Method for co-producing n-butyl isocyanate from chloroformic acid-2-ethylhexyl ester
A technology of butyl isocyanate and ethyl hexyl ester is applied in the field of co-production of n-butyl isocyanate with 2-ethylhexyl chloroformate, which can solve problems such as unfavorable FNC phosgenation reaction, and achieve economical and environmental protection effects. Remarkably, reduce the treatment cost, and the effect of tail gas separation is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
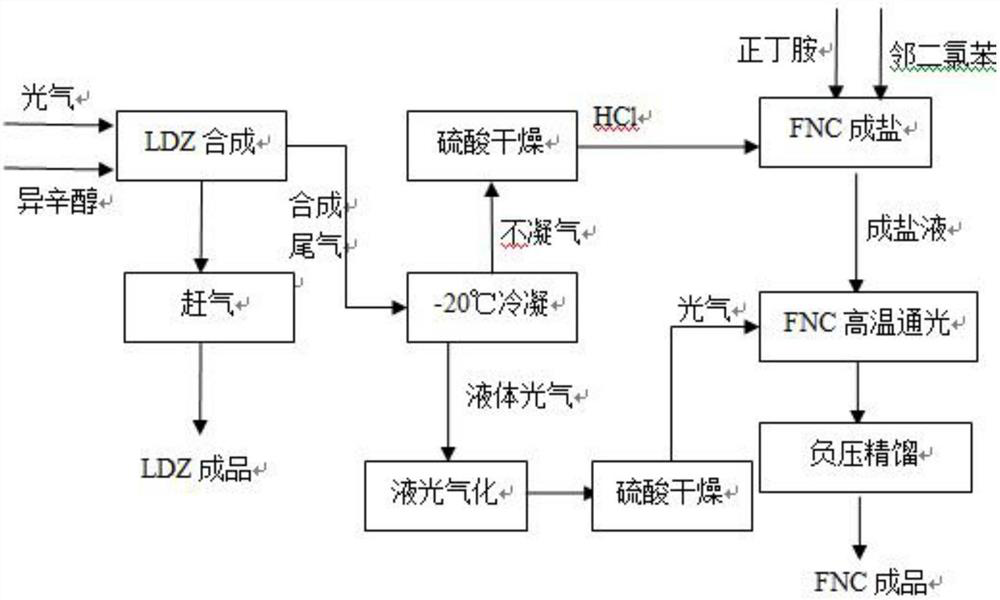
[0026] Such as figure 1 As shown, (1) Add 50 mol of iso-octanol to the LDZ synthesis kettle, the mol ratio of phosgene to iso-octanol is 1.3:1, the reaction temperature is 10°C, and the reaction time is 3 hours. The reaction tail gas is condensed at -20°C, the non-condensable gas is dried by sulfuric acid, and the HCl of the tail gas is 45mol. The normalized content of LDZ is 98.1% and the yield is 97.2%.
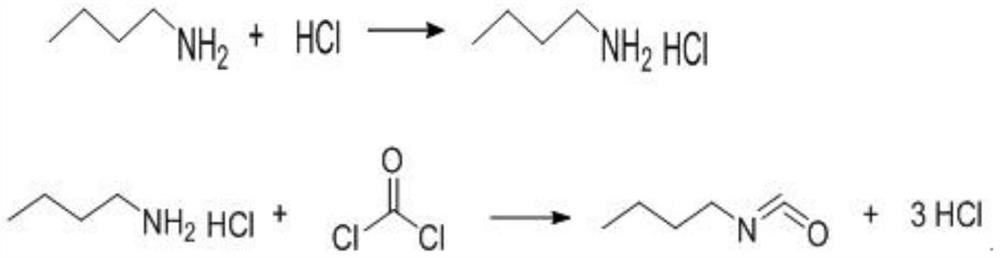
[0027] (2) Add 40 mol of n-butylamine to the FNC synthesis kettle, and the mass ratio of n-butylamine to o-dichlorobenzene is 1:2. Pass in hydrogen chloride in step (1), the molar ratio of hydrogen chloride to n-butylamine is 1.12:1, and the salt-forming reaction temperature is 10°C. After salt formation, the temperature is raised to 80°C, phosgene is introduced in (1) and the light is supplemented until the material liquid is clarified. The light passage time is 5 hours, and the actual amount of phosgene used is 48mol. The amine molar ratio was 1.2:1. The FNC content o...
Embodiment 2
[0029] Such as figure 1 As shown, (1) Add 50 mol of iso-octanol to the LDZ synthesis kettle, the phosgene: iso-octanol mol ratio is 1.5:1, the reaction temperature is 10°C, and the reaction time is 3 hours. The tail gas of the reaction was condensed at -20°C, the non-condensable gas was dried with sulfuric acid, and the HCl in the tail gas was 46 mol. The normalized content of LDZ is 98.7% and the yield is 97.7%.
[0030] (2) Add 40 mol of n-butylamine to the FNC synthesis kettle, and the mass ratio of n-butylamine to o-dichlorobenzene is 1:3. Pass in hydrogen chloride in step (1), the molar ratio of hydrogen chloride to n-butylamine is 1.15:1, and the salt-forming reaction temperature is 10°C. After salt formation, raise the temperature to 90°C, pass through (1) phosgene and supplement light, and pass light for 7 hours. The actual amount of phosgene used is 52 mol, the supplementary phosgene in the process is 29.5 mol, and the molar ratio of phosgene to n-butylamine is 1.3...
Embodiment 3
[0032] Such as figure 1 As shown, (1) Add 50 mol of iso-octanol to the LDZ synthesis kettle, the phosgene:iso-octanol mol ratio is 1.7:1, the reaction temperature is 15°C, and the reaction time is 3 hours. The tail gas of the reaction was condensed at -20°C, the non-condensable gas was dried with sulfuric acid, and the HCl in the tail gas was 48mol. The normalized content of LDZ is 99.2% and the yield is 98.5%.
[0033] (2) Add 40 mol of n-butylamine to the FNC synthesis kettle, and the mass ratio of n-butylamine to o-dichlorobenzene is 1:5. Pass in hydrogen chloride in step (1), the molar ratio of hydrogen chloride to n-butylamine is 1.2:1, and the salt-forming reaction temperature is 15°C. After salt formation, raise the temperature to 90°C, pass through (1) phosgene and supplement light, and pass light for 5 hours. The actual amount of phosgene used is 50mol, the supplementary phosgene is 18.5mol in the process, and the molar ratio of phosgene to n-butylamine is 1.25:1. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com