Genetically engineered bacterium for high-yielding L-valine and method for producing L-valine by fermentation
A technology of genetically engineered bacteria and valine, applied in the field of microorganisms, can solve the problems of unbalanced coenzyme supply and demand, low sugar-acid conversion rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
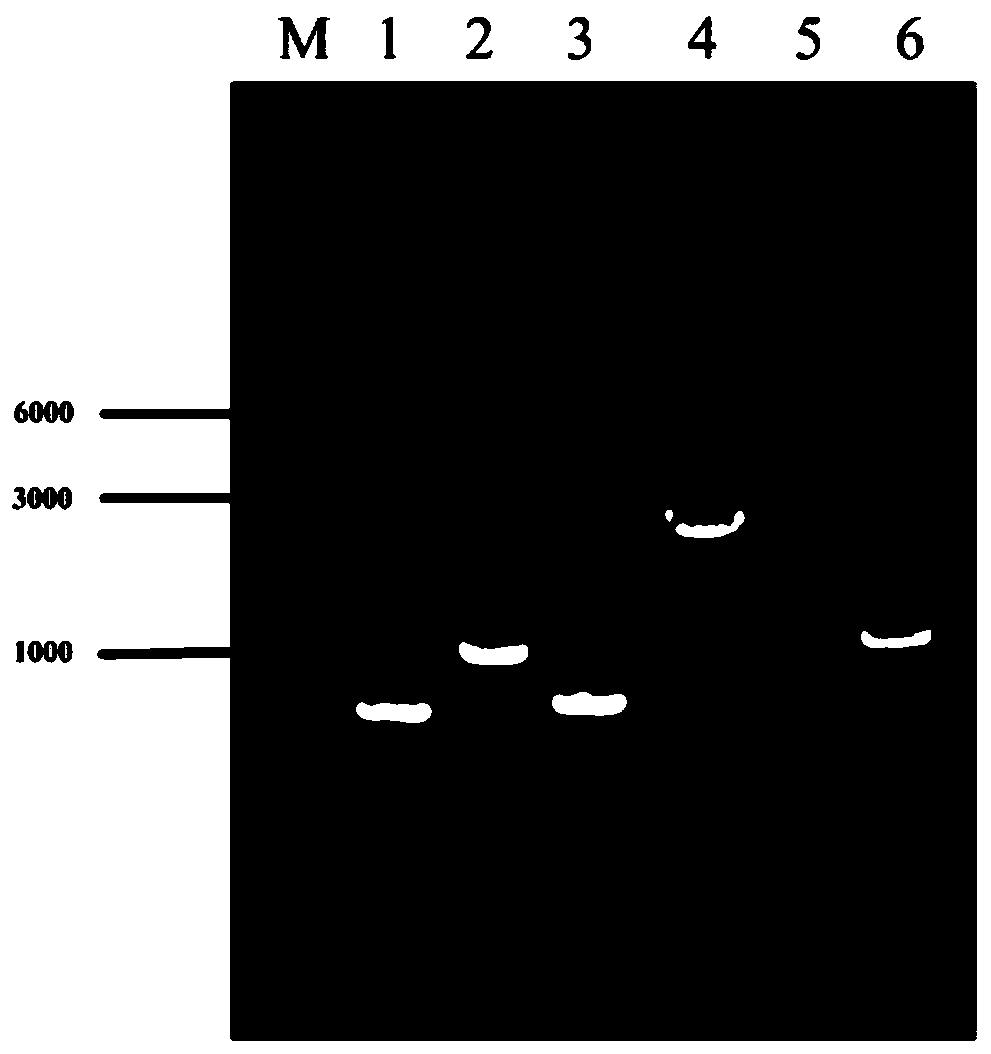
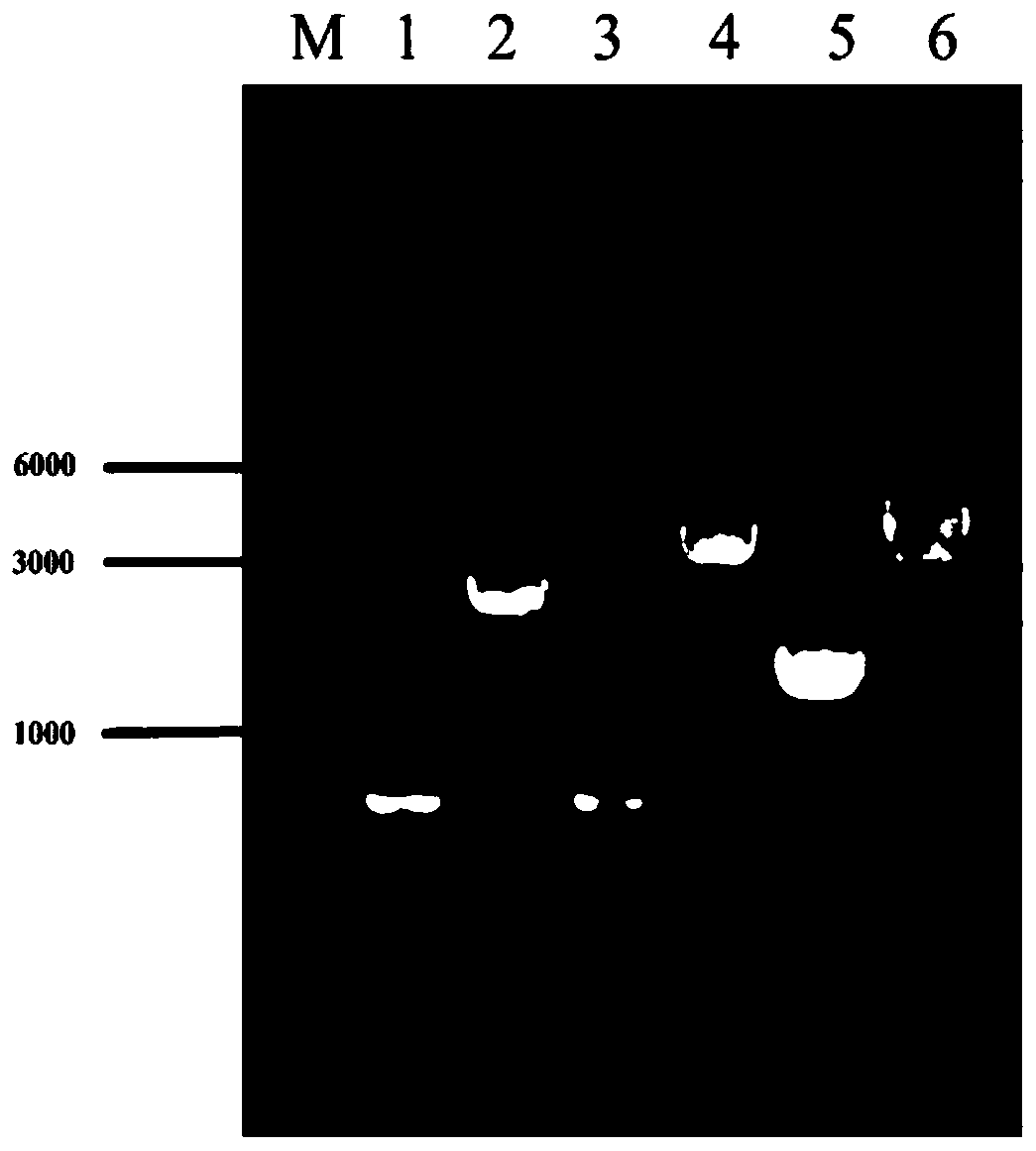
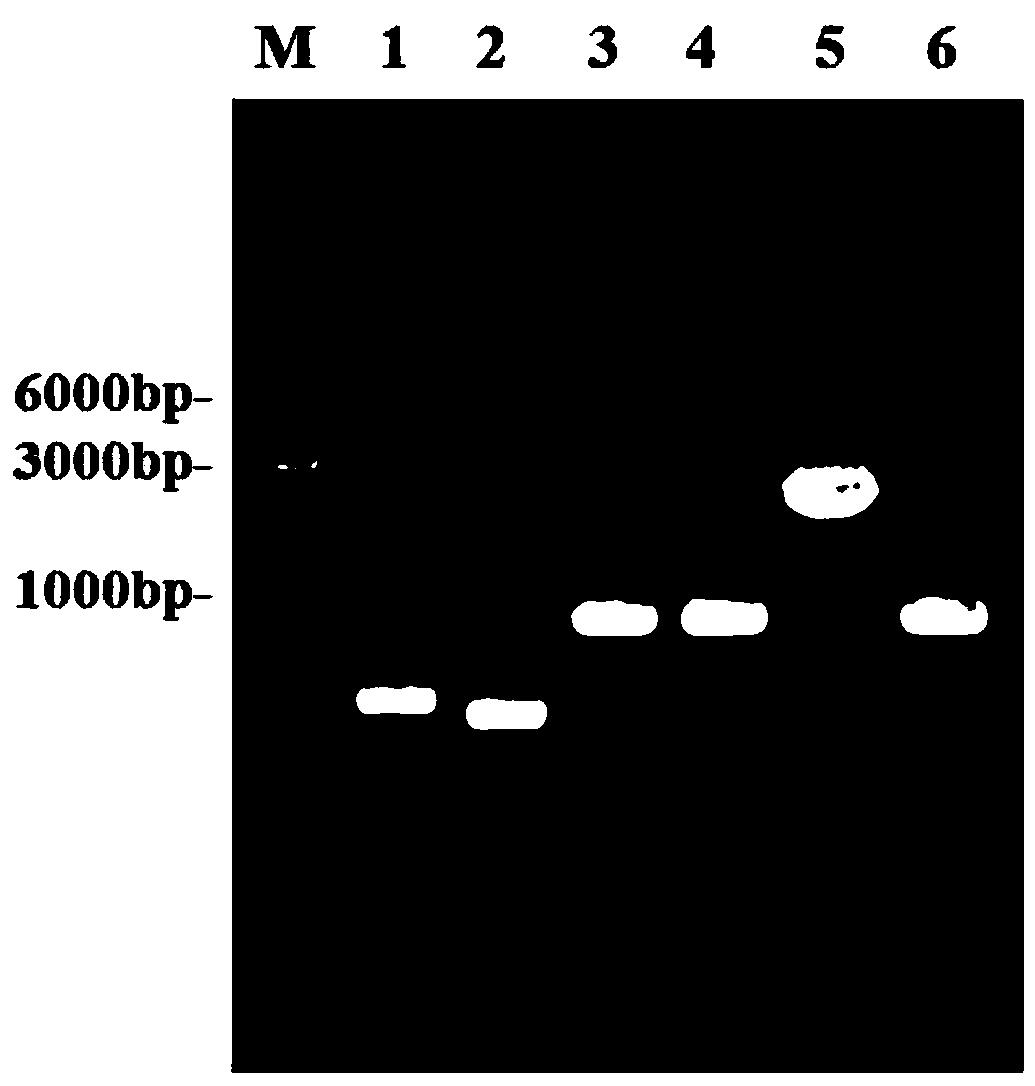
[0038] The efficient L-valine production strains VXR01, VXR02, and VXR03 were constructed, and the gene editing method used was referred to the literature (Li Y, Lin Z, Huang C, et al. Metabolic engineering of Escherichia coli using CRISPR–Cas9meditated genome editing. Metabolic engineering, 2015 ,31:13-21.), the specific method is as follows:
[0039] 1 The acetolactate synthase coding gene alsS was integrated into the pseudogene site ydeU to construct strain VXR01
[0040] 1.1 Preparation of recombinant DNA fragments
[0041] The alsS gene was integrated into the ydeU pseudogene locus. Using the genome of Bacillus subtilis B.subtilis 168 as a template, the gene alsS (NCBI-Protein ID: NP_391482.2) was amplified by PCR; using the genome of Escherichia coli W3110 as a template, the upstream and downstream homology arm fragments of ydeU (primer UP -ydeU-S, UP-ydeU-A; DN-ydeU-S, DN-ydeU-A); promoter P trc Designed in the downstream primer of the upstream homology arm and the u...
Embodiment 2
[0103] Shake flask fermentation of strains VXR02 and VXR05:
[0104] Slant culture: Streak inoculation of -80°C preserved strains on the activated slant, culture at 37°C for 12 hours, and subculture twice;
[0105] Shake flask seed culture: use the inoculation loop to scrape two rings of slant seeds and inoculate them into a 500mL Erlenmeyer flask containing 30mL of seed medium, culture at 37°C and 200rpm for 8-10h;
[0106] Shake flask aerobic fermentation: Inoculate 10-15% of the inoculum into a 500mL Erlenmeyer flask with fermentation medium (final volume is 30mL), culture at 37°C with shaking at 200r / min, maintain pH by adding ammonia water during fermentation At 6.7-7.0, add 60% (m / v) glucose solution to maintain the fermentation, and the fermentation period is 24h;
[0107] Aerobic-anaerobic two-stage fermentation: Inoculate 10-15% of the inoculum into a 500mL Erlenmeyer flask with fermentation medium (final volume is 30mL), 37°C, 200r / min shaking culture; after 12-16h,...
Embodiment 3
[0115] 5L fermenter fermentation of strains VXR02 and VXR05:
[0116] Aerobic fermentation: activate the strains on the slope for two generations, insert 15-20% of the inoculum into the fermentation medium, and start fermentation. During the fermentation process, the pH is controlled to be stable at about 6.7, the temperature is maintained at 35°C, and the dissolved oxygen is controlled at 25-30%; when the glucose in the medium is consumed, add 80% (m / v) glucose solution to maintain the glucose concentration in the fermentation medium at 0.1-5g / L; the fermentation period is 40h;
[0117] Aerobic-anaerobic two-stage fermentation: Activate the strains for two generations on a slant, insert 15-20% of the inoculum into the fermentation medium, and start fermentation. During the fermentation process, the pH is controlled to be stable at around 6.7, and the temperature is maintained at 35°C , control dissolved oxygen at 25-30%; when the glucose in the medium is consumed, add 80% (m / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com