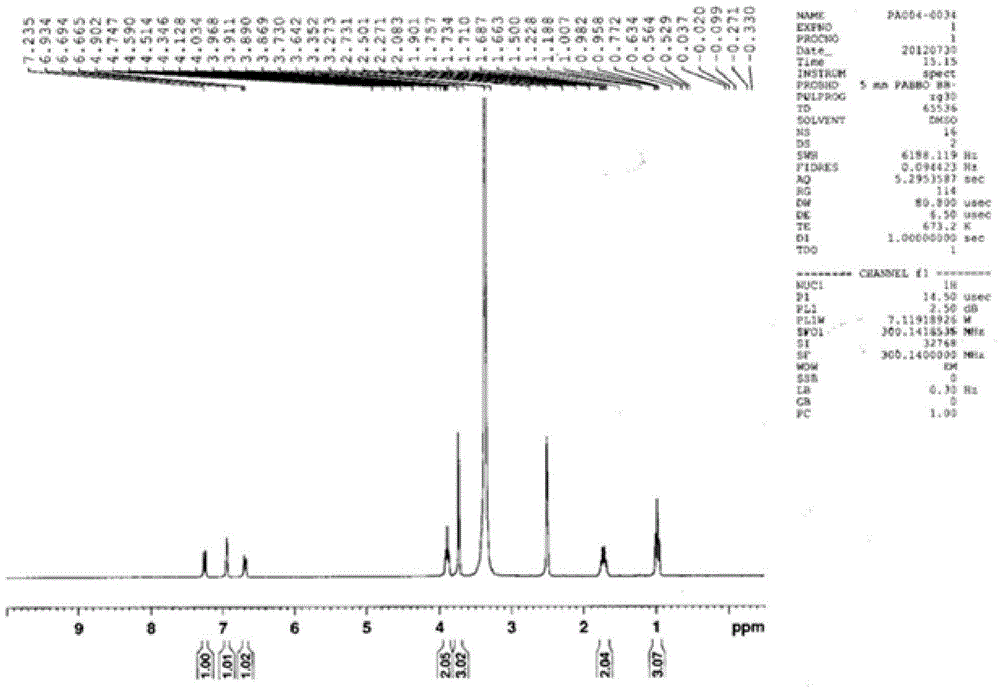
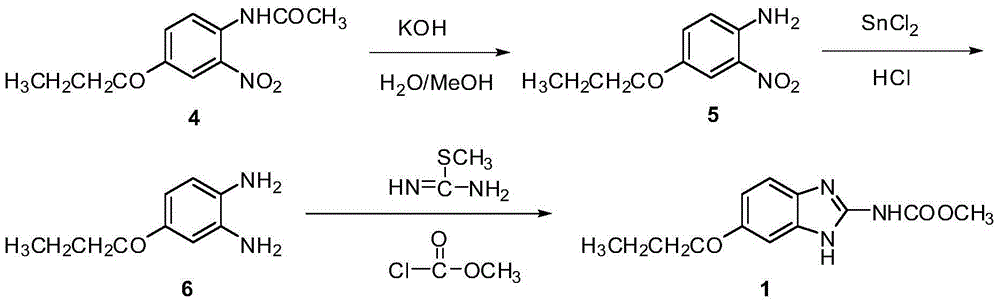
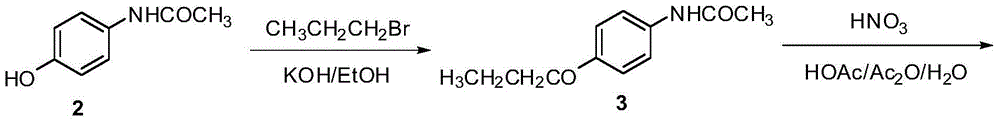Preparation method of oxibendazole
A technology of propoxyimidazole and n-propanol, which is applied in the field of chemistry or medicine, can solve the problems of human injury, high price of acetaminophen, and great environmental hazards, and achieve the effect of small environmental and personal hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 12, the preparation of 4-dichloronitrobenzene
[0036] In a 500ml four-necked flask, add 100.0g (0.68mol) of m-dichlorobenzene, 90.0g (0.90mol) of concentrated sulfuric acid, and 48.1g (0.75mol) of nitric acid, and keep the reaction at 5-10°C for 3 hours, then remove the waste from the lower layer. The acid layer was washed by adding 100 g of 5% aqueous sodium hydroxide solution, stirred and washed twice with 100 ml of water, and the lower layer was separated to obtain 125.0 (0.65 mol) g of 2,4-dichloronitrobenzene, which was directly used in the next reaction. This step yield is 95.7%, and content is 98.7%.
[0037] Step 22 - Preparation of nitro-5-chloroaniline
[0038] Add 100.0g (0.52mol) of 2,4-dichloronitrobenzene obtained in the previous step, 350g methanol, and 88.4g (5.2mol) of liquid ammonia to a 1000ml autoclave. The temperature is controlled at 130-140°C and the reaction pressure is 2.5 ~3.0MPa, react for 12 hours, cool down, release ammonia, slowly a...
Embodiment 2
[0047] Step 1 The preparation of 2,4-dichloronitrobenzene, the specific operation steps are the same as in Example 1, and the data are shown in Table 1;
[0048] Step 2 The preparation of 2-nitro-5-chloroaniline, the specific operation steps are the same as in Example 1, and the data are shown in Table 1;
[0049] Step 3 Preparation of 2-nitro-5-propoxyaniline
[0050] Add 80.0 g of n-propanol, 26.0 g (0.15 mol) of 2-nitro-5-chloroaniline and 14.8 g (0.18 mol) of sodium n-propoxide into a 500 ml four-necked flask. Heat up to 97°C and reflux for 5 hours, cool down to 60°C, slowly add 240ml of water, continue to cool down to 25°C and keep stirring for 3 hours. Filter, wash with water until neutral, and dry to obtain 25.1 g of 2-nitro-5-propoxyaniline. The yield is 85.2%, and the content is 97.5%.
[0051] Step 4 4-propoxy-1,2-phenylenediamine
[0052] In a 500ml four-necked flask with a thermometer and a stirring device, add isopropanol and 23.5g of 2-nitro-5-propoxyaniline ...
Embodiment 3
[0056] Step 1 The preparation of 2,4-dichloronitrobenzene, the specific operation steps are the same as in Example 1, and the data are shown in Table 1;
[0057] Step 2 The preparation of 2-nitro-5-chloroaniline, the specific operation steps are the same as in Example 1, and the data are shown in Table 2;
[0058] Step 3 The preparation of 2-nitro-5-propoxyaniline, the specific operation is the same as in Example 1, and the data are shown in Table 3;
[0059] Step 4 The preparation of 4-propoxy-1,2-phenylenediamine, the specific operation is the same as in Example 1, and the data are shown in Table 4;
[0060] Step 5 The preparation of propoxyimidazole, the specific operation is the same as in Example 1, and the data are shown in Table 5;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com