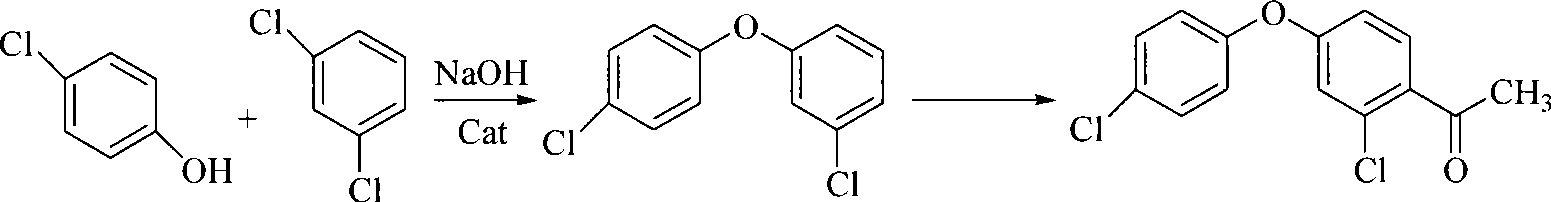
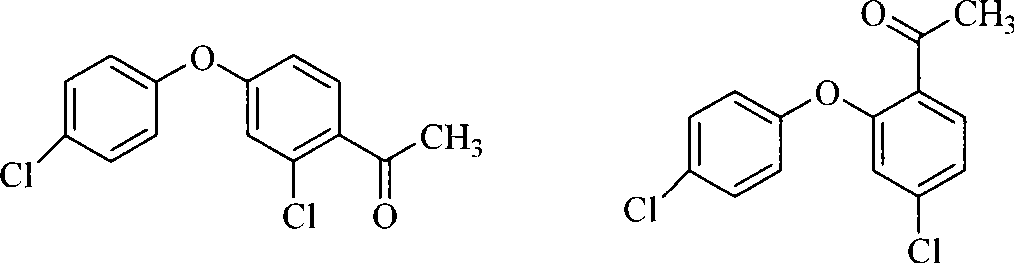
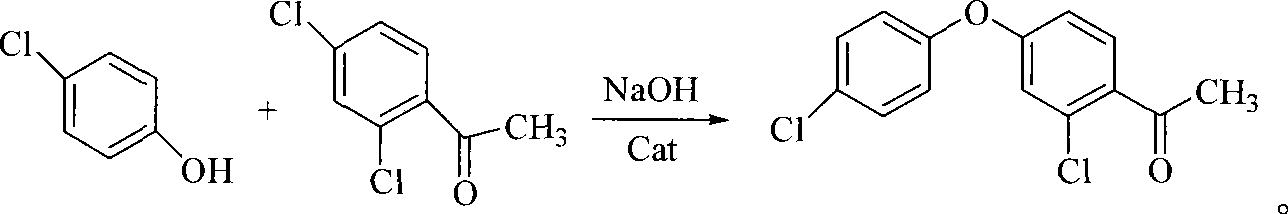Method for preparing 2-chlorine-4-(4-chlorophenoxy)-hypnone
A kind of technology of chlorophenoxy and acetophenone, applied in the field of organic synthesis, can solve problems such as long reaction period and reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Add 64.3 grams (0.5 moles) of p-chlorophenol into the reaction flask, heat up to 80° C., and after it is completely dissolved, add 22.4 grams (0.4 moles) of potassium hydroxide, and stir for 2 hours to complete the reaction. Add 200 ml m-dichlorobenzene, heat to reflux to separate water. After the water is separated, 0.5 g of copper oxide is added, heated to reflux for reaction, and analyzed by gas chromatography until the normalized content of p-chlorophenol is ≤1.0%. Cool down after the reaction is over, add 300 milliliters of water, stir, filter, stand to separate layers, extract the water layer once with 50 milliliters of m-dichlorobenzene, then wash the organic layer with dilute alkali, water, dilute hydrochloric acid and water successively, and steam under negative pressure. After removing the solvent and the previous fraction, the fraction at 190-200°C (20mmHg) was collected to obtain 82.0 g of 3,4'-dichlorodiphenyl ether.
[0037] Add 300 milliliters of dichlor...
example 2
[0039] 300 milliliters of dichloromethane, 80.0 g (0.60 moles) of aluminum trichloride, 72.0 grams (0.3 moles) of 3,4'-dichlorodiphenyl ether (prepared by the method in Example 1, the same below) were added to the belt in sequence In the reaction flask with stirrer, thermometer, dropping funnel and condenser, add dropwise a solution of 31.0 g (0.30 moles) of acetic anhydride in dichloromethane (50 ml) under stirring, react at room temperature for 6 hours after the addition, and gas phase Chromatographic tracking analysis to the normalized content of 3,4'-dichlorodiphenyl ether ≤ 0.5%, the reaction ends. The post-treatment operation was the same as above to obtain 71.9 g of 2-chloro-4-(4-chlorophenoxy)-acetophenone, a white solid, mp54-56°C, mass fraction of 99.4% according to gas chromatography, yield 85.2%.
example 3
[0041]Add 300 milliliters of dichloromethane, 160.0 grams (1.2 moles) of aluminum trichloride, and 72.0 grams (0.3 moles) of 3,4'-dichlorodiphenyl ether into the mixture with agitator, thermometer, dropping funnel, and condenser in sequence. In a reaction flask, add 62.0 grams (0.60 moles) of acetic anhydride in dichloromethane (50 milliliters) dropwise under stirring, react at room temperature for about 6 hours after the addition, and follow up the analysis by gas chromatography. When 3,4'-di When the normalized content of chlorodiphenyl ether is ≤0.5%, the reaction ends. The post-treatment operation was the same as above to obtain 70.7 g of 2-chloro-4-(4-chlorophenoxy)-acetophenone, a white solid, mp54-56°C, mass fraction of 99.1% according to gas chromatography, yield 84.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com