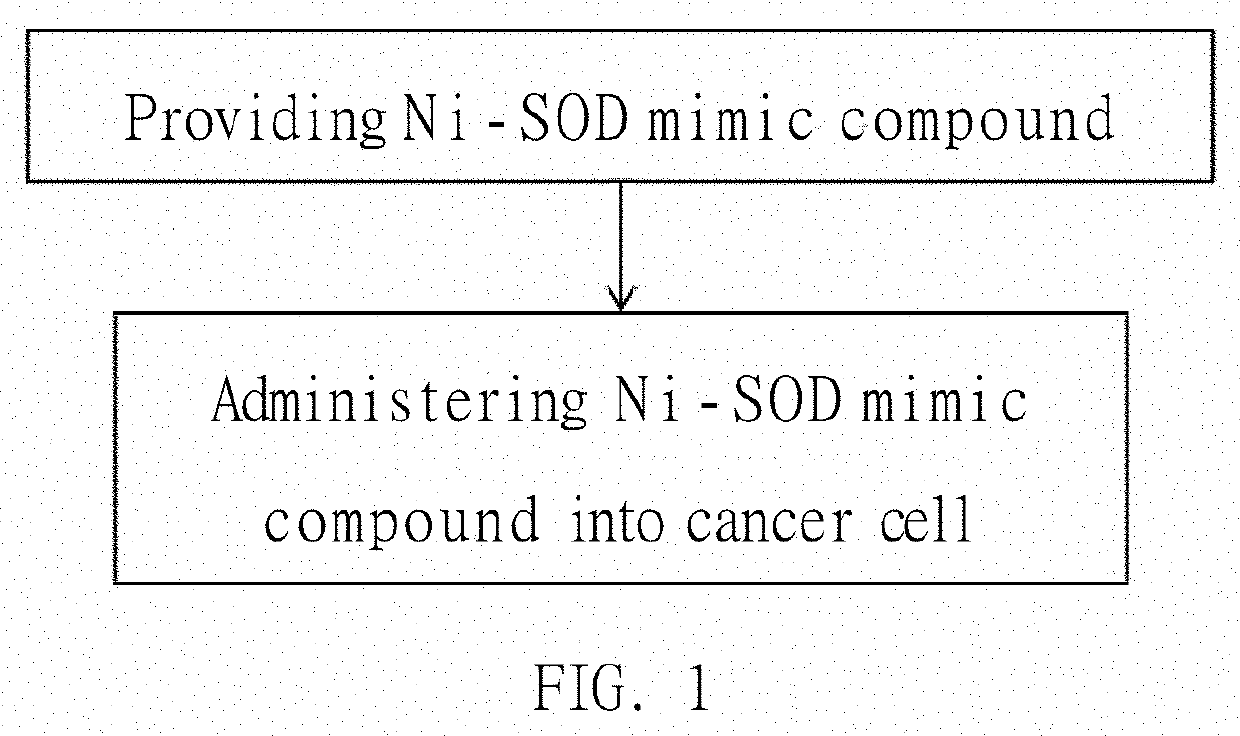
CANCER TREATMENT METHOD USING Ni-SOD MIMIC COMPOUND
InactiveUS20190350939A1Simple manufacturing processLow costOrganic active ingredientsPharmaceutical delivery mechanismCancer cellTert-butyl isocyanate
A cancer treatment method using a Ni-SOD mimic compound is provided, which includes administrating the Ni-SOD compound to a cancer cell of a cancer. The structure of the Ni-SOD mimic compound is represented as follows:wherein R1 represents H or A-R′; A represents a bond, O or N; L represents acetonitrile, water, or t-butyl isocyanate; R′ represents H, unsubstituted or substituted alkyl, polyalkoxy, polydimethylsiloxane, polyurethane or other polymer materials or amino acid groups; R2 represents unsubstituted or substituted alkyl, alkoxy, siloxy, amino, alkylamine or hydrocarbyl groups; R3 represents unsubstituted or substituted amino, alkylamine, oxyalkylamine groups or magnetic nanoparticles attached oxyalkylamine. Ni is bivalent or trivalent.
Owner:NAT CHIAO TUNG UNIV
Method for preparing bensulfuron methyl
ActiveCN103483274AReduce decompositionImprove conversion rateOrganic chemistryChemical recyclingBensulfuron methylN-butylisocyanide
The invention discloses a method for preparing bensulfuron methyl. The method comprises the following steps: adding phosgene, benzylsulfamide xylene and normal-butyl isocyanate into a photochemical kettle I to synthesize o-methoxy carboxyl benzyl sulfamide; after the o-methoxy carboxyl benzyl sulfamide enters a photochemical kettle II, adding phosgene to synthesize o-methoxy carboxyl benzylsulfo isocyanate; enabling o-methoxy carboxyl benzylsulfo isocyanate to enter a stripping tower to strip normal-butyl isocyanate, dimethyl benzene and phosgene; adding o-methoxy carboxyl benzylsulfo isocyanate subjected to gas stripping and 2-amino-4,6-dimethoxy pyrimidine into a synthesis kettle; after the material enters an automatic centrifuger, performing centrifugal separation to obtain the product bensulfuron methyl. The method has the benefits that a catalyst is recycled in the photochemical process, so that the consumption is reduced, and the cost and the treatment cost are both lowered; the stripping tower is adopted to remove phosgene in a dimethyl benzene solution, so that the consumption of dimethyl benzene is reduced; when the method provided by the invention is adopted for synthesis, the synthesis yield and the product quality are improved and the synthesis yield can be up to 98%.
Owner:JIANGSU KUAIDA AGROCHEM
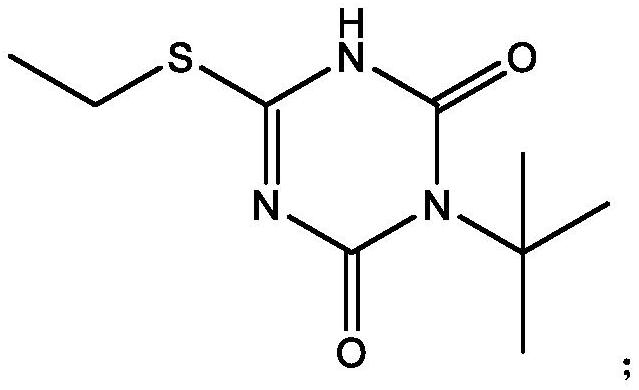
Preparation method of 3-tert-butyl-6-ethylthio-1, 3, 5-triazine-2, 4 (1H, 3H)-diketone
The invention belongs to the field of organic synthesis, and particularly relates to a preparation method of 3-tert-butyl-6-ethylthio-1, 3, 5-triazine-2, 4 (1H, 3H)-diketone. The method comprises the following steps: adding S-ethyl isothiourea into an organic solvent, slowly dropwise adding tert-butyl isocyanate, then carrying out a first-stage heat preservation reaction, then adding triethylamine and dropwise adding an organic solution of triphosgene, carrying out a second-stage heat preservation reaction after slowly dropwise adding to obtain a stock solution, and carrying out a second-stage heat preservation reaction after slowly dropwise adding; and carrying out post-treatment on the stock solution to obtain the target product 3-tert-butyl-6-ethylthio-1, 3, 5-triazine-2, 4 (1H, 3H)-diketone. The preparation of the 3-tert-butyl-6-ethylthio-1, 3, 5-triazine-2, 4 (1H, 3H)-diketone is realized in a milder and more efficient manner, and compared with the existing preparation scheme, the purity can reach 99% or above, and the product yield is also obviously improved to 84% or above.
Owner:杭州布朗生物医药科技有限公司
Preparation process of sulfonyl isocyanate with high conversion rate
PendingCN113634213ARealize the heating functionRealize the cooling functionSulfonic acid amide preparationChemical/physical/physico-chemical stationary reactorsXylyleneChlorobenzene
The invention discloses a preparation process of sulfonyl isocyanate with a high conversion rate, relates to the technical field of sulfonyl isocyanate preparation, and aims to solve the problems that an existing sulfonyl isocyanate preparation process is complex and relatively long in period, and the introduction precision of phosgene is relatively low during preparation. The preparation process comprises the following steps: step 1, opening a second valve, adding chlorobenzene sulfonamide and xylene into a first reaction kettle through a feeding pipe, closing the second valve, regulating and controlling the temperature of a heating and cooling sleeve to 100 DEG C through a temperature controller, carrying out heating azeotropic distillation to remove moisture, driving a mixing rod and an auxiliary rod to rotate by a motor, and mixing 4-chlorobenzenesulfonamide and xylene to obtain sulfanilamide slurry; and step 2, enabling a pressure pump of the first reaction kettle to work, applying pressure to the first reaction kettle through a pressure pipe, opening a first valve of the first reaction kettle, discharging the sulfanilamide slurry through a discharging pipe, then placing the sulfanilamide slurry in a second reaction kettle, and adding xylene and an n-butyl isocyanate catalyst.
Owner:ANHUI GUANGXIN AGROCHEM
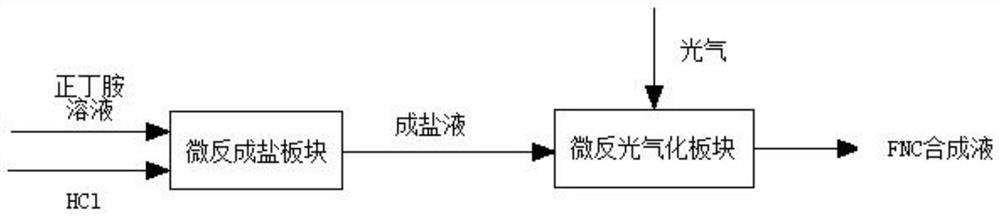
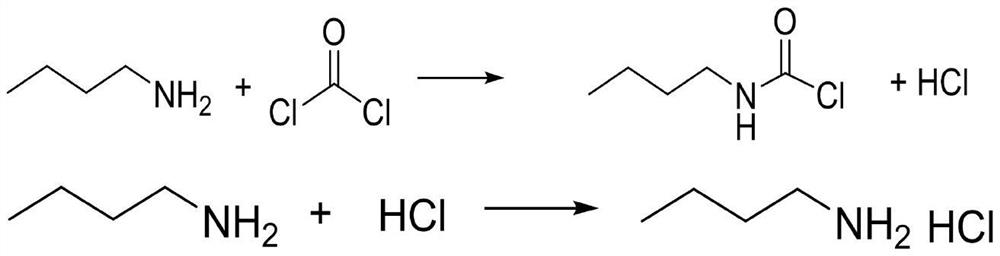
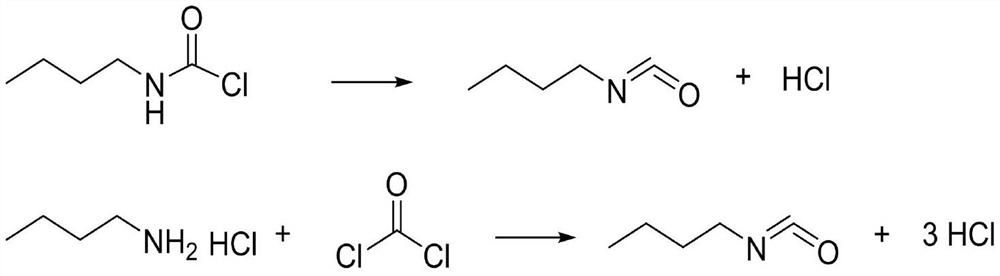
Method for synthesizing n-butyl isocyanate by adopting micro-channel reactor
PendingCN113831263AShort processReduce labor intensityProcess control/regulationIsocyanic acid derivatives preparationReaction temperatureN-Butylamine
The invention discloses a method for synthesizing n-butyl isocyanate by adopting a micro-channel reactor, the method comprises the following steps: preparing n-butylamine and a solvent into an n-butylamine solution, and simultaneously introducing the n-butylamine solution and hydrogen chloride into a salifying plate of the micro-channel reactor at a reaction temperature for salifying reaction to generate a salifying solution; and introducing the salifying liquid generated in the steps and phosgene into a phosgenation plate of the micro-channel reactor at reaction temperature and pressure to carry out phosgenation reaction, and sampling and analyzing the content of the synthetic liquid after the synthetic liquid is subjected to phosgenation. According to the invention, the continuous salification and phosgenation reaction is realized through the micro-channel reactor, and the n-butyl isocyanate can be continuously prepared. The method has the advantages of high phosgene utilization rate, few side reactions, high product yield, small liquid holding volume of the microchannel reactor, and greatly improved reaction safety compared with the traditional kettle reaction.
Owner:NINGXIA RUITAI TECH +1
Refining method for n-butyl isocyanate intermediate
InactiveCN105254508AAffect production efficiencyAffect purityIsocyanic acid derivatives preparationAmino compound purification/separationActivated carbonFiltration
The invention provides a refining method for an n-butyl isocyanate intermediate. The refining method comprises the steps that firstly, ammonium hydroxide is added into a reaction kettle, and then molybdenum oxide, being the catalyst, is added to the ammonium hydroxide; secondly, n-butyl alcohol steam is led into the reaction kettle, and meanwhile ammonia gas is led into the reaction kettle without stop; thirdly, nitrogen is added for primary gas expelling of reacted materials, and a primary collection tank is adopted to collect primarily expelled gas; fourthly, the gas in the primary collection tank is cooled; fifthly, nitrogen is led into the cooled primary collection tank for secondary gas expelling; sixthly, gas in a secondary collection tank is cooled, nitrogen is led into the secondary collection tank for third gas expelling, and liquid in the secondary collection tank is extracted; lastly, activated carbon is added to the extracted liquid for drying, and filtrate is reserved after filtration is conducted. By the adoption of the production process, the purity of n-butylamine, the n-butyl isocyanate intermediate, can be improved to 78.9-83.5%, and the yield is improved by 10-25%.
Owner:ANHUI GUANGXIN AGROCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com