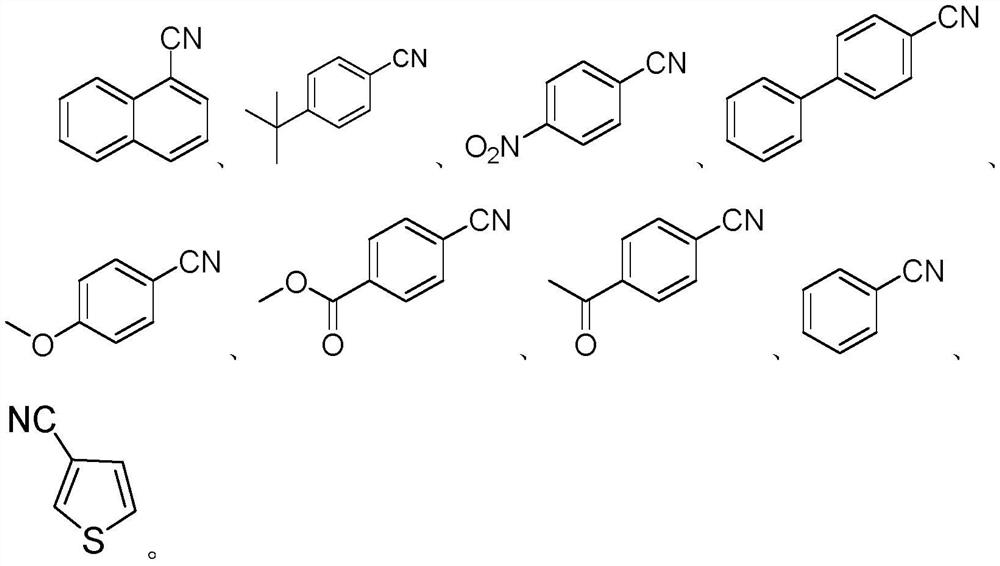
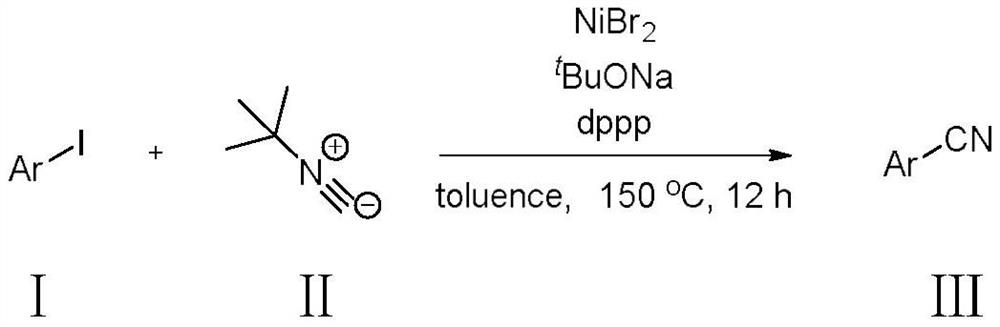
Method for synthesizing aryl nitrile compound from tert-butyl isonitrile
A technology of tert-butyl isonitrile and compound, which is applied in the field of synthesizing aryl nitrile substances, and achieves the effect of reducing reaction cost, low cost and abundant reserves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Under the protection of nitrogen, 0.4mmol (61.2mg) 1-iodonaphthalene, NiBr 2 (1equiv, 87.4mg), sodium tert-butoxide (1equiv, 38.4mg), phosphine ligand dppp (20mol%, 33mg) were added to a 25mL Schlenk tube, 2mL of toluene was added as a solvent, followed by 0.6mmol (49.9mg) tert-Butylisonitrile. Next, the reaction was stirred in an oil bath at 150 °C for 12 hours. After the reaction, cool the reaction solution to room temperature, dilute the reaction solution with 2 mL of ethyl acetate, then add 500 mg of silica gel to the reaction solution, mix well, and rotate to evaporate, use n-hexane: ethyl acetate = 50: 1 to wash through the silica gel column After the target product was obtained, 39.78 mg of pure product was obtained by concentration under reduced pressure in vacuo, with a yield of 65%.
[0025] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ8.24(dq, J=8.3,0.9Hz,1H),8.08(dd,J=8.3,1.2Hz,1H),7.92(td,J=7.7,7.2,1.0Hz,2H),7.70(ddd ,J=8.3,6.9,1.3Hz,1H...
Embodiment 2
[0027]
[0028] Under nitrogen protection, 0.4mmol (240mg) 4-tert-butyl iodobenzene, NiBr 2 (1equiv, 87.4mg), sodium tert-butoxide (1equiv, 38.4mg), phosphine ligand dppp (20mol%, 33mg) were added to a 25mL Schlenk tube, 2mL of toluene was added as a solvent, followed by 0.6mmol (49.9mg) tert-Butylisonitrile. Next, the reaction was stirred in an oil bath at 150 °C for 12 hours. After the reaction, cool the reaction solution to room temperature, dilute the reaction solution with 2 mL of ethyl acetate, then add 500 mg of silica gel to the reaction solution and mix well, then rotate and evaporate, and use n-hexane: ethyl acetate = 80: 1 to wash the reaction solution through a silica gel column. After decompression, the target product was obtained and concentrated under reduced pressure to obtain 43.9 mg of pure product with a yield of 69%.
[0029] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ7.62–7.57(m,2H),7.52–7.47(m,2H),1.34(s,9H).
Embodiment 3
[0031]
[0032] Under nitrogen protection, 0.4mmol (99.6mg) p-nitroiodobenzene, NiBr 2 (1equiv, 87.4mg), sodium tert-butoxide (1equiv, 38.4mg), phosphine ligand dppp (20mol%, 33mg) were added to a 25mL Schlenk tube, 2mL of toluene was added as a solvent, followed by 0.6mmol (49.9mg) tert-Butylisonitrile. Next, the reaction was stirred in an oil bath at 150 °C for 12 hours. After the reaction, cool the reaction solution to room temperature, dilute the reaction solution with 2mL ethyl acetate, then add 500 mg of silica gel to the reaction solution, mix well and then rotatively evaporate, use n-hexane:ethyl acetate=50:1 to conduct After elution, the target product was obtained and concentrated under reduced pressure to obtain 18.4 mg of pure product with a yield of 31%.
[0033] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ8.41–8.35(m,2H),7.96–7.88(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com