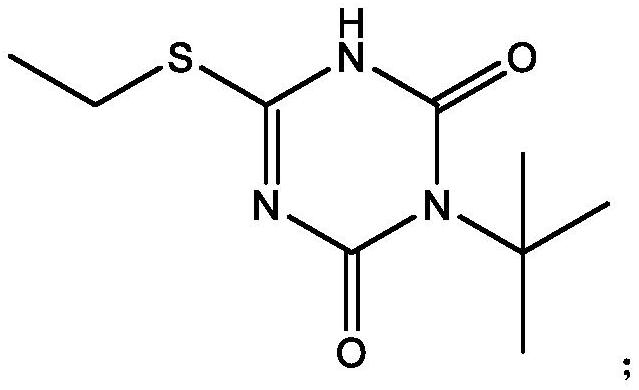
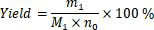Preparation method of 3-tert-butyl-6-ethylthio-1, 3, 5-triazine-2, 4 (1H, 3H)-diketone
An ethylthio and tert-butyl technology, applied in the field of organic synthesis, can solve the problems of low yield and limited purity of the target product, and achieve the effects of improving product yield and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A preparation method of 3-tert-butyl-6-ethylthio-1,3,5-triazine-2,4(1H,3H)-dione, the specific steps are:
[0057]1 mol (185 g) S-ethylisothiourea was added to 300 g acetonitrile, the temperature of the water bath was controlled at 32-36 °C, and 1.2 mol (100 g) tert-butyl isocyanate was slowly added dropwise within 1 h After the reaction was kept for 2.5 h, 2.17 mol (220 g) triethylamine was added, then the temperature was increased and the temperature was also controlled to 53-56 °C with a water bath, and 0.40 mol (120 g) triphosgene was dissolved in 500 g of acetonitrile to prepare a solution, Slowly added dropwise within 150 min and incubated for 4.5 h to obtain a crude product solution after the reaction was completed;
[0058] Under the pressure of 1.5 atm, it was heated to 90 °C and concentrated until the crude product solution volume was about 71% of the original volume. Then 1000 mL of dilute hydrochloric acid with a concentration of 1 wt% was slowly added dropw...
Embodiment 2
[0067] A preparation method of 3-tert-butyl-6-ethylthio-1,3,5-triazine-2,4(1H,3H)-dione, the specific steps are:
[0068] 1 mol (185 g) S-ethylisothiourea was added to 300 g acetonitrile, the temperature of the water bath was controlled at 10-20 °C, and 1.15 mol (96 g) tert-butyl isocyanate was slowly added dropwise within 45 min After the reaction was completed, 2.00 mol (202 g) of triethylamine was added after 4 h of incubation, and then the temperature was increased and the temperature was also controlled to 10-20 °C in a water bath, and 0.35 mol (104 g) of triphosgene was dissolved in 500 g of acetonitrile to prepare a solution. Slowly added dropwise within 90 min and incubated for 6 h to obtain a crude product solution after the reaction was completed;
[0069] Under the pressure of 1.5 atm, it was heated to 90 °C and concentrated until the crude product solution volume was about 74% of the original volume. Then 1000 mL of dilute hydrochloric acid with a concentration of ...
Embodiment 3
[0073] A preparation method of 3-tert-butyl-6-ethylthio-1,3,5-triazine-2,4(1H,3H)-dione, the specific steps are:
[0074] 1 mol (185 g) S-ethylisothiourea was added to 300 g acetonitrile, the temperature was controlled at 0-10 °C in a water bath, and 1.25 mol (104 g) tert-butyl isocyanate was slowly added dropwise within 75 min After the reaction was kept for 2 h, 2.6 mol (263 g) triethylamine was added, and then the temperature was increased and the temperature was controlled to 0-10 °C in a water bath. 1.0 mol (297 g) triphosgene was dissolved in 500 g of dichloromethane to prepare The solution was slowly added dropwise within 180 min and incubated for 4 h to obtain a crude product solution after the reaction was completed;
[0075] Under the pressure of 1.5 atm, it was heated to 90 °C and concentrated until the crude product solution volume was about 72% of the original volume, then 1000 mL of dilute hydrochloric acid with a concentration of 1 wt% was slowly added dropwise,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com