Preparation method of amicarbazone
A technology of amfentrazone and amino, which is applied in the field of preparation of amfentrazone, can solve the problems of poor reaction selectivity, unsatisfactory reaction results, low boiling point of methyl acetate, etc., and achieves mild reaction conditions and strong reaction operability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
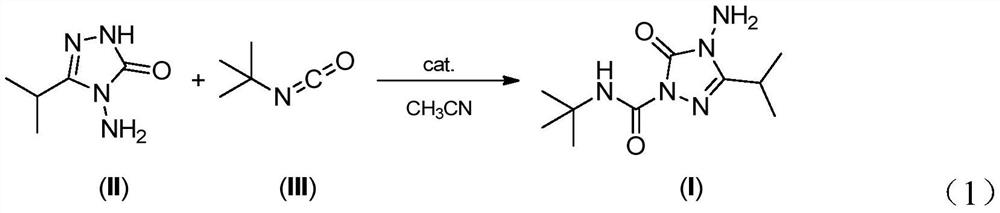
[0018] The invention provides a method for the preparation of amifentrazone, which comprises, under the catalysis of lithium hydroxide, 3-isopropyl-4-amino-1,2,4-triazolin-5-one (compound II ) and tert-butyl isocyanate (compound III) react chemically in acetonitrile to obtain the target product amenzazone. The reaction is characterized by high selectivity and high yield.
[0019] The applicant found that the lithium chloride in the composite catalyst reported in the patent US5708184 is almost insoluble in solvents such as methyl acetate, acetone, tetrahydrofuran, ethylene glycol dimethyl ether, and acetonitrile. Therefore, lithium chloride cannot effectively coordinate with the reaction substrate, resulting in poor reaction selectivity. Surprising discovery is, when using the lithium hydroxide of catalytic amount instead as catalyzer, when adopting acetonitrile as reaction medium simultaneously, compound (II) and compound (III) can take place chemical reaction smoothly, and t...
Embodiment 1
[0028] Under the protection of nitrogen flow, 360.00g of acetonitrile, 57.29g (0.575mol) of tert-butyl isocyanate, 0.71g (0.030mol) of powdered lithium hydroxide and 71.80g (0.500mol) of 3-isocyanate were successively put into the reaction flask. Propyl-4-amino-1,2,4-triazolin-5-one. Start stirring, heat up to 60° C., and keep the temperature for 60 minutes to complete the reaction. 6.00 g of acetic acid was added to neutralize the alkali in the reaction system, and samples were taken for high-performance liquid chromatography (HPLC) analysis. The reaction conversion rate was 97.9%, and the reaction selectivity was 98.2%.
[0029] After concentration and precipitation under negative pressure, 175ml of water was added, stirred, cooled to 10°C, and filtered. The filter cake was dried to obtain 116.19 g of amenzazone in the form of a white powdery solid with a purity of 97.6% and a yield of 94.0%.
Embodiment 2
[0031] Under the protection of nitrogen flow, 360.00g of acetonitrile, 0.71g (0.030mol) of powdered lithium hydroxide and 71.80g (0.500mol) of 3-isopropyl-4-amino-1,2,4- Triazolin-5-one. Start stirring, raise the temperature to 60° C., drop 57.29 g (0.575 mol) tert-butyl isocyanate into the reaction solution within 30 minutes, and then keep the temperature for 60 minutes. After the reaction was completed, 6.00 g of acetic acid was added to neutralize the alkali in the reaction system, and samples were taken for HPLC analysis. The reaction conversion rate was 97.8%, and the reaction selectivity was 98.7%.
[0032] After concentration and precipitation under negative pressure, 175ml of water was added, stirred, cooled to 10°C, and filtered. The filter cake was dried to obtain 116.78 g of amentrazone in the form of a white powdery solid with a purity of 97.7% and a yield of 94.6%.
[0033] The reactant and consumption of embodiment 1 and 2 are identical, and difference is only ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com