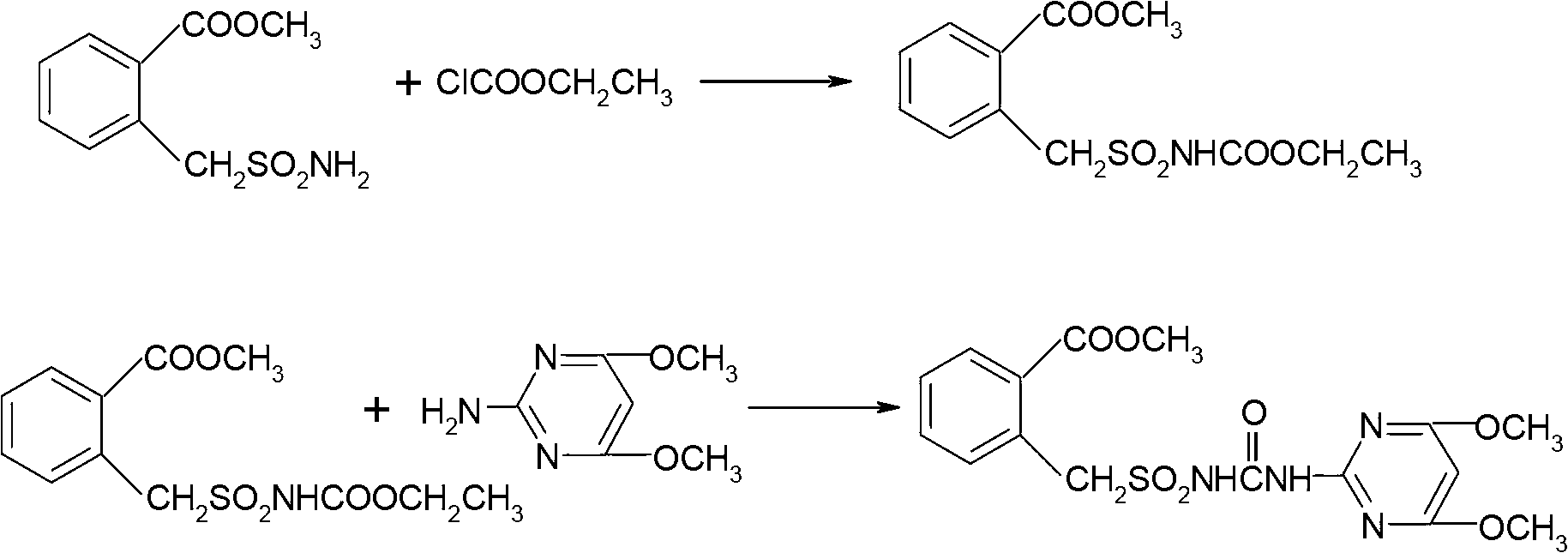
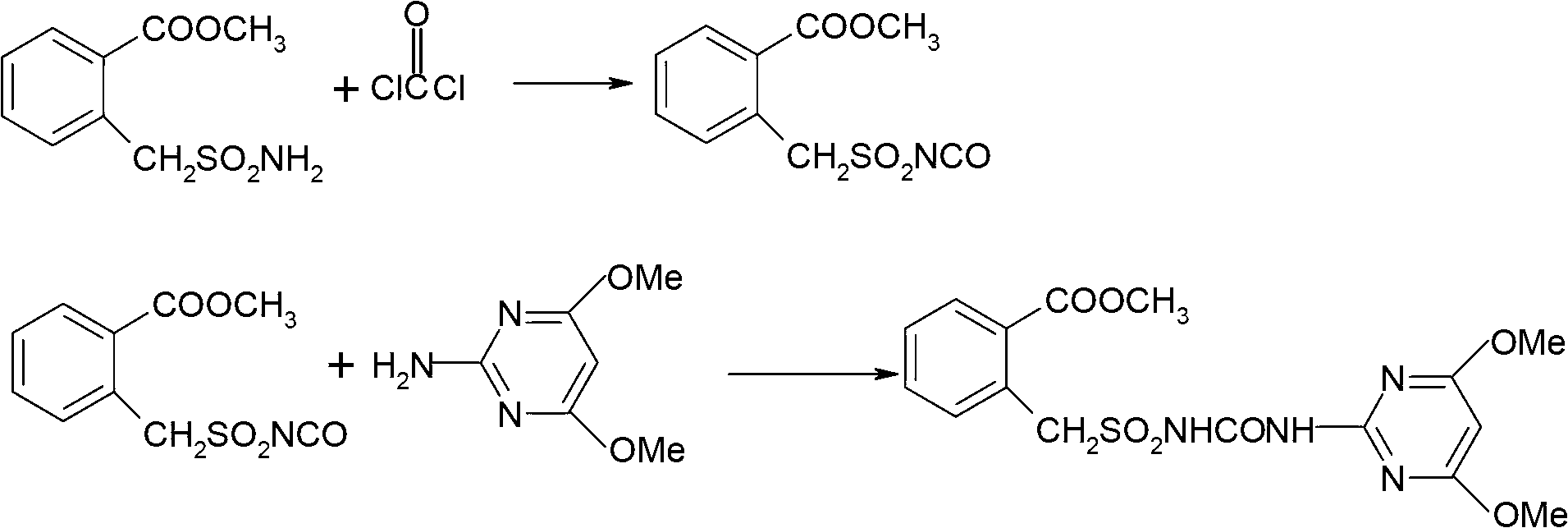
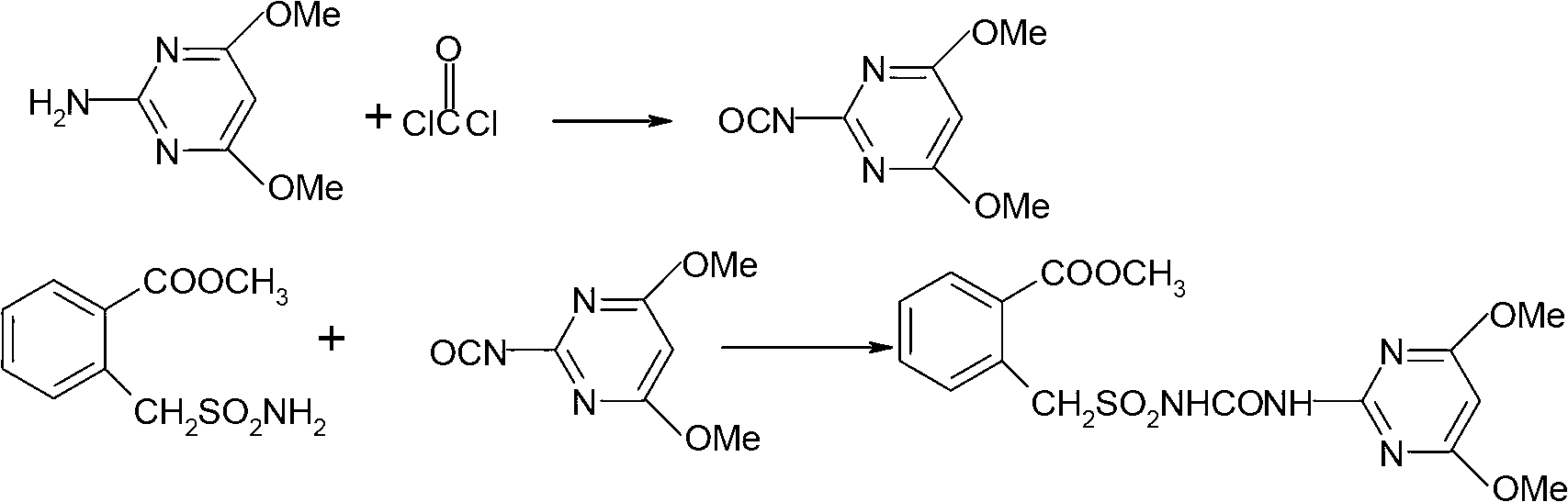
Method for preparing bensulfuron methyl
A technology of bensulfuron-methyl and methyl benzsulfonamide, which is applied in the field of synthesis of the pesticide bensulfuron-methyl, and can solve the problems of low yield, low content and unstable intermediates of bensulfuron-methyl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Put 50 grams (98%) of methyl o-formate benzylsulfonamide, 12 grams of n-butyl isocyanate, 300 ml of xylene and 35 grams of bis(trichloromethyl)carbonate (100 percent) into a 500 ml four-necked reaction flask, stir Accompanied by raising the temperature to 80°C, start to heat up at a rate of 0.5°C / min, and keep warm for 10 minutes every time the temperature rises by 10°C. When the temperature rises to 120°C, add 7 grams of trichloromethyl chloroformate dropwise, and keep warm for 2 to 3 hours. After the heat preservation is over, remove the solvent by distillation under reduced pressure, keep a vacuum of -0.09MPa during distillation, evaporate the solvent to dryness, add 70ml of toluene, cool to 40°C, add 31 grams of 2-amino-4,6-dimethoxypyrimidine evenly, The temperature was raised to 70°C, stirred for 1 hour, cooled to room temperature, centrifuged at high speed, and dried to obtain bensulfuron-methyl, with a weight of 86.3 g, a mass content of 97.2%, and a molar yield ...
Embodiment 2
[0033] Put 50 grams (98%) of methyl o-formate benzylsulfonamide, 12 grams of n-butyl isocyanate, 300 ml of xylene and 37 grams of bis(trichloromethyl)carbonate (100 percent) into a 500 ml four-necked reaction flask, stir Accompanied by raising the temperature to 80°C, start to heat up at a rate of 0.5°C / min, and keep warm for 10 minutes every time the temperature rises by 10°C. When the temperature rises to 120°C, add 5 grams of trichloromethyl chloroformate dropwise, and keep warm for 2 to 3 hours. After the heat preservation is over, remove the solvent by distillation under reduced pressure, keep a vacuum of -0.09MPa during distillation, evaporate the solvent to dryness, add 70ml of toluene, cool to 40°C, add 30g of 2-amino-4,6-dimethoxypyrimidine evenly, The temperature was raised to 70°C, stirred for 1 hour, cooled to room temperature, centrifuged at high speed, and dried to obtain bensulfuron-methyl, with a weight of 86.2 g, a mass content of 97.1%, and a molar yield of 90...
Embodiment 3
[0035] Put 50 grams (98%) of methyl o-formate benzylsulfonamide, 12 grams of n-butyl isocyanate, 300 ml of xylene and 37 grams of bis(trichloromethyl)carbonate (100 percent) into a 500 ml four-necked reaction flask, stir Accompanied by raising the temperature to 80°C, start to heat up at a rate of 0.5°C / min, and keep warm for 10 minutes every time the temperature rises by 10°C. When the temperature rises to 120°C, add 5 grams of trichloromethyl chloroformate dropwise, and keep warm for 2 to 3 hours. After the heat preservation is over, remove the solvent by distillation under reduced pressure, keep a vacuum of -0.09MPa during distillation, evaporate the solvent to dryness, add 50ml of acetonitrile, cool to 40°C, and evenly add 29 grams of 2-amino-4,6-dimethoxypyrimidine, The temperature was raised to 70°C, stirred for 1 hour, cooled to room temperature, centrifuged at high speed, and dried to obtain bensulfuron-methyl, with a weight of 85.2 g, a mass content of 98.0%, and a mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com