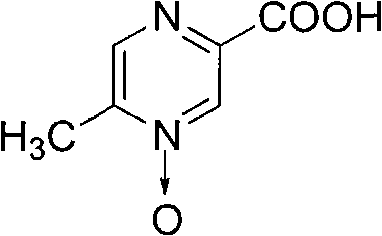
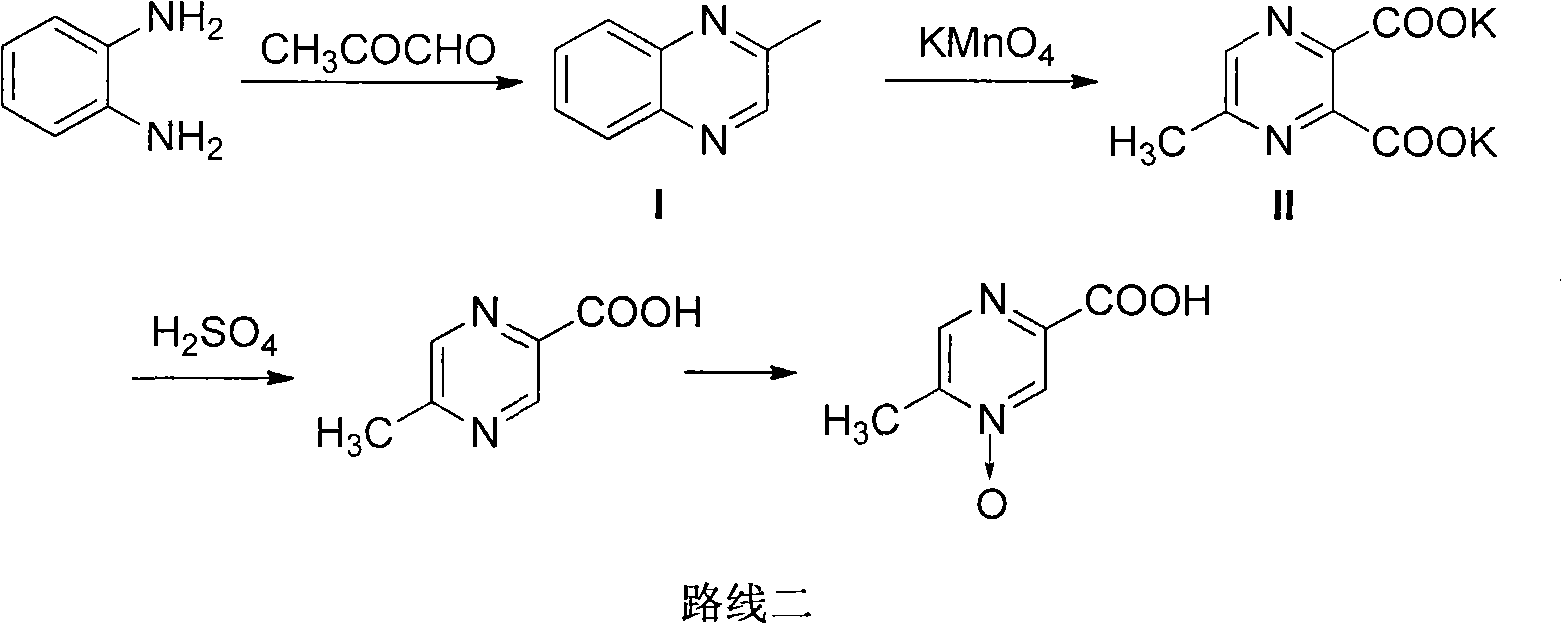
Preparation method of acipimox
A technology of methylpyrazine and dimethylpyrazine, applied in the field of medicine, can solve the problems of harsh reaction conditions, many decarboxylation by-products, unfavorable and the like, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
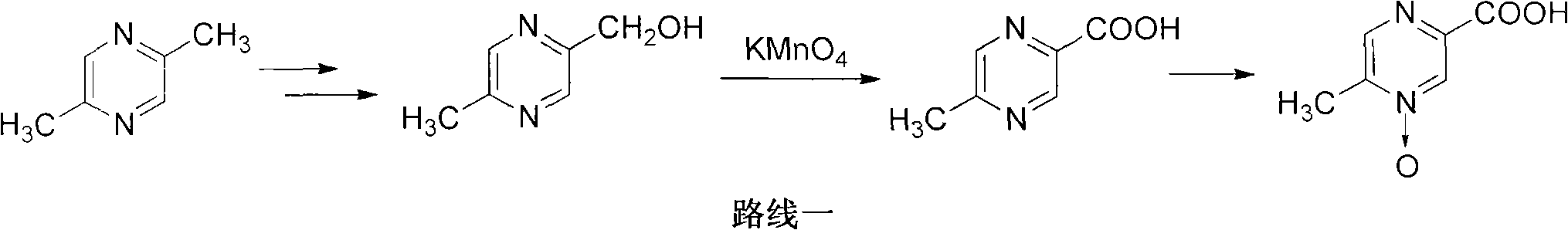
[0023] Embodiment 1: the preparation of 2,5-dimethylpyrazine-1-oxide
[0024] Add 20g of 2,5-dimethylpyrazine and 20mL of 30% hydrogen peroxide to a 500mL three-strand bottle, heat to 80°C for 10h, cool down to room temperature naturally, extract with dichloromethane (100mL x3), dry over anhydrous sodium sulfate, and filter with suction , The solvent was removed from the filtrate under reduced pressure, and the residue was recrystallized from toluene to obtain 18 g of white solid with a yield of 78%.
Embodiment 2
[0025] Embodiment 2: Preparation of 2-acetoxymethyl-5-methylpyrazine
[0026] Add 20g of 2,5-dimethylpyrazine-1-oxide, 40mL of acetic anhydride, 10g of sodium acetate and 250mL of acetic acid into a 500mL Sanjin bottle, heat to reflux for 5h, and remove the solvent under reduced pressure to obtain a residue of 23g, which is used directly for Next reaction.
Embodiment 3
[0027] Embodiment 3: the preparation of 2-hydroxymethyl-5-methylpyrazine
[0028] The product from the previous step and 50 mL of 1N sodium hydroxide solution were heated to 50°C, stirred for 3 h, extracted with ethyl acetate (100 mL x 3), concentrated under reduced pressure to remove about 2 / 3 of the solvent, and the remaining liquid was directly used for the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com