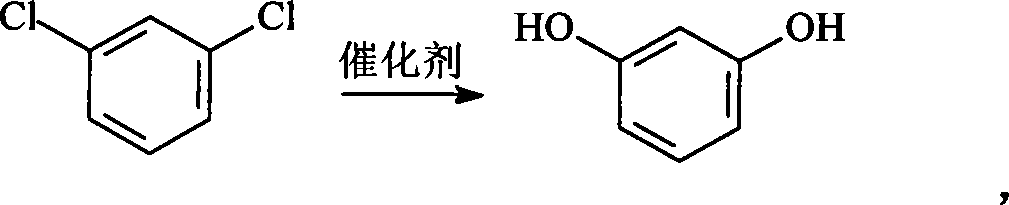
Method for preparing resorcin
A technology for resorcinol and m-dichlorobenzene is applied in the field of preparation of resorcinol by hydrolysis, can solve the problems of high requirements for production equipment and high price of m-phenylenediamine, and achieves easy separation and purification and low price , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 1L stainless steel autoclave equipped with stirring, add 50g m-dichlorobenzene, 5g sodium hydroxide, 5g sodium carbonate, 2g lanthanum oxide, and 200g water. After leak test, heat to 220℃, control temperature 220~230℃, and react. After 2 hours, the temperature was lowered to below 30°C after the reaction, and gas chromatograph was used for sampling. In the reaction solution: the content of m-dichlorobenzene is 40.42%; the content of resorcinol is 20.01%; the content of m-chlorophenol is 39.57%. The conversion rate of m-dichlorobenzene was 59.58%. The reaction solution was taken out, filtered, adjusted to pH 2 to 3 with hydrochloric acid, and filtered again. The reaction liquid was extracted with a solvent, and the extractant was distilled under reduced pressure to recover the extractant and m-chlorophenol to obtain 10.01 g of resorcinol, and the yield of resorcinol was 27.1%.
Embodiment 2
[0027] In a 1L stainless steel autoclave equipped with stirring, add 60g m-dichlorobenzene, 30g potassium hydroxide, 5g calcium oxide, 3g lanthanum oxide, and 200g water. After the leak test, heat to 250℃, control the temperature at 245~255℃, and react. After 4 hours, the temperature was lowered to below 30°C after the reaction, and gas chromatograph was used for sampling. In the reaction solution: the content of m-dichlorobenzene is 4.15%; the content of resorcinol is 42.23%; the content of m-chlorophenol is 53.62%. The conversion rate of m-dichlorobenzene was 95.85%. The reaction solution was taken out, filtered, adjusted to pH 2 to 3 with hydrochloric acid, and filtered again. The reaction liquid was extracted with a solvent, and the extractant was distilled under reduced pressure to recover the extractant and m-chlorophenol to obtain 25.33 g of resorcinol, and the yield of resorcinol was 57.1%.
Embodiment 3
[0029] In a 1L stainless steel autoclave equipped with stirring, add 80g m-dichlorobenzene, 30g sodium hydroxide, 5g lanthanum oxide, 2g cuprous chloride, and 300g water. After the leak test, heat to 250℃ and control the temperature to 240~260℃. After the reaction was completed for 8 hours, the temperature was lowered to below 30°C after the reaction, and gas chromatography was used for sampling. In the reaction solution: the content of m-dichlorobenzene is 20.7%; the content of resorcinol is 35.34%; the content of m-chlorophenol is 43.96%. The conversion rate of m-dichlorobenzene was 79.3%. The reaction solution was taken out, filtered, adjusted to pH 2 to 3 with hydrochloric acid, and filtered again. The reaction solution was extracted with a solvent, and the extractant was distilled under reduced pressure to recover the extractant and m-chlorophenol to obtain 28.2 g of resorcinol, and the yield of resorcinol was 41.29%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com