Synthetic process of difenoconazole
A difenoconazole and synthesis process technology, applied in the direction of organic chemistry, can solve the problems of pollution, high content of condensation reaction isomers, large amount of catalyst AICl3, etc., and achieve the effect of simple process and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
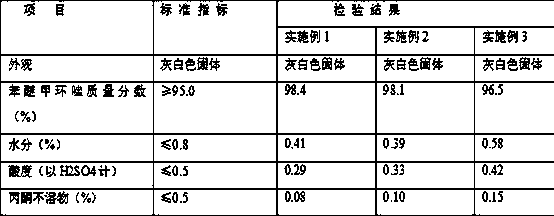
Embodiment 1
[0019] Acetylation reaction: Add 300 grams (Bmim) of Cl-FeCl3 ionic liquid to a 1000ml four-necked reaction flask with stirring, reflux condenser and thermometer, and add 147 grams (1.0mol) of m-dichlorobenzene and 94.2 grams of m-dichlorobenzene under stirring (1.2 mol) acetyl chloride, stirred and heated up, and kept at 50°C±2°C for 4 hours. After the reaction was completed, it was extracted with 200 ml×2 cyclohexane, the organic phase was combined, and the solvent was distilled off under reduced pressure to obtain liquid 2,4- Dichloroacetophenone 181.1 grams, purity 97.6%, yield 95.8%. The ionic liquid was washed with cyclohexane and dried for use.
[0020] Bromination reaction: add 300 milliliters of dichloromethane into 1000 milliliters of four-neck flasks with stirrer, thermometer, condenser and constant pressure titration funnel, add 189 grams of 2,4-dichloroacetophenone successively under stirring, 120 Milliliter of 27.5% hydrogen peroxide, 15 grams of sodium percarbo...
Embodiment 2
[0025] Acetylation reaction: Add 300 grams (Bmim) of Cl-FeCl3 ionic liquid to a 1000ml four-necked reaction flask with stirring, reflux condenser and thermometer, and add 147 grams (1.0mol) of m-dichlorobenzene and 94.2 grams of m-dichlorobenzene under stirring (1.2mol) acetyl chloride, stirred and heated up, and kept at 60°C ± 2°C for 4 hours. After the reaction, extracted twice with 200 ml cyclohexane, combined the organic phase, and evaporated the solvent under reduced pressure to obtain liquid 2,4 -Dichloroacetophenone 181.5 grams, content 96.9%, yield 96.0%. The ionic liquid was washed with cyclohexane and dried for use.
[0026] Bromination reaction: add 300 milliliters of dichloromethane into 1000 milliliters of four-neck flasks with stirrer, thermometer, condenser and constant pressure titration funnel, add 189 grams of 2,4-dichloroacetophenone successively under stirring, 150 Milliliter of 27.5% hydrogen peroxide, 15 grams of sodium percarbonate; then slowly add 80 g...
Embodiment 3
[0031] Acetylation reaction: Add 300 grams (Bmim) of Cl-FeCl3 ionic liquid to a 1000ml four-necked reaction flask with stirring, reflux condenser and thermometer, and add 147 grams (1.0mol) of m-dichlorobenzene and 94.2 grams of m-dichlorobenzene under stirring (1.2 mol) acetyl chloride, stirred and heated up, and kept at 40°C±2°C for 4 hours. After the reaction, extracted twice with 200 ml cyclohexane, combined the organic phase, and evaporated the solvent under reduced pressure to obtain liquid 2,4 -148.4 grams of dichloroacetophenone, content 93.1%, yield 78.5%. The ionic liquid was washed with cyclohexane and dried for use.
[0032] Bromination reaction: 300 milliliters of dichloromethane is added in 1000 milliliters four-neck flasks with stirrer, thermometer, condenser and constant pressure titration funnel, add 189 grams of 2,4-dichloroacetophenone under stirring, then in Slowly add 80 grams of bromine dropwise at room temperature (a large amount of hydrogen bromide gas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com