Method for preparing bensulfuron methyl
A technology of bensulfuron-methyl and bensulfuron-methyl, which is applied in the field of preparation of bensulfuron-methyl, can solve the problems of unsafe operation, too many raw materials of bensulfuron-methyl, high risk of chlorobutane, etc., so as to improve conversion rate and synthesis yield The efficiency and product quality, the effect of reducing product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
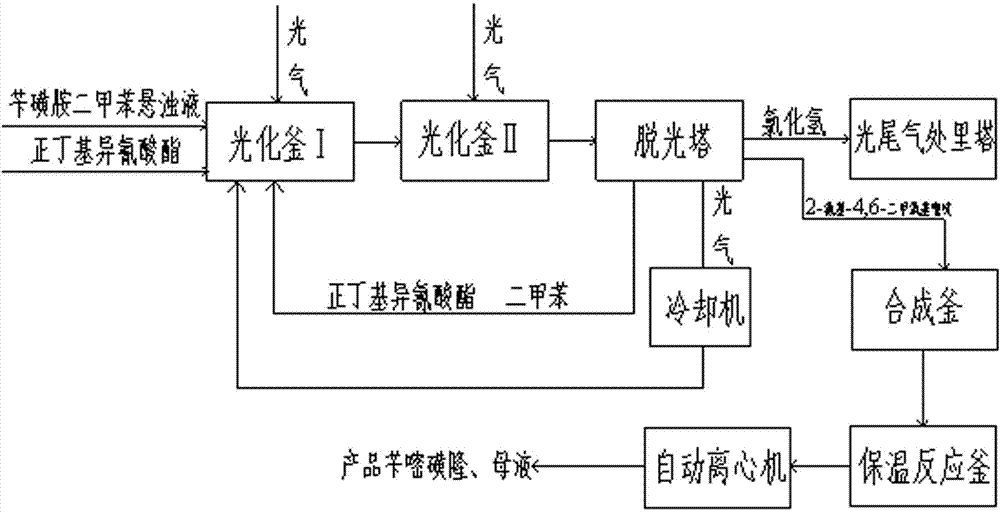
[0017] Add phosgene 33 kg / h, 30% benzsulfonamide xylene suspension 127.3 kg / h and catalyst n-butyl isocyanate in a molar ratio of 40:20:1 to the photochemical kettle Ⅰ continuously for photochemical reaction. The temperature in kettle I was 105°C, and the reaction residence time was 2 hours to synthesize o-methoxycarboxybenzylsulfonamide; o-methoxycarboxybenzylsulfonamide was continuously fed into photochemical kettle II, and 8.3kg / h of phosgene was added, The molar ratio of phosgene to benzylsulfonamide xylene solution is 1:4, the temperature in photochemical kettle II is 120°C, and the reaction residence time is 1h to synthesize o-methoxycarboxybenzylsulfoisocyanate; o-methoxycarboxybenzylsulfonate The butyl isocyanate continuously enters the light removal tower, and the n-butyl isocyanate and xylene that are removed are returned to the photochemical kettle Ⅰ for recycling. The excess phosgene passes through the cooling separator, and the separated phosgene is continuously re...
Embodiment 2
[0019] Add phosgene 33kg / h, 30% benzsulfonamide xylene suspension 127.3kg / h and catalyst n-butyl isocyanate in a molar ratio of 30:10:1 to the photochemical reactor Ⅰ continuously for photochemical reaction. The temperature in I is 110°C, the reaction residence time is 2.5h, and o-methoxycarboxybenzylsulfonamide is synthesized; o-methoxycarboxybenzylsulfonamide enters photochemical kettle II continuously, and 8.3kg / h of phosgene is added, The molar ratio of phosgene to benzylsulfonamide xylene suspension is 1.2:4, the temperature in photochemical kettle II is 125°C, and the reaction residence time is 1.5h to synthesize o-methoxycarboxybenzylsulfoisocyanate; o-methoxy Carboxybenzylsulfoisocyanate continuously enters the de-lighting tower, and the desorbed n-butyl isocyanate and xylene are returned to the photochemical reactor Ⅰ for recycling, and the excess phosgene passes through the cooling separator, and the separated phosgene is continuously returned to the photochemical rea...
Embodiment 3
[0021] Add phosgene 33kg / h, 30% benzsulfonamide xylene suspension 127.3kg / h and catalyst n-butyl isocyanate in a molar ratio of 60:20:3 to the photochemical kettle Ⅰ continuously for photochemical reaction. The temperature in Ⅰ is 115°C, the reaction residence time is 3h, and the o-methoxycarboxybenzylsulfonamide is synthesized; the o-methoxycarboxybenzylsulfonamide enters the photochemical kettle II continuously, 8.3kg / h of phosgene is added, and the Gas and benzylsulfonamide xylene suspension 1.5:4, the temperature in photochemical kettle II is 127°C, the reaction residence time is 2h, and o-methoxycarboxybenzylsulfoisocyanate is synthesized; o-methoxycarboxybenzylsulfoisocyanate enters The stripping tower, the n-butyl isocyanate and xylene that are removed are returned to the photochemical kettle Ⅰ for recycling, and the excess phosgene passes through the cooling separator, and the separated phosgene is continuously returned to the photochemical kettle Ⅰ for recycling, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com