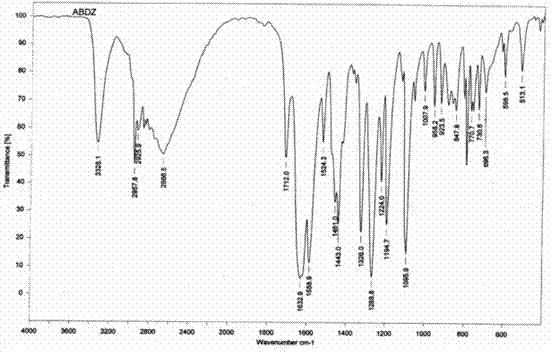
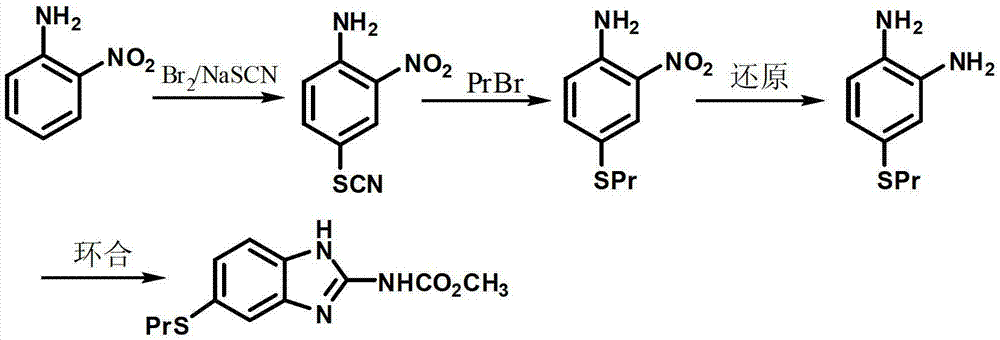
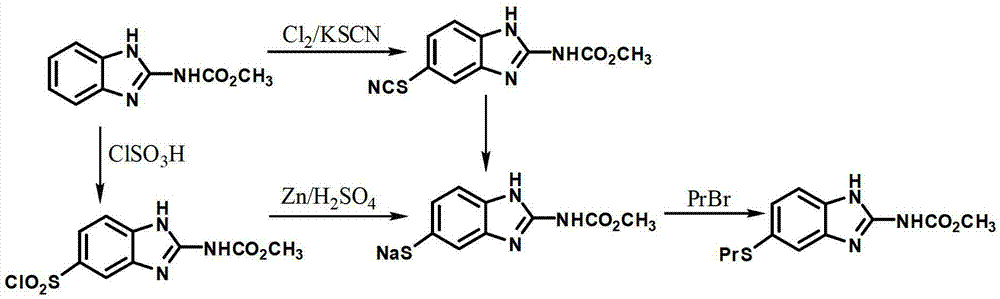
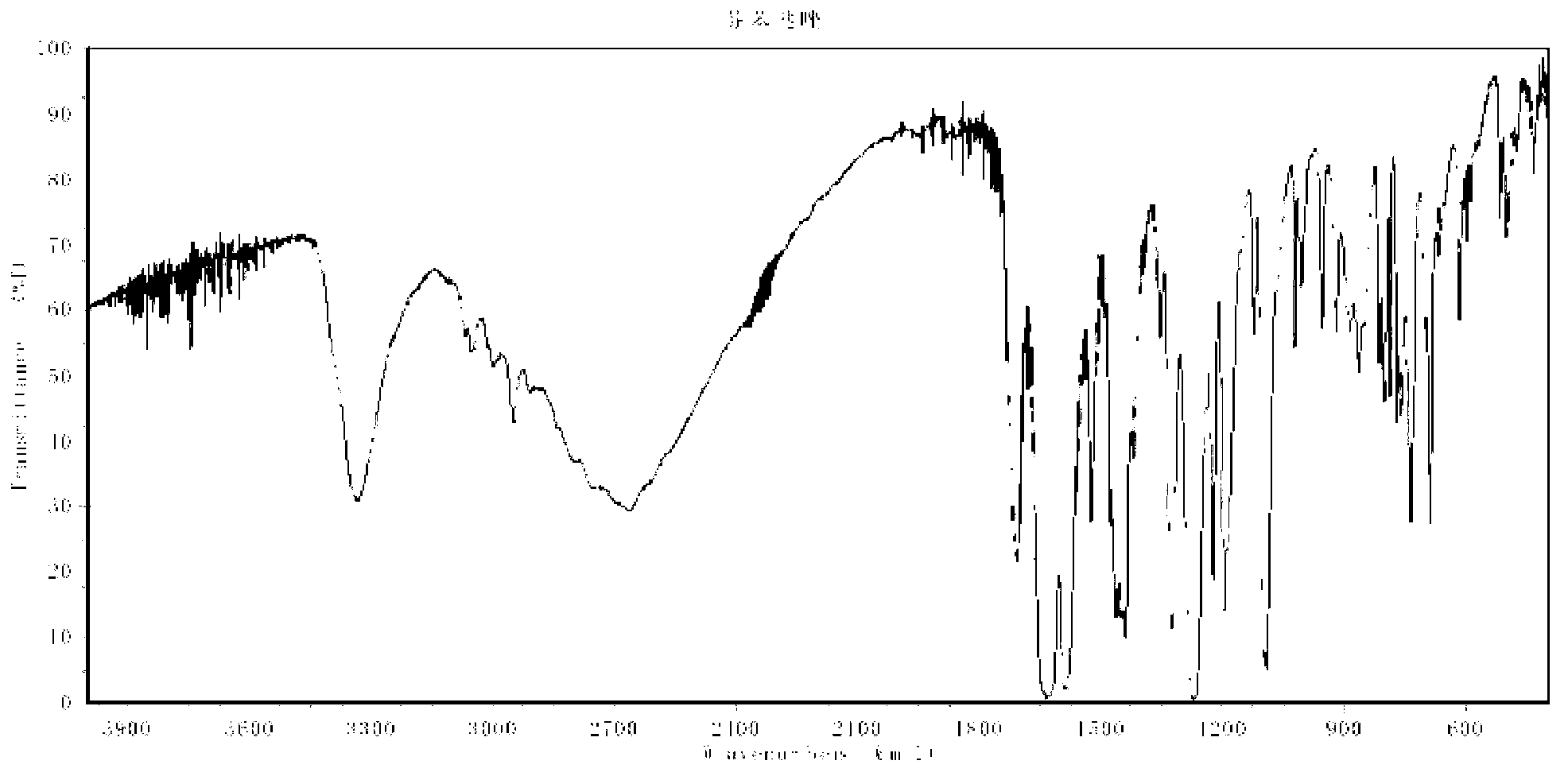
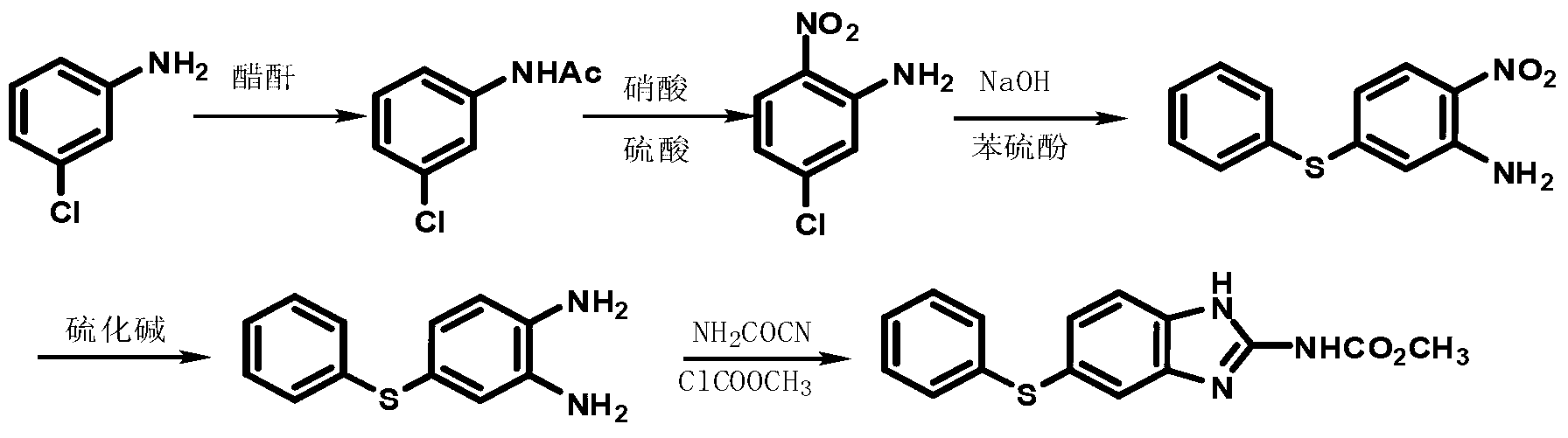
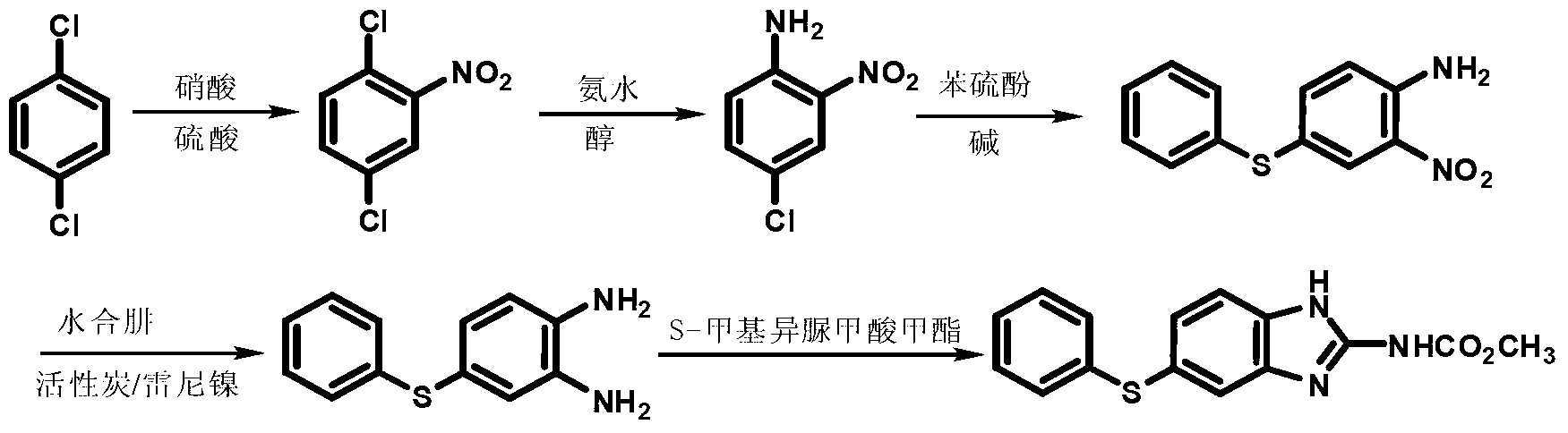
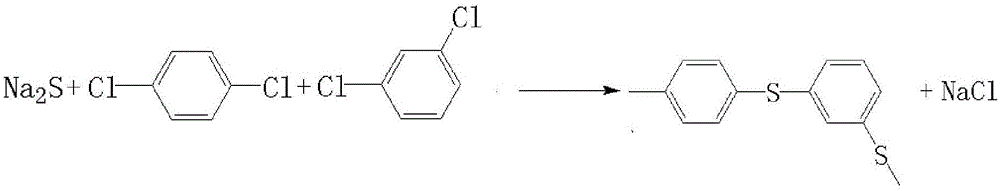
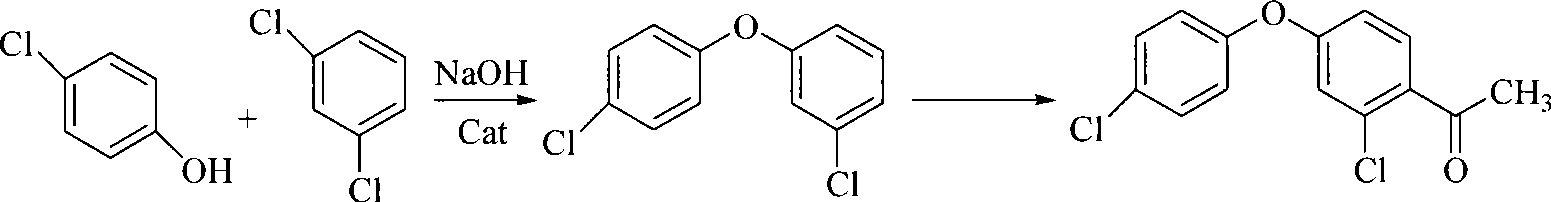
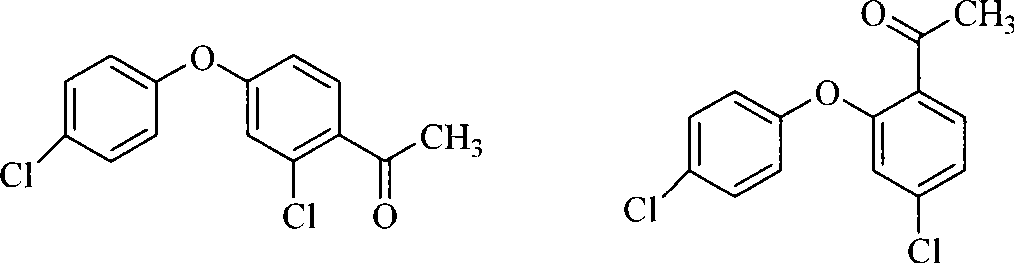
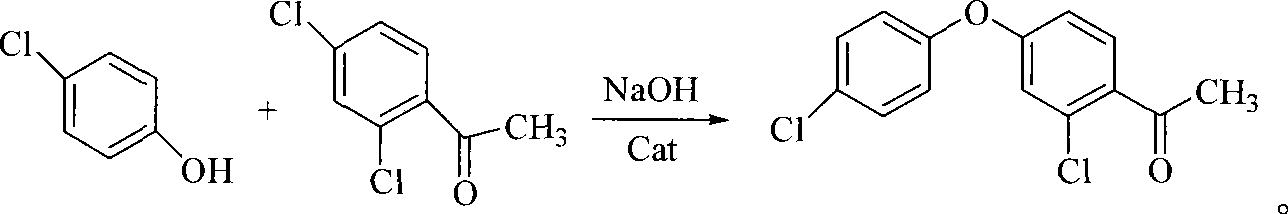Patents
Literature
96 results about "M-dichlorobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
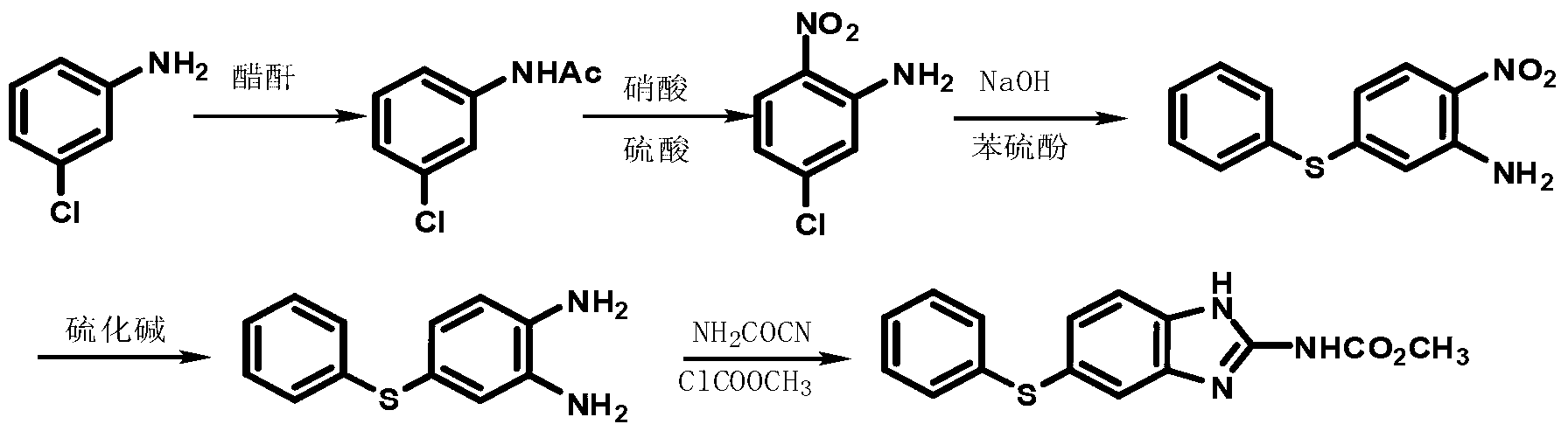
Synthetic method of m-dichlorobenzene
InactiveCN101696151ASolution to short lifeImprove conversion rateChemical recyclingHalogenated hydrocarbon preparationAutomatic controlGas phase
The invention discloses a synthetic method of m-dichlorobenzene which is obtained by transposition in the presence of a catalyst by taking o-dichlorobenzene and / or p-dichlorobenzene as raw materials. The catalyst is a composite catalyst comprising a solid phase catalyst and a gas phase catalyst, wherein the solid phase catalyst is aluminum trichloride or halide of transition metal, and the gas phase catalyst is anhydrous hydrogen chloride or other anhydrous halogen acids. The raw materials have no limitation of water, have arbitrary ortho-para proportion and react under normal pressure; the solid catalyst has long service life, and the gas catalyst can be recycled after gas-liquid separation and has high conversion ratio; and the synthetic method can be used for large-scale industrial production. The process realizes the automatic control easily and has energy saving, safety, environment protection and low cost.
Owner:JIANGSU EQUALCHEM
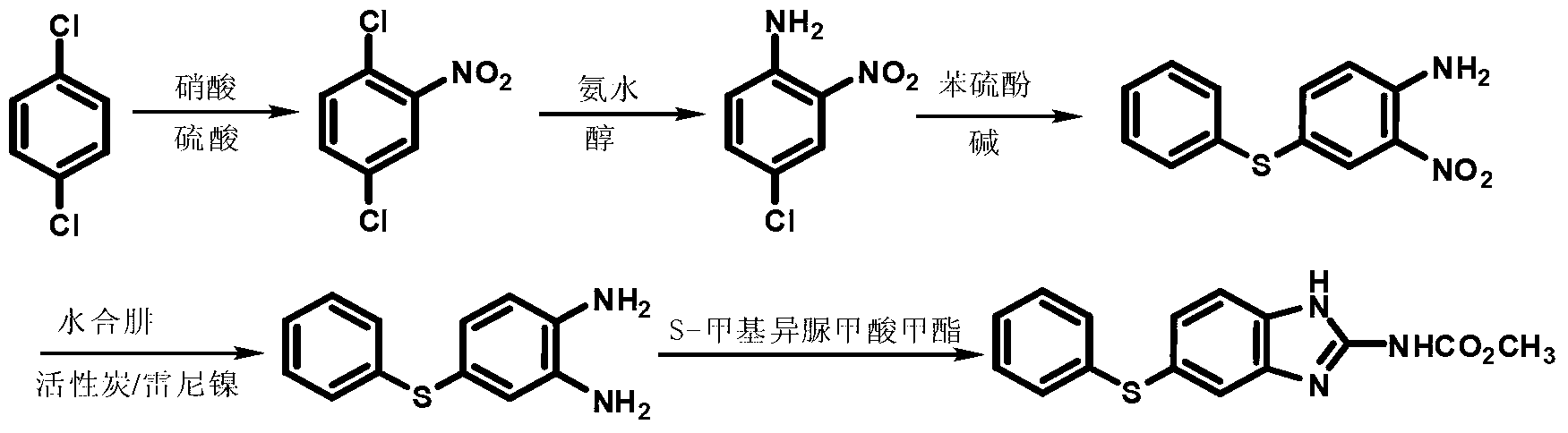
New preparation method of insect repellent albendazole
The invention discloses a new preparation method of an insect repellent albendazole and provides a brand-new synthesis route of albendazole. The new preparation method adopting m-dichlorobenzene as the starting material is used for obtaining high-purity albendazole through nitration, amination, condensation, reduction and cyclization reaction; the new preparation method is characterized in that an unstable intermediate condensation compound 2-nitryl-4-propyl sulfenyl aniline in the existing industrial route is changed into 2-nitryl-5-propyl sulfenyl aniline with stable quality. The reducing process is changed into a clean and efficient reducing process by virtue of sodium sulfide. The synthesis process is simple and efficient, less in pollution, high in quality and suitable for industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM +1
Method for producing 2,4-dichloroacetophenone by using solid waste chlorobenzene tar as raw material
InactiveCN101898947ASolve your worriesAlleviate supply and demand pressureCarbonyl compound preparation by condensationAcetyl chlorideM-dichlorobenzene
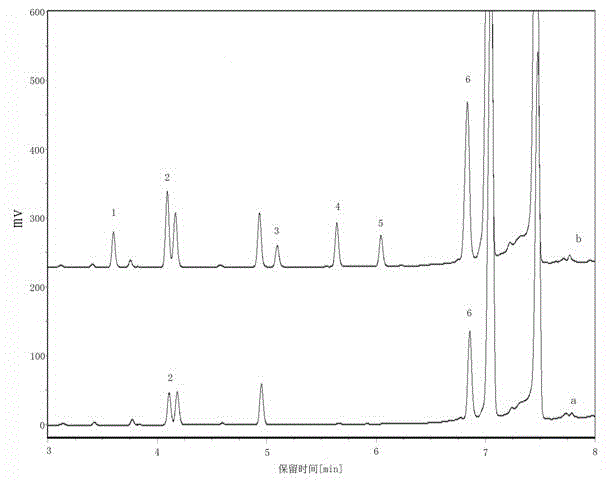
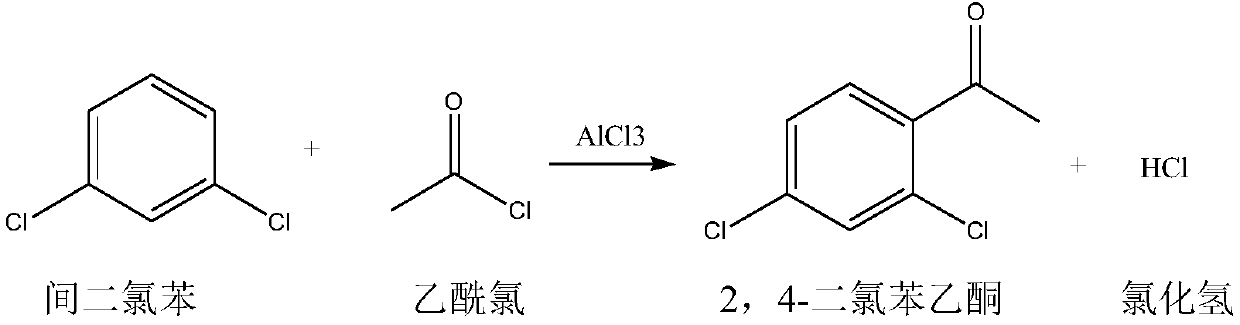
The invention discloses a method for producing 2,4-dichloroacetophenone by using solid waste chlorobenzene tar as a raw material. The method mainly comprises the following steps of: extracting m-dichlorobenzene from the solid waste chlorobenzene tar, and then performing five steps of acylation, washing, distillation, crystallization and packing in the 2,4-dichloroacetophenone production process. The method is characterized in that: the acylation reaction comprises that: the m-dichlorobenzene and acetyl chloride undergo the acylation reaction under the action of anhydrous aluminum trichloride, the dripping temperature of the acetyl chloride is kept between 50 and 60 DEG C during reaction, the temperature is gradually raised to between 90 and 95 DEG C after the dripping is finished, and reflux stirring reaction is performed for about 4 hours to obtain the 2,4-dichloroacetophenone. The method has the advantages that: the m-dichlorobenzene is reclaimed from the solid waste chlorobenzene tar during production, and the reclaimed m-dichlorobenzene is used as the raw material for producing the 2,4-dichloroacetophenone, so by the method, the 2,4-dichloroacetophenone is produced by using the m-dichlorobenzene as the raw material while extra worries of m-dichlorobenzene and o-dichlorobenzene production enterprises are solved, the supply and demand pressure of chlorobenzene markets is relieved and extended products are further developed and utilized.
Owner:JIANGSU LONGCHANG CHEM
2-4-dichloronitrobenzene synthesis method utilizing micro-channel reactor
ActiveCN104478730AImprove heat transfer efficiencyImprove conversion rateNitro compound preparationTemperature controlHigh flux
The invention discloses a 2,4-dichloronitrobenzene synthesis method. The synthesis method is characterized in that m-dichlorobenzene and a mixed acid prepared from concentrated nitric acid and concentrated sulfuric acid are used as starting materials, and the precooling and reaction processes of the m-dichlorobenzene and the mixed acid are carried out in a high-flux micro-channel reactor. The synthesis method achieves continuous nitration reaction, and has the characteristics of high stability in temperature control and high process safety.
Owner:ZHEJIANG YONGTAI TECH CO LTD
Method for preparing 2, 4-dichloroacetophenone
InactiveCN102675073AImprove securityThe operation process is safe and controllableCarbonyl compound preparation by condensationAcetic anhydrideDistillation
The invention discloses a method for producing 2, 4-dichloroacetophenone. The method mainly includes acylation, hydrolysis, washing, distillation and crystallization, and m-dichlorobenzene and acetic anhydride are in acylation reaction under the effects of anhydrous aluminum trichloride. The method has the advantages that the m-dichlorobenzene is used as a raw material, the acetic anhydride is used as an acylating agent, A1C13 is used as a catalyst to prepare the 2, 4-dichloroacetophenone by means of reaction, and yield of obtained products is higher than that of products prepared by using acetyl chloride as an acylating agent; as the acetic anhydride is used as the acylating agent, the A1C13 is used as the catalyst and the m-dichlorobenzene which is extracted from chlorinated aromatics waste is comprehensively used as the raw material to prepare the 2, 4-dichloroacetophenone, by-products in a benzene chlorination process are disposed, reusing rate of chlorinated aromatics is increased, and the method has high economical and economical benefits.
Owner:JIANGSU LONGCHANG CHEM
Method for producing 5-chloro-2-nitroaniline
InactiveCN102531923ASimple methodEasy to operateOrganic compound preparationAmino compound preparationNitrationOil phase
The invention discloses a method for producing 5-chloro-2-nitroaniline. The method comprises the following steps of: performing nitration on m-dichlorobenzene by using mixed acid of sulfuric acid and nitric acid at the temperature of between 45 and 55 DEG C for 4 to 5 hours, then layering after the nitration is finished, washing an oil phase of the upper layer until the oil phase of the upper layer is neutral, performing soda boiling by using a 4 percent sodium hydroxide solution, standing and layering, washing an oil phase of a lower layer until the oil phase of the lower layer is neutral to obtain 2,4-dichloronitrobenzene; and adding 2,4-dichloronitrobenzene into a high-pressure amination kettle, introducing liquid ammonia, preserving heat at the temperature of between 140 and 150 DEG C under the pressure of 7.0 to 8.5MPa for 5 to 6 hours, relieving pressure until the pressure is 0.5MPa, pressing materials into a washing kettle, washing off ammonium chloride, and suction-filtering to obtain a filter cake containing a product 5-chloro-2-nitroaniline. The method is simple and convenient, and is easy to operate, and the content of a finished product reaches over 98 percent.
Owner:江苏优普生物化学科技股份有限公司
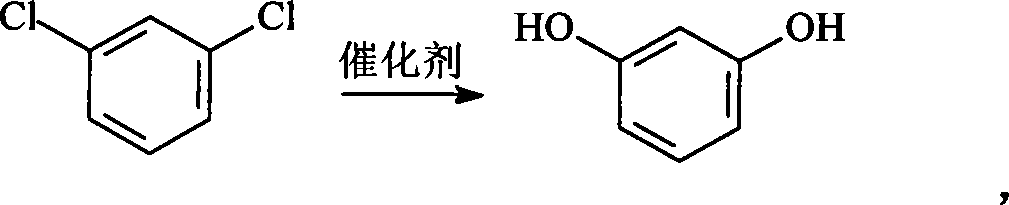
Method for preparing resorcin
ActiveCN101092333AEmission reductionReduce pollutionPreparation by hydrolysisPtru catalystM-dichlorobenzene
This invention relates to a method for preparing high-purity resorcinol. The method comprises: adding m-dichlorobenzene and NaOH aqueous solution of a certain concentration into a high-pressure reaction kettle, adding alkaline catalyst, metal oxide, metal halide and phase-transfer catalyst, starting a stirrer, heating to 220-300 deg.C, keeping for 2-8 h to obtain resorcinol, cooling the reaction solution to normal temperature, filtering, adjusting pH value to 2-3 with HCl, filtering again, extracting the reaction solution with a solvent, distilling the extract to recover the solvent and obtain byproduct m-chlorophenol. Resorcinol remains in the column reactor. The method has such advantages as abundant raw materials, high catalyst performance, high product selectivity, and little pollution.
Owner:溧阳常大技术转移中心有限公司
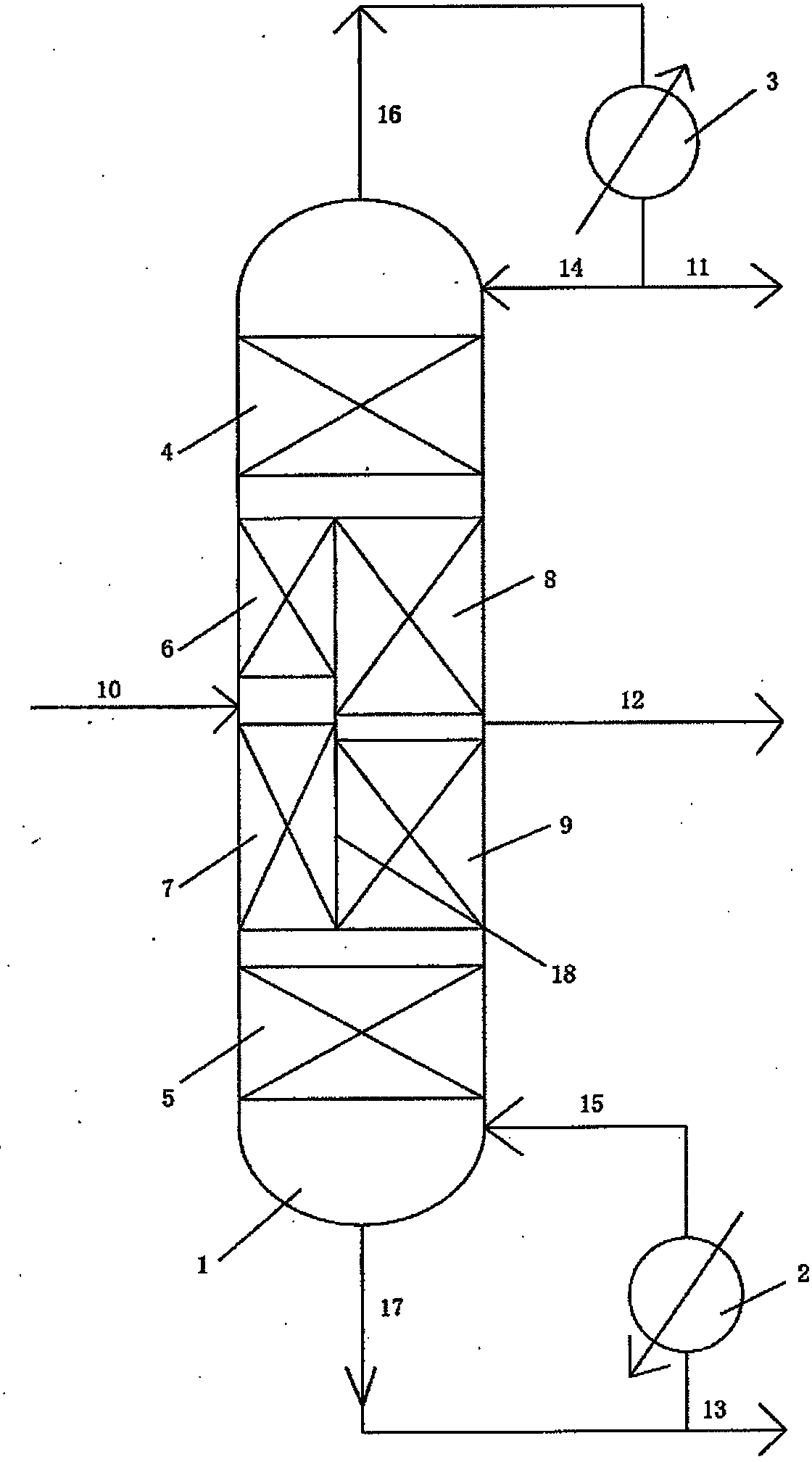
Distillation equipment and method for separating dichlorobenzene isomers
The invention discloses distillation equipment and method for separating dichlorobenzene isomers. The distillation equipment comprises a dividing wall tower, a tower kettle reboiler and a tower top condenser, wherein the dividing wall tower comprises a middle tower region, a tower top distillation section arranged above the middle tower region and used for performing distillation and separation on m-dichlorobenzene and p-dichlorobenzene, and a tower kettle stripping section arranged below the middle tower region and used for stripping and separating m-dichlorobenzene and o-dichlorobenzene; the middle tower region is divided into a pre-separation tower region and a finished product separation tower region by a baffle plate arranged in a longitudinal direction; the pre-separation tower region is parallel to the finished product separation tower region; the pre-separation tower region comprises an upper feeding distillation section and a lower feeding stripping section; the finished product separation tower comprises an upper m-dichlorobenzene / p-dichlorobenzene stripping section and a lower p-dichlorobenzene / o-dichlorobenzene distillation section. According to the distillation equipment, the dichlorobenzene isomers, namely the m-dichlorobenzene, the p-dichlorobenzene and o-dichlorobenzene can be separated in a distillation tower through the dividing wall tower, so that the energy consumption, the equipment investment and the occupied area are reduced.
Owner:李群
New preparation method for anthelmintic fenbendazole
InactiveCN103242237AAvoid pollutionThe new synthesis process is simple and efficientOrganic chemistryFenbendazoleM-chloroaniline
The invention discloses a new preparation method for anthelmintic fenbendazole, and provides a new synthetic route for fenbendazole. Fenbendazole is obtained by taking m-dichlorobenzene as a starting raw material through the reactions of nitration, amination, condensation, reduction and cyclization. The new preparation method is characterized in that m-chloroaniline serving as the starting raw material in the conventional industrial route is changed into m-dichlorobenzene which is low in cost; and a reduction process which uses sodium sulfide is changed into a clean and efficient reduction process. The new synthetic process is simple and efficient, low in pollution, high in quality and applicable to industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM
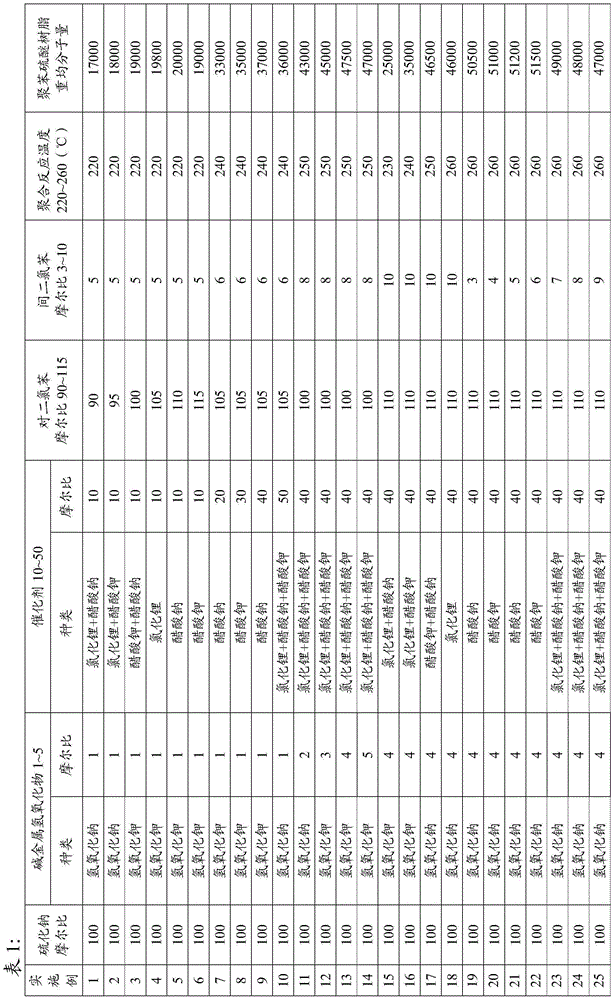
Film-grade polyphenylene sulfide resin and preparation method thereof
The invention discloses film-grade polyphenylene sulfide resin. The film-grade polyphenlene sulfide resin is mainly prepared from sodium sulfide, alkali metal hydroxides, a catalyst, p-dichlorobenzene and meta dichlorobenzene which are reacted in the environment of 220-260 DEG C for 3-6 hours. The invention further provides a preparation method of the resin, and the method comprises the steps that 1, the sodium sulfide and the alkali metal hydroxides are stirred and dispersed in solvent NMP, and after dehydration is conducted, a mixture is obtained; 2 the solvent NMP, the catalyst, the p-dichlorobenzene and the meta dichlorobenzene are added to the mixture, heating is conducted to make the temperature range from 220 DEG C to 260 DEG C to conduct polymerization for 3-6 hours, and the film-grade polyphenylene sulfide resin is obtained. According to the film-grade polyphenylene sulfide resin, an adequate amount of the meta dichlorobenzene is added when the PPS resin is compounded by taking the p-dichlorobenzene as the raw material, a linear type polyphenylene sulfide molecular chain is changed into an elastic polyphenylene sulfide molecular chain with a bend structure, and the transverse tensile capacity of a polyphenylene sulfide film prepared from the resin is enhanced. In addition, the weight-average molecular weight of a polymer is controlled by controlling the reaction temperature and the reaction time, and the thickness of the formed film is more even.
Owner:GUANGZHOU GAOBAER PLASTIC
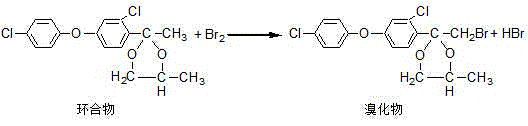
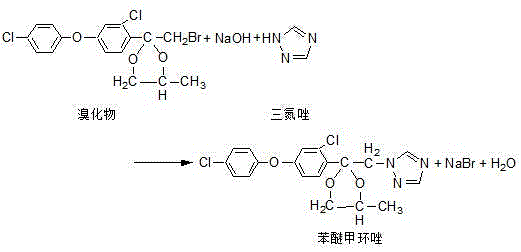
Production process of difenoconazole
InactiveCN104803990AReduce process reaction timeHigh yieldOrganic chemistryMethylene DichloridePotassium hydroxide
The invention discloses a production process of difenoconazole. The production process comprises steps as follows: etherification: parachlorophenol and solid potassium hydroxide which are well weighed and metered m-dichlorobenzene are put into an etherification reaction kettle, and etherate is obtained and transferred to a next procedure; acylation: etherate obtained in the etherification step, a small amount of aluminum trichloride and metered methylene dichloride are added to an acylation kettle to be stirred and mixed at the room temperature, acetyl chloride is dropwise added for a reaction, and acylate is obtained and transferred to a next procedure; cyclization: acylate obtained in the acylation step as well as propylene glycol and cyclohexane which are metered is put into a cyclization kettle at the room temperature, and materials are transferred to a next procedure after the materials are cooled to the room temperature by cooling water; bromination: a cyclization material is introduced into a bromination kettle at the room temperature, is bromized by dropwise adding bromine and is added with water for washing, waste water obtained through washing is sent for waste water treatment, and bromide is obtained and transferred to a next procedure; condensation: the bromide obtained in the bromination step, triazole, caustic soda flakes and dimethyl sulfoxide are put into a condensation reaction kettle. According to the production process of the difenoconazole, the reaction time of the process is shortened, the yield is increased, three wastes are fewer, and clean and environment-friendly production is realized.
Owner:JIANGSU CHANGQING AGROCHEM NANTONG
Method for preparing 2-chlorine-4-(4-chlorophenoxy)-hypnone
InactiveCN101434528AOrganic compound preparationCarbonyl compound preparationAcetic anhydrideCopper oxide
The invention discloses a method for preparing 2-chloro-4-(4-chlorophenoxy)-hypnone, which comprises reactions of two steps: firstly, m-dichlorobenzene is adopted as a raw material and the etherification reaction is carried out to p-chlorophenol salt under the catalysis of copper oxide or cupric salt to generate 3, 4'-dichloro-diphenyl ether, and then acylation reaction is carried out between the 3, 4'-dichloro-diphenyl ether and acylation reagent acetic anhydride or acetyl chloride to prepare 2-chloro-4-(4-chlorophenoxy)-hypnone, wherein, the acylation reaction is completed within 1 to 10 hours at the temperature from 0 DEG C to reflux temperature of reaction materials in an organic solvent and with the presence of Lewis acid. When the acetic anhydride is selected as the acylation reagent, mole ratio of materials is that 3, 4'-dichloro-diphenyl ether: acetic anhydride: catalyst is equal to 1:1 to 2:2 to 4; when the acetyl chloride is adopted as the acylation reagent, the mole ratio of the materials is that 3, 4'-dichloro-diphenyl ether: acetyl chloride: catalyst is equal to 1:1 to 2:1 to 2. According to the method, 2-chloro-4-(4-chlorophenoxy)-hypnone which is an important intermediate product of an agricultural fungicide-difenoconazole can be prepared with high yield and high quality.
Owner:中国中化股份有限公司 +1
Preparation and application of 1,2,4,5-tetra amino benzene and hydrochloride thereof
InactiveCN103102274AReduce manufacturing costEasy to compressOrganic compound preparationMonocomponent synthetic polymer artificial filamentAminolysisSide reaction
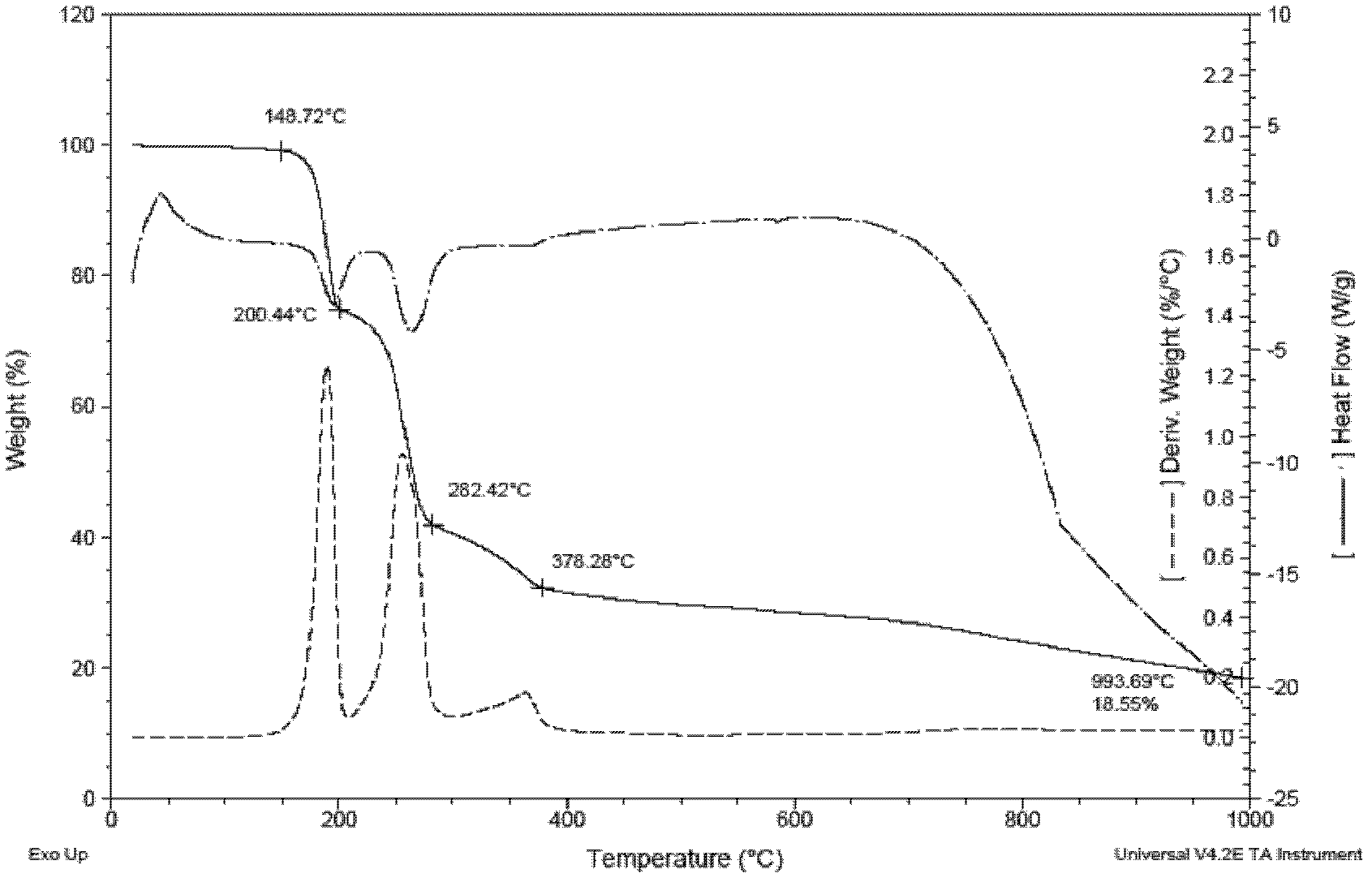
The invention provides a method of preparing 1,2,4,5-tetra amino benzene and hydrochloride thereof. 4,6-dinitro m-dichlorobenzene is used as a raw material, and the 1,2,4,5-tetra amino benzene is prepared through aminolysis and catalysis hydrogenation reduction and the hydrochloride of the 1,2,4,5-tetra amino benzene is prepared by adding hydrochloric acid. The method has advantages of easily-available raw materials for the reaction, few side reactions and controllable amount of byproducts. The hydrochloride of the 1,2,4,5-tetra amino benzene has a purity up to a polymer grade requirement, and a total yield of more than 75 %. A thermal decomposition temperature of the 1,2,4,5-tetra amino benzene is 200-282 DEG C detected by a thermal gravimetric method. The 1,2,4,5-tetra amino benzene is good in thermal stability. As a new synthetic material monomer, the 1,2,4,5-tetra amino benzene with a purity of more than 99 % is used to polymerize with 2,5-dihydroxy terephthalic acid to prepare poly-1,4-(2.5-dihydroxy) phenylene benzo diimidazole resin with an intrinsic viscosity Eta of more than 12 dL*g<-1> and a good spinnability.
Owner:ZHEJIANG UNIV OF TECH +1
Synthetic process of difenoconazole
The invention discloses a synthetic process of difenoconazole, comprising the steps of synthesizing 2,4-dichloroacetophenone through ionic liquid acylation using m-dichlorobenzene as a raw material; then synthesizing alpha-bromo-2,4-dichloroacetophenone through a green bromination method; subjecting the alpha-bromo-2,4-dichloroacetophenone and 1,2-propanediol to cyclization to generate a ketal compound that is 2-(2,4-dichlorophenyl)-2-bromomethyl-4-methyl-1,3-dioxolane; subjecting the ketal compound and 1,2,4-triazole potassium to condensation to generate 1-[[2-(2,4-dichlorophenyl)-4-methyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4-triazole; and finally subjecting the 1-[[2-(2,4-dichlorophenyl)-4-methyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4-triazole and parachlorophenol to etherification to obtain the difenoconazole. The process has advantages of easily available raw materials, a high reaction conversion ratio, few byproducts, capability of being friendly to production environment and a low cost.
Owner:CHANGSHA XINBEN CHEM
Method for producing m-dichlorobenzene with aromatic hydrocarbon chlorination waste as raw material
InactiveCN103896727AEfficient use ofImprove protectionHalogenated hydrocarbon separation/purificationMolecular sieveIsomerization
The invention discloses a method for producing m-dichlorobenzene with aromatic hydrocarbon chlorination waste as a raw material. The method comprises the following steps: separating mixed dichlorobenzene stoste from the aromatic hydrocarbon chlorination waste, performing directional isomerization on the mixed dichlorobenzene stoste under catalysis of an HZSM 5 molecular sieve to translocate mixed dichlorobenzene into m-dichlorobenzene to obtain mixed dichlorobenzene of m-dichlorobenzene, p-dichlorobenzene and o-dichlorobenzene, selectively adsorbing p-dichlorobenzene by using an MFI hydrophobic amorphous silica zeolite molecular sieve as an adsorbent to obtain a mixed solution of m-dichlorobenzene and o-dichlorobenzene, and performing rectification separation to obtain m-dichlorobenzene with mass fraction higher than 99.5%. In the method disclosed by the invention, resource utilization is performed on the aromatic hydrocarbon chlorination waste to separate and extract the mixed dichlorobenzene stoste, p-dichlorobenzene whit higher content is separated by using two high-performance molecular sieves, and high-concentration m-dichlorobenzene is prepared by rectification technology; by adopting the production method disclosed by the invention, m-dichlorobenzene with mass fraction higher than 99.5% can be obtained, resources are saved and the environment is protected.
Owner:JIANGSU LONGCHANG CHEM
Method for detecting chlorobenzene in sludge through ultrasonic assisted dispersive liquid-liquid microextraction-gas chromatography
The invention discloses a method for detecting chlorobenzene in sludge through ultrasonic assisted dispersive liquid-liquid microextraction-gas chromatography. The method comprises the steps of (1) preparation of a standard solution, namely preparing 0.1milligram / ml of standard mixed solution comprising m-dichlorobenzene, o-dichlorobenzene, 1,2,4-trichlorobenzene, 1,2,3-trichlorobenzene, 1,2,4,5-tetrachlorobenzene, and 1,2,3,4-tetrachlorobenzene; (2) ultrasound-assisted extraction, namely putting 1.0g of a sludge sample into a cone centrifuging tube with a turncap, adding acetone, sealing, extracting by ultrasounds, centrifuging extract liquor, then filtering supernate by a microfiltration membrane, and moving into the cone centrifuging tube; (3) dispersive liquid-liquid microextraction; and (4) gas chromatography. The method for detecting the chlorobenzene compounds in the sludge has the high sensitivity. Compared with conventional detection methods such as a soxhlet extraction method, the method saves a large amount of organic solvent being toxic and harmful to human bodies, and little time is used in analysis, so that the method is applicable to the detection on a large quantity of sludge samples.
Owner:ZHEJIANG UNIV
Preparation method of dichlorodiphenylene ether ketone
InactiveCN103073408AImprove recycling ratesMild reaction conditionsCarbonyl compound preparation by condensationKetoneSolvent
The invention discloses a preparation method of dichlorodiphenylene ether ketone. The method includes the steps as follows: (1) condensation reaction: adding p-chlorophenol and a sodium hydroxide solution into a reactor to prepare phenoxide; dehydrating and adding a copper acetate catalyst, assistants and m-dichlorobenzene; and subjecting phenoxide to a condensation reaction with m-dichlorobenzene under the effects of the copper acetate catalyst and the assistants to prepare dichlordiphenyl ether; (2) acylation reaction: dissolving the prepared dichlordiphenyl ether in a solvent, and then subjecting dichlordiphenyl ether to an acylation reaction under the catalysis of an anhydrous aluminum trichloride catalyst to generate dichlorodiphenylene ether ketone. The preparation method has the advantages of short and simple synthetic route, mild reaction conditions, high conversion rate, low production cost, little discharge of three wastes, and high waste liquor recycling rate.
Owner:扬州市天平化工厂有限公司
Synthetic method for 2,4-dichloroacetophenone
InactiveCN109721480AReduce unit consumptionSimple processChemical industryCarbonyl compound preparation by condensationAcetic anhydrideM-dichlorobenzene
The invention discloses a synthetic method for 2,4-dichloroacetophenone. The method comprises the step of performing an electrophilic substitution reaction by using m-dichlorobenzene and acetic anhydride as raw materials to synthesize the 2,4-dichloroacetophenone. According to the method provided by the invention, the electrophilic substitution reaction is performed by adopting a reactive rectification manner, a vacuum degree of a reaction rectification column is adjusted, temperature of the reaction rectification column is controlled, reactive rectification is performed under the optimal temperature condition, so that the catalyst unit consumption and the acetic anhydride unit consumption are reduced, and the production process is simplified.
Owner:XINCHANG COUNTY TAIRU TECH CO LTD
Process for producing m-dichlorobenzene by using chloridized aromatic hydrocarbon waste as raw materials
InactiveCN102675039AIncrease contentHalogenated hydrocarbon preparationHigh concentrationSeparation technology
The invention discloses a process for producing m-dichlorobenzene by using chloridized aromatic hydrocarbon waste as raw materials. The process mainly comprises the following steps of: rectifying the chloridized aromatic hydrocarbon waste to obtain high-concentration paradichlorobenzene; preparing high-content paradichlorobenzene by a crystallization technology by using a paradichlorobenzene solution; inverting the high-content paradichlorobenzene into low-concentration paradichlorobenzene by a normal pressure inversion technology; washing with water; and crystallizing and purifying. According to the process, the chloridized aromatic hydrocarbon waste is used as the raw materials, the high-content paradichlorobenzene is separated by a separation technology, the high-concentration paradichlorobenzene is prepared by normal-pressure acylation reaction, and the high-content m-dichlorobenzene is extracted by the crystallization technology.
Owner:JIANGSU LONGCHANG CHEM
Method for preparing m-dichlorobenzene by o-dichlorobenzene isomerization
InactiveCN107311837AImprove conversion rateImprove stabilityHalogenated hydrocarbon preparationIsomerizationM-dichlorobenzene
The invention relates to a method for preparing m-dichlorobenzene by o-dichlorobenzene isomerization. O-dichlorobenzene and a modified HZSM-5 catalyst react for 5-6 h at a packing ratio of 1:2, a vapor liquid ratio of 60, an airspeed of 0.5-1 h<-1> and a reaction temperature of 400-420 DEG C. The method has the advantages that in the method for preparing m-dichlorobenzene by o-dichlorobenzene isomerization, an appropriate solid acid catalyst is adopted, that is, a modified HSM-5 catalyst is adopted, and the catalyst is high in stability and reusable, can greatly improve m-dichlorobenzene conversion rate and also greatly reduces corrosion to a device.
Owner:JIANGSU LONGCHANG CHEM
Preparation method of oxibendazole
InactiveCN103601685AAvoid it happening againNo pollution in the processOrganic chemistryBromineM-dichlorobenzene
The invention relates to the field of chemistry or medicine, particularly a preparation method of oxibendazole. The invention provides a brand-new oxibendazole synthesis route: m-dichlorobenzene used as an initial raw material is subjected to nitration, ammonification, condensation, reduction and cyclization reaction to obtain the oxibendazole. The initial raw material acetaminophen in the existing industrial route is changed into the cheap m-dichlorobenzene; the condensation adopts alkali metal salt of npropanol or npropanol, and avoids using bromopropane to generate bromine-containing wastewater, thereby being environment-friendly; and the reduction technique uses a clean efficient reduction technique instead of sodium sulfide. The new synthesis technique is simple and efficient, has the advantages of low pollution and high quality, and is suitable for industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM
Preparation method of fenbendazole
ActiveCN103242238AAvoid pollutionThe new synthesis process is simple and efficientOrganic chemistryM-chloroanilineFenbendazole
The invention discloses a preparation method of fenbendazole and provides a brand-new synthesis route of the fenbendazole. The fenbendazole is prepared from m-dichlorobenzene as a starting material through the steps of nitrification, condensation, amination, reduction and cyclization. The preparation method is characterized in that the starting material m-chloroaniline in the existing industrial route is changed to the cheap m-dichlorobenzene; the existing reduction technology with sodium sulfide dihydrate is changed to the clean and high-efficiency reduction technology; and the new synthesis technology is concise and simple, high in efficiency, slight in pollution, high in quality, and suitable to industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM +2
Process for extracting high-purity m-dichlorobenzene from solid waste chlorobenzene tar
The invention discloses a process for extracting high-purity m-dichlorobenzene from solid waste chlorobenzene tar, which is a process for extracting the m-dichlorobenzene from a mixture of m-dichlorobenzene, o-dichlorobenzene and p-dichlorobenzene separated from the solid waste chlorobenzene tar by adopting indirect adsorption of zeolite. The process comprises the following steps of: preparing mixed solution of alkanes, then separating a mixture of p-dichlorobenzene and o-dichlorobenzene by using the adsorption of the zeolite, and finally obtaining the m-dichlorobenzene. The process has the advantages that: the process for extracting the m-dichlorobenzene from the isomer mixture is an extraction process implemented by adopting a physical method, and the product obtained by the process hashigh purity and high yield.
Owner:JIANGSU LONGCHANG CHEM
Recycling treatment method for separating byproduct rich meta-position from mixed dichlorobenzene
InactiveCN107141203AIncrease profitImprove recycling ratesCarbonyl compound preparation by condensationSocial benefitsMolecular sieve
The invention relates to a recycling treatment method for separating a byproduct rich meta-position from mixed dichlorobenzene. The method comprises the following steps: performing acylation reaction for the mixed dichlorobenzene comprising m-dichlorobenzene and acetic anhydride in the presence of anhydrous aluminum chloride, and then hydrolyzing, washing, distilling and crystallizing to obtain 2,4-dichloroacetophenone and little dichlorobenzene; performing catalytic orientation isomerization for the little dichlorobenzene by utilizing a high-efficiency HZSM-5 molecular sieve, enabling p / o-dichlorobenzene to be transpositioned to m-dichlorobenzene, wherein the obtained m-dichlorobenzene can be used for producing 2,4-dichloroacetophenone. The recycling treatment method has the advantages that since the price of the m-dichlorobenzene is high, the m-dichlorobenzene in the mixed dichlorobenzene is sufficiently utilized to be treated at the process route, the raw material utilization rate can be effectively increased, not only can the environment be protected, but also partial market demand on the 2,4-dichloroacetophenone can be satisfied, and the social benefit and the economic value are relatively high.
Owner:JIANGSU LONGCHANG CHEM
Clean production method for improving reaction yield of 2, 4-dichloroacetophenone
InactiveCN110922322AImprove recycling ratesLow production costCarbonyl compound preparation by condensationCarbonyl compound separation/purificationBiochemical engineeringM-dichlorobenzene
The invention relates to a clean production method for improving the reaction yield of 2, 4-dichloroacetophenone, which comprises the following steps: S1, separating and extracting m-dichlorobenzene by using chlorinated aromatic hydrocarbon waste; S2, preparing crude 2, 4-dichloroacetophenone; and S3, separating and purifying the crude 2, 4-dichloroacetophenone to obtain a dichloroacetophenone product. The method has the advantages that the optimal reaction conditions are found through the influence of the feeding ratio, the reaction temperature, the reaction time, different Lewis acids and the AlCl3 dosage on the product yield, the purity and the yield of the product are guaranteed, the purity of the product can reach 99.5% or above, and the yield of the product can reach 65% or above.
Owner:JIANGSU LONGCHANG CHEM
Preparation method of 2',2',4'-trichloroacetophenon
InactiveCN103613491ASimple separation and purificationRaw materials are easy to getCarbonyl compound preparation by condensationOrganic synthesisOil phase
The invention discloses a preparation method of 2',2',4'-trichloroacetophenon and belongs to the field of organic synthesis. The technical scheme adopted by the invention is as follows: the preparation method of 2',2',4'-trichloroacetophenon comprises the following steps: adding 450-550 parts by weight of m-dichlorobenzene and 600-750 parts by weight of aluminum chloride into an acylation reactor, then raising the temperature of materials to 58-62 DEG C; dropwise adding 380-420 parts by weight of chloroacetyl chloride into the acylation reactor in 2-4 hours, and keeping the temperature at 58-62 DEG C; after dropwise adding, raising the temperature of the materials to 80-100 DEG C, and thermally reacting for 2-3 hours; putting the feed liquid after the thermal reaction into a hydrolysis reactor for hydrolyzing at a temperature not higher than 90 DEG C, and standing for 30-40 minutes after the hydrolysis, wherein 1800-2200 parts by weight of dilute hydrochloric acid is held in the hydrolysis reactor in advance; extracting an oil layer in the feed liquid after the reaction into a rinsing reactor for rinsing till the PH value of the feed liquid is 4-6, extracting an oil phase into a distiller, distilling a course product through negative pressure, dissolving by using a solvent, crystallizing, centrifuging and drying to acquire a finished product. The preparation method of 2',2',4'-trichloroacetophenon has the advantages that raw materials are easily available, the product yield is high, the product quality is good, resource conservation is facilitated, the environment friendliness is achieved.
Owner:TAIZHOU KEYAN FINE CHEM CO LTD
Preparation method for 5-chloro-2-nitroaniline
InactiveCN108329211AEasy to cleanReduce pollutionAmino preparation by hydrogen substitutionNitro compound preparationFiltrationNitration
The invention discloses a preparation method for 5-chloro-2-nitroaniline. The preparation method comprises the following steps: performing nitration by using m-dichlorobenzene as a starting material to prepare 2,4-dichloronitrobenzene, adding the 2,4-dichlorobenzene and a solvent into an autoclave, adding ammonia, performing high-pressure amination, after the amination is completed, performing cooling, performing pressure filtration, removing the solvent from a filtrate, and performing refining to obtain the target product 5-chloro-2-nitroaniline. The method provided by the invention has the following advantages: (1) nitrogen dioxide is used as a nitrating reagent to replace a traditional nitric acid-sulfuric acid mixed acid nitrating agent, so that no waste acid is produced, cleanliness of an industrial synthesis reaction is improved, and environmental pollution is reduced; and (2) after the amination is completed, by-product ammonium chloride is directly removed by pressure filtration, so that an amount of waste water is small, and an amination yield is high.
Owner:江苏优普生物化学科技股份有限公司
Synthesis method of 2, 4-dichloroacetophenone
InactiveCN111116336AReduce usageImprove conversion rateOrganic compound preparationCarbonyl compound preparation by condensationAcetyl chlorideDistillation
The invention discloses a synthesis method of 2, 4-dichloroacetophenone. The method comprises the following steps: carrying out electrophilic substitution reaction on m-dichlorobenzene and acetyl chloride in an ionic liquid (Bmin)Cl-FeCl3 medium to synthesize the 2, 4-dichloroacetophenone; adding m-dichlorobenzene and acetyl chloride into the (Bmin)Cl-FeCl3 ionic liquid according to a molar ratioof 1:(1.1-1.2), carrying out a stirring reaction for 4h at a temperature of 40-60 DEG C, extracting 2, 4-dichloroacetophenone with cyclohexane, and carrying out reduced pressure distillation to removethe solvent so as to obtain 2, 4-dichloroacetophenone. The weight ratio of the feeding amount of the ionic liquid (Bmim)Cl-FeCl3 to the total feeding amount of m-dichlorobenzene and acetyl chloride is 1:(1-0.8). The method has the advantages that the use of anhydrous aluminum chloride is avoided, the conversion rate of raw materials is improved, byproducts are reduced, and green and environment-friendly production is realized.
Owner:长沙鑫本助剂有限公司
Method for synthesizing fiber-grade polyphenylene sulfide through adding composite accelerant in intermediate stage
The invention discloses a method for synthesizing fiber-grade polyphenylene sulfide through adding a composite accelerant in an intermediate stage. The method comprises the step: with p-dichlorobenzene and waterish sodium sulfide as reaction raw materials, synthesizing polyphenylene sulfide through polycondensation, wherein the composite accelerant is added in the intermediate stage of the reaction and is formed by mixing the four components: lithium chloride, p-dichlorobenzene, trichlorobenzene and / or m-dichlorobenzene and N-methylpyrrolidone. Compared with the prior art, the method disclosed by the invention is simple in process and low in production cost, and the synthesized polyphenylene sulfide is high in molecular weight, good in appearance and favorable in quality.
Owner:ZHUHAI CHANGXIAN CHEM TECH
Method for preparing m-dichlorobenzene by catalyzing isomerization of p-dichlorobenzene by using nano ZSM-12 molecular sieve
InactiveCN104610014AHigh selectivityStrong resistance to carbon deposition and deactivationMolecular sieve catalystsHalogenated hydrocarbon preparationIsomerizationM-dichlorobenzene
The invention discloses a method for preparing m-dichlorobenzene by catalyzing isomerization of p-dichlorobenzene by using a nano ZSM-12 molecular sieve and belongs to the field of production of fine chemicals to solve the technical problems that an anhydrous aluminum chloride homogeneous catalyst which is used in preparation of m-dichlorobenzene by virtue of isomerization of p-dichlorobenzene cannot be regenerated for use and generates a lot of acidic wastewater, and the problem of relatively low selectivity of m-dichlorobenzene prepared by adopting a ZSM-5 molecular sieve. The method for preparing m-dichlorobenzene comprises the following steps: 1, preparing a raw material solution; and 2, filling SiO2, Al2O3 and the nano ZSM-12 molecular sieve into a constant-temperature zone of a fixed bed reactor, activating, and injecting the raw material solution by adopting a continuous injecting manner to perform catalytic reaction. According to the method disclosed by the invention, an environment-friendly nano ZSM-12 molecular sieve solid acid catalyst is used, so that a continuous heterogeneous catalysis reaction process can be realized, the selectivity to the target product namely m-dichlorobenzene is high, the reaction product can be easily separated from the catalyst, and large-scale industrial production can be realized.
Owner:HEILONGJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com