Preparation and application of 1,2,4,5-tetra amino benzene and hydrochloride thereof
A technology of tetraaminobenzene hydrochloride and tetraaminobenzene, which is applied in the application field of high-performance materials PDBI, can solve the problems of high TAP monomer preparation cost, difficulty in industrialization implementation and promotion, and restrictions on the preparation of polymerization-grade monomers. , to enhance the effect of realizing industrialized production, controllable amount of by-products, and good fiber spinnability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation and application of a kind of 1,2,4,5-tetraaminobenzene hydrochloride
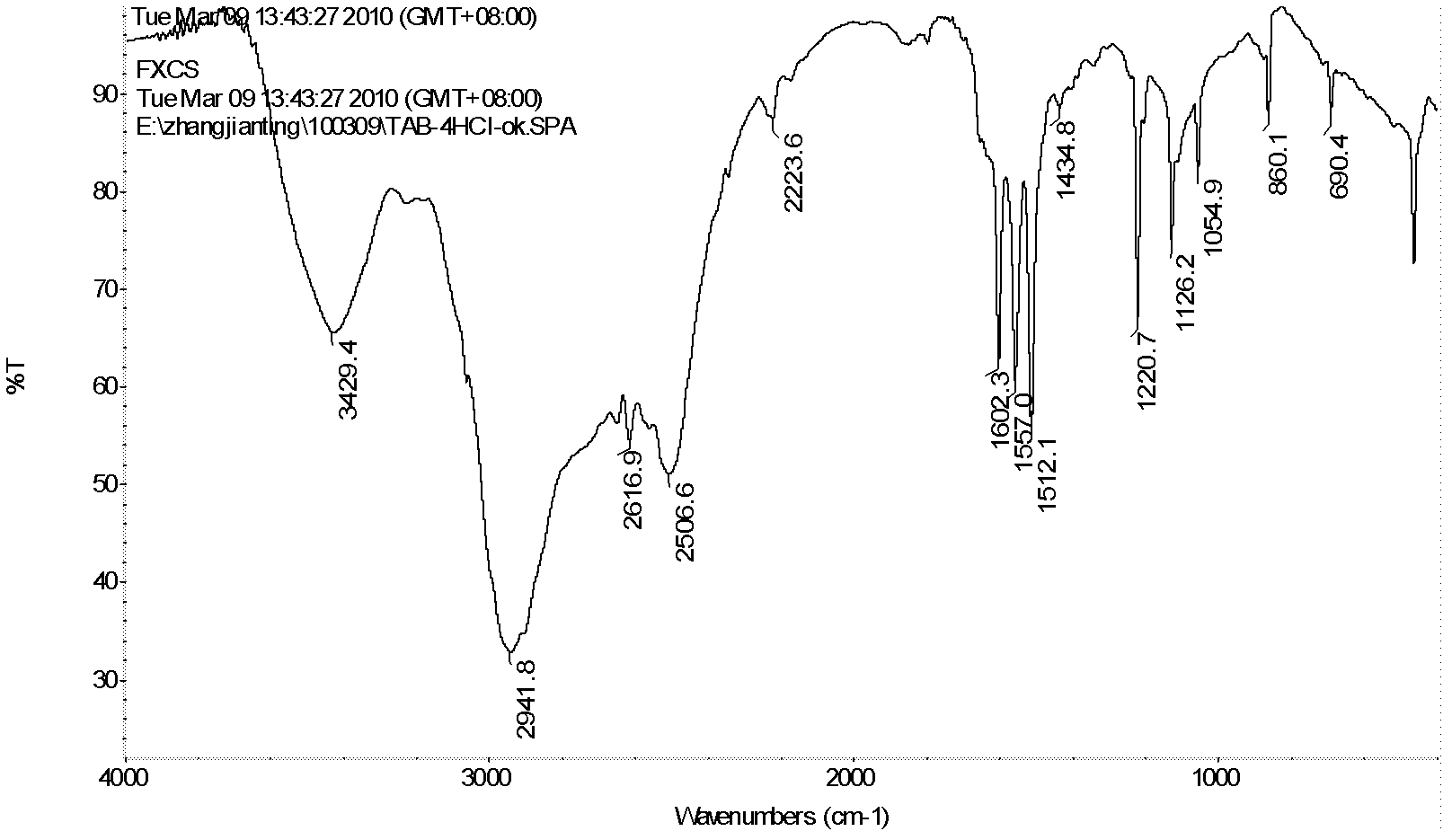
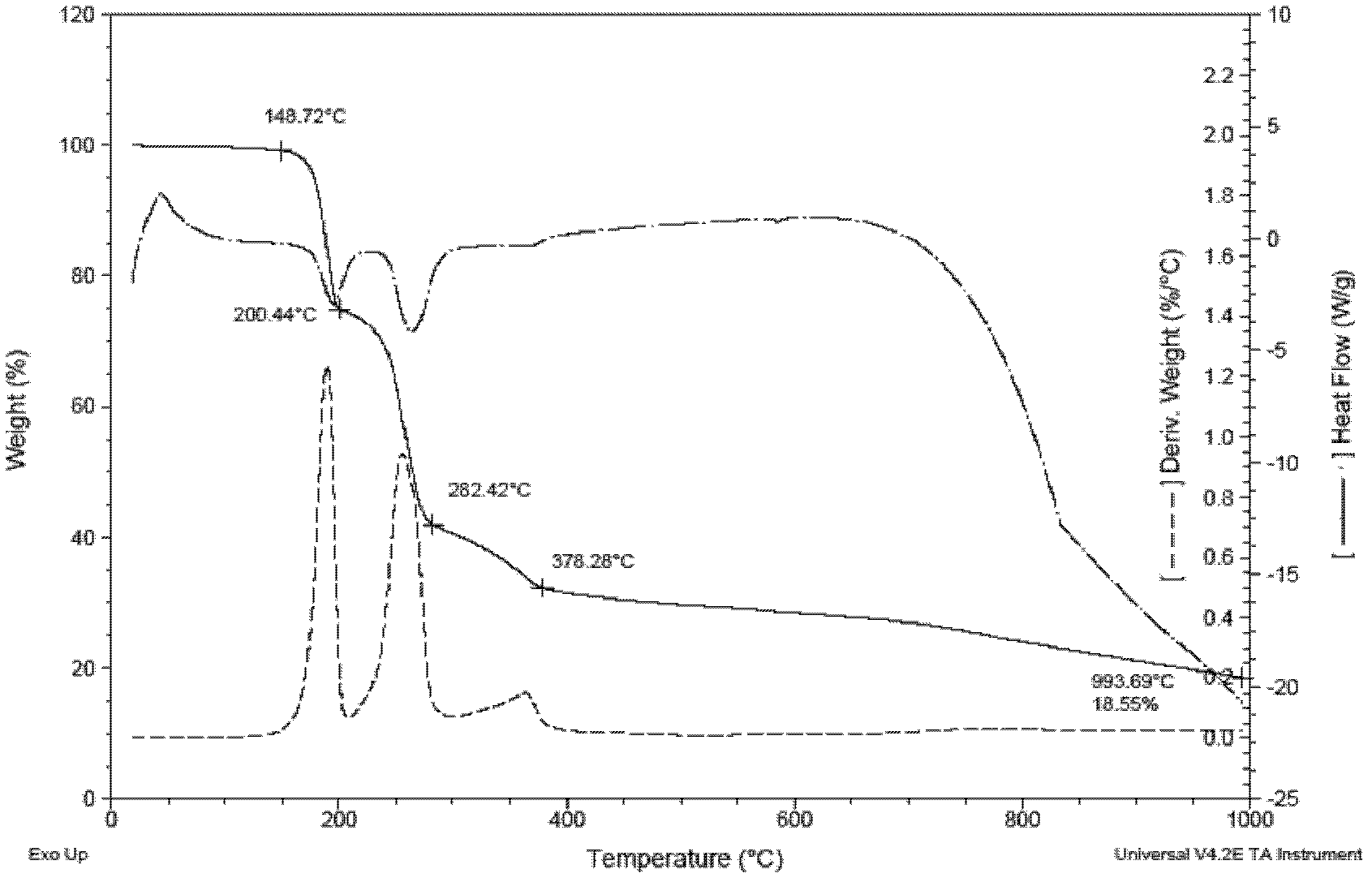
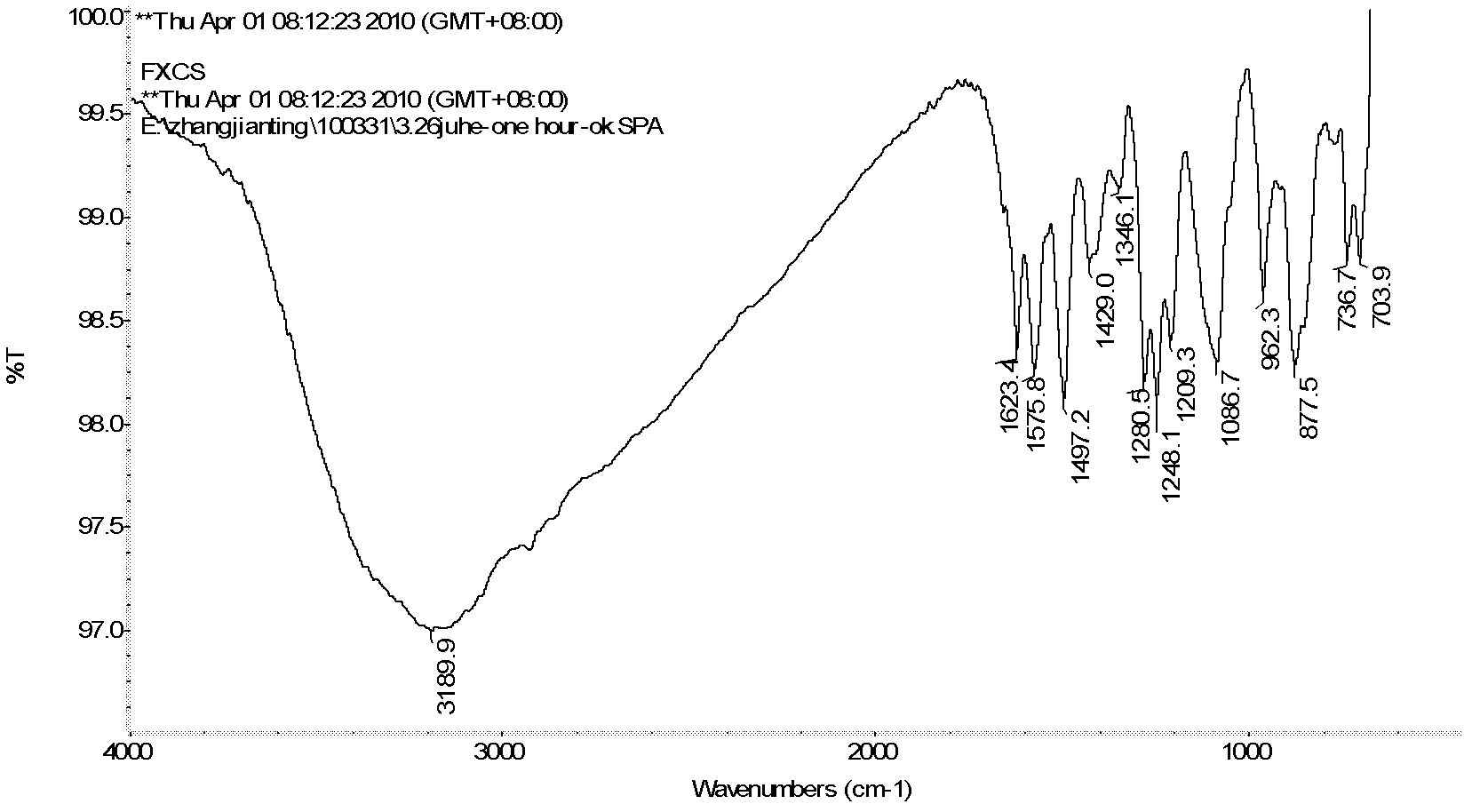
[0037] 1) Weigh 17.5g (0.0735mol) of 4,6-dinitro-m-dichlorobenzene (DCDNB) in a 500mL autoclave, add 1,4-dioxane 175mL, weigh ammonia water (29%) 42g ( 0.70mol), the glass rod was stirred evenly and then the kettle was installed to ensure its sealing, and the nitrogen gas was passed three times to remove the air, the stirring speed was controlled to be 500 rpm, and the ammonolysis reaction was heated to 150°C for 5h (the maximum temperature and pressure were respectively 160°C and 1.5MPa). After the reaction was completed, the temperature was cooled, the reaction solution was taken out, a large amount of water was added, and a yellow solid was precipitated. After filtration and drying, 13.3 g of the product 4,6-dinitrom-phenylenediamine (DADNB) was obtained, with a melting point of 150° C. and a purity of 96.3%. The rate is 90.9%. IR (KBr, cm -1 ): 3476.6(s), 3367.5(s), 1605...
Embodiment 2
[0042] Embodiment 2: intermediate 4, the technical scope of 6-dinitro-m-diphenylamine (V) synthesis
[0043] Change the molar ratio RO of ammonia water and 4,6-dinitro-m-dichlorobenzene in step 1) in Example 1, the reaction temperature T (℃), and the reaction preset pressure P 1 (MPa), reaction control pressure P 2 (MPa), reaction solvent, reaction time t (h), all the other steps are with the step 1 in embodiment 1), synthetic 4, the test result list 1 of 6-dinitro-m-diphenylamine (V):
[0044] Table 1:
[0045]
Embodiment 3
[0046] Embodiment 3: Intermediate 1,2,4,5-tetraaminobenzene (I) synthetic process scope
[0047] Change the percentage ω1 of reaction solvent, catalyzer and raw material weight in step 2) in embodiment 1, reaction temperature T (℃), reaction pressure P (MPa), reaction time t (h), all the other steps are with the steps in embodiment 1 1), step 2), the results are shown in Table 2.
[0048] Table 2
[0049]
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com