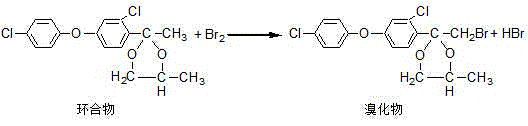
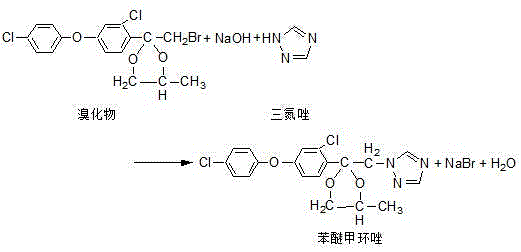
Production process of difenoconazole
A technology of difenoconazole and production process, applied in the field of production technology of difenoconazole, can solve problems such as difficulty in achieving quality and yield, air pollution, stimuli, etc., and achieve shortened process reaction time, cleanliness Environmentally friendly production, the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The production technology of difenoconazole comprises the following steps:
[0031] a. Etherification: Put the weighed p-chlorophenol, solid potassium hydroxide and measured m-dichlorobenzene into the etherification reaction kettle, heat up the jacket steam to 100°C, and perform dehydration reaction at normal pressure for 10 hours. After sampling and analysis are qualified , the cooling water drops to room temperature and then filtered, the filter residue containing potassium chloride is sent to solid waste treatment, the filtrate is returned to the reaction kettle and the jacket is heated to 140°C, first at normal pressure and then depressurized to -0.090 MPa to remove excess m-dichloride Benzene is recovered and used mechanically, and the etherified compound obtained is transferred to the next process;
[0032] b. Acylation: At room temperature, add the etherified product obtained in the previous step, a small amount of aluminum trichloride and metered dichloromethane...
Embodiment 2
[0037] The production technology of difenoconazole comprises the following steps:
[0038] a. Etherification: Put the weighed p-chlorophenol, solid potassium hydroxide and measured m-dichlorobenzene into the etherification reaction kettle, heat up the jacketed steam to 102°C, and perform dehydration reaction under normal pressure for 11 hours. After sampling and analysis are qualified , the cooling water drops to room temperature and then filtered, the filter residue containing potassium chloride is sent to solid waste treatment, the filtrate is returned to the reaction kettle and the jacket is heated to 140°C, first at normal pressure and then decompressed to -0.091 MPa to remove excess m-dichloride Benzene is recovered and used mechanically, and the etherified compound obtained is transferred to the next process;
[0039] b. Acylation: At room temperature, add the etherified product obtained in the previous step, a small amount of aluminum trichloride and metered dichloromet...
Embodiment 3
[0044] The production technology of difenoconazole comprises the following steps:
[0045] a. Etherification: Put the weighed p-chlorophenol, solid potassium hydroxide and measured m-dichlorobenzene into the etherification reaction kettle, heat up the jacket steam to 105°C, and perform dehydration reaction under normal pressure for 12 hours. After sampling and analysis are qualified , the cooling water drops to room temperature and then filtered, the filter residue containing potassium chloride is sent to solid waste treatment, the filtrate is returned to the reaction kettle and the jacket is heated to 142°C, first at normal pressure and then depressurized to -0.092MPa to remove excess m-dichloride Benzene is recovered and used mechanically, and the etherified compound obtained is transferred to the next process;
[0046] b. Acylation: At room temperature, add the etherified product obtained in the previous step, a small amount of aluminum trichloride and metered dichlorometha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com