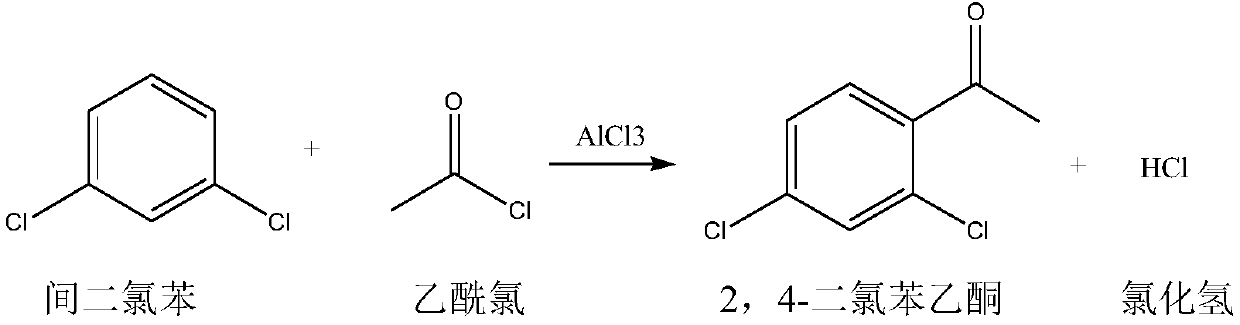
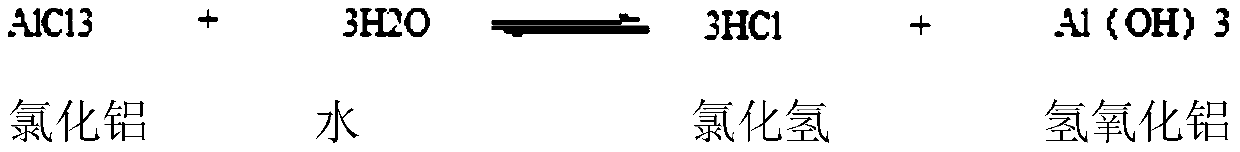
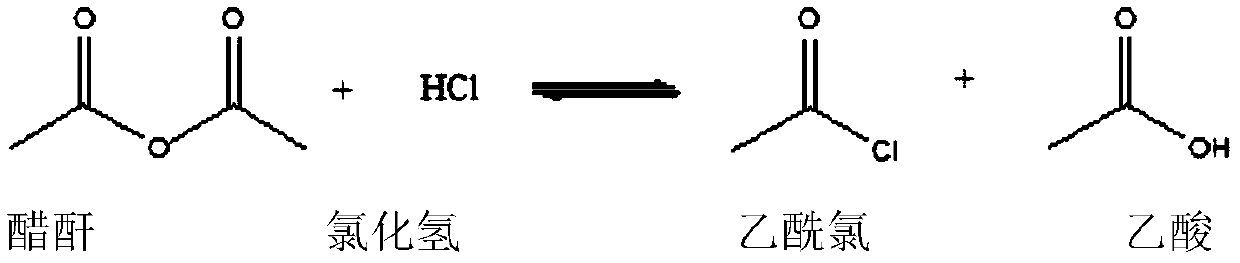
Synthetic method for 2,4-dichloroacetophenone
A technology of dichloroacetophenone and its synthesis method, which is applied in the field of pharmaceutical intermediates 2, can solve the problem that there is no clear data on the effect of the electrophilic substitution reaction of m-dichlorobenzene, and achieve convenient operation, low unit consumption, and equipment investment little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Put acetic anhydride: 200.0g, anhydrous AlCl3: 100.0g into a dry 500ml four-necked flask equipped with a stirring paddle, a thermometer, a constant pressure dropping funnel, and a rectification tower. Add m-dichlorobenzene: 100.0 g (98.3%, 0.6687 mol) into the constant pressure dropping funnel. The outer wall of the four-necked flask was kept warm with a water bath, and the temperature of the hot water in the water bath was set at 55°C. Turn on stirring, turn on the vacuum at the top of the rectification tower, and adjust the vacuum at the top of the rectification tower to ≥0.085MPa. When the internal temperature reaches 40°C, slowly add the m-dichlorobenzene in the constant pressure dropping funnel into the four-neck flask dropwise, and the dropping time is controlled within 0.5-1.0 hours. During the dropwise addition process, reflux slowly appears at the top of the rectification tower, and the acetic acid formed by the reaction is extracted by controlling the reflux ...
Embodiment 2
[0037] Put 112.0 g of acetic anhydride and 95.0 g of anhydrous AlCl3 into a dry 500 ml four-necked flask equipped with a stirring paddle, a thermometer, a constant pressure dropping funnel, and a rectification tower. Add m-dichlorobenzene: 100.0 g (98.3%, 0.6687 mol) into the constant pressure dropping funnel. The outer wall of the four-necked flask was kept warm with a water bath, and the temperature of the hot water in the water bath was set at 55°C. Turn on stirring, turn on the vacuum at the top of the rectification tower, and adjust the vacuum at the top of the rectification tower to ≥0.085MPa. When the internal temperature reaches 40°C, slowly add the m-dichlorobenzene in the constant pressure dropping funnel into the four-neck flask dropwise, and the dropping time is controlled within 0.5-1.0 hours. During the dropwise addition process, reflux slowly appears at the top of the rectification tower, and the acetic acid formed by the reaction is extracted by controlling th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com