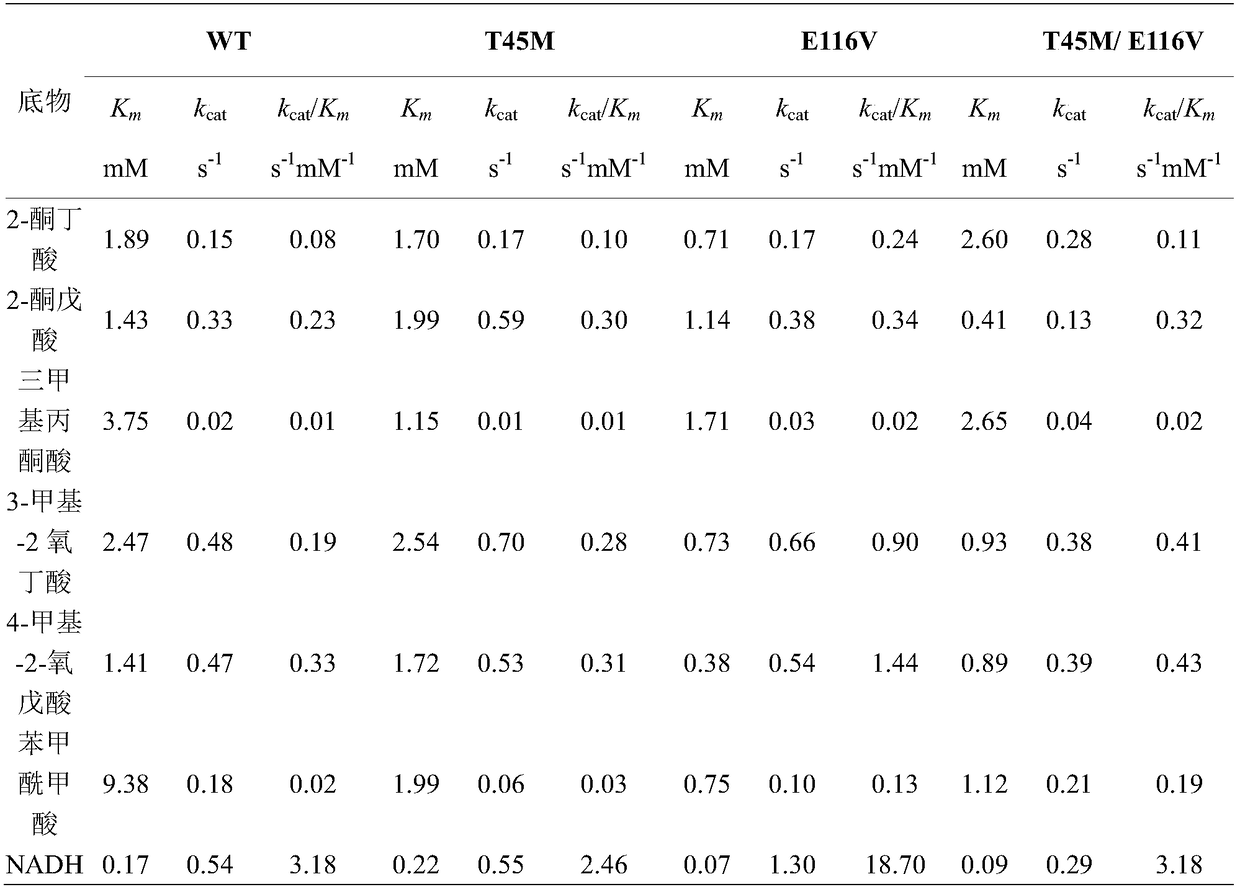
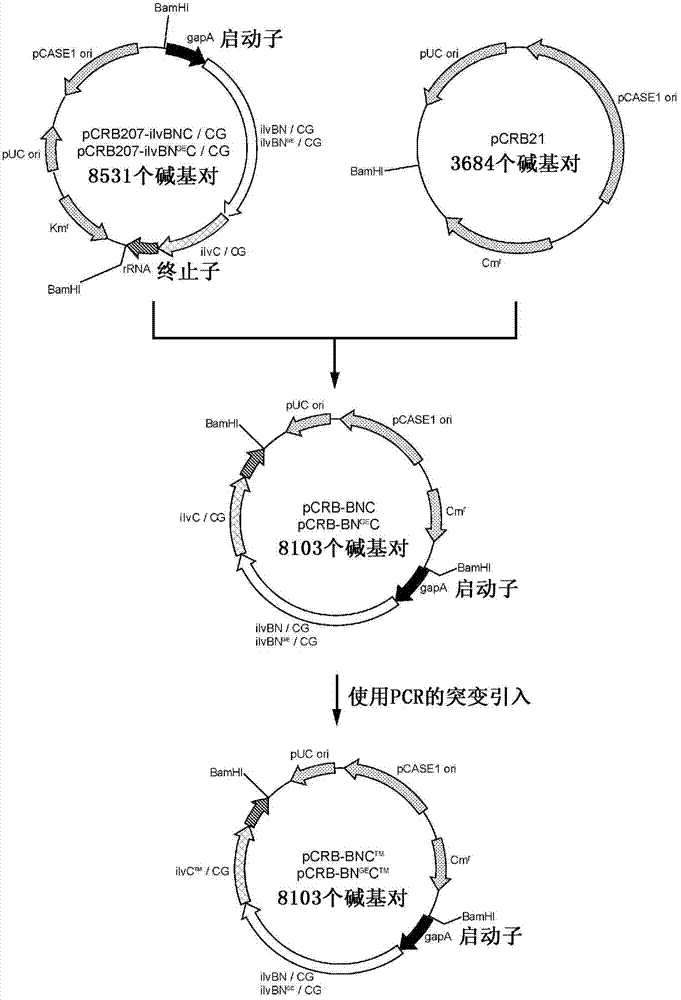
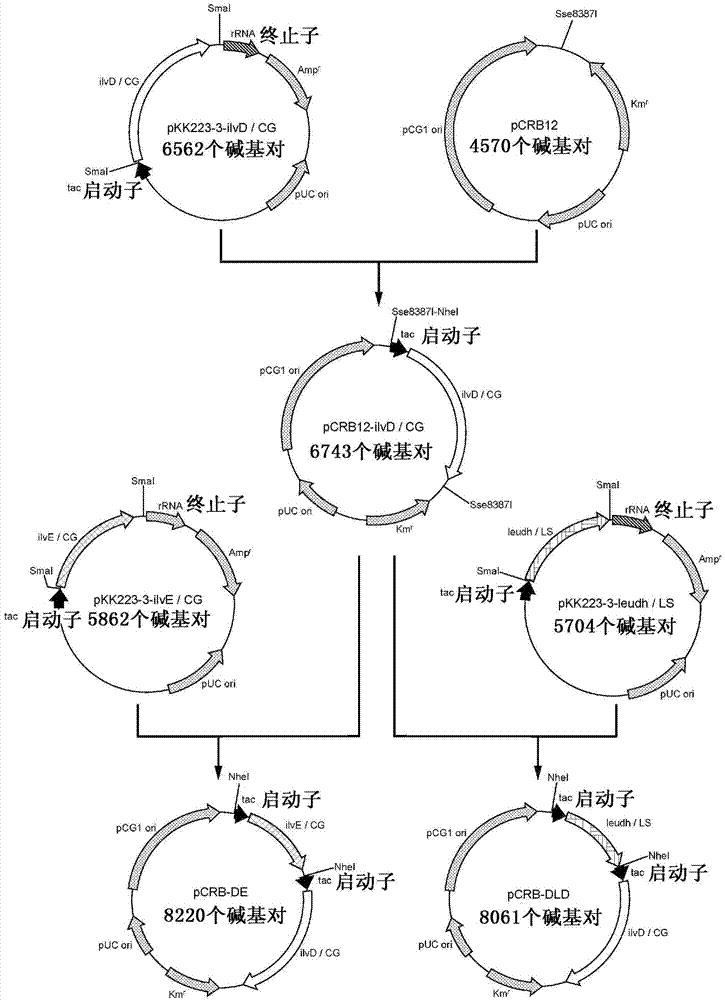
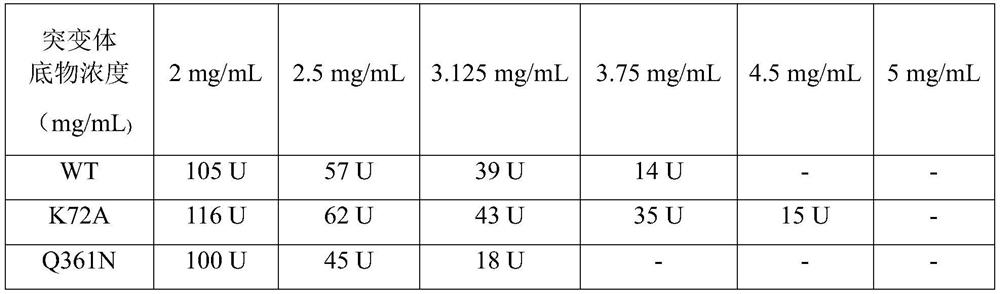
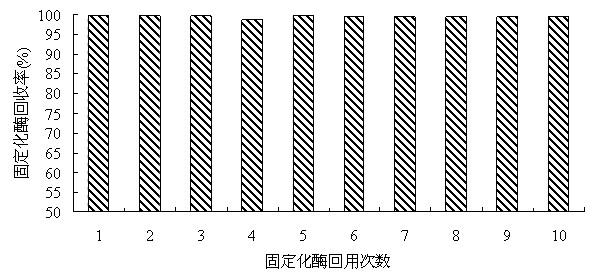
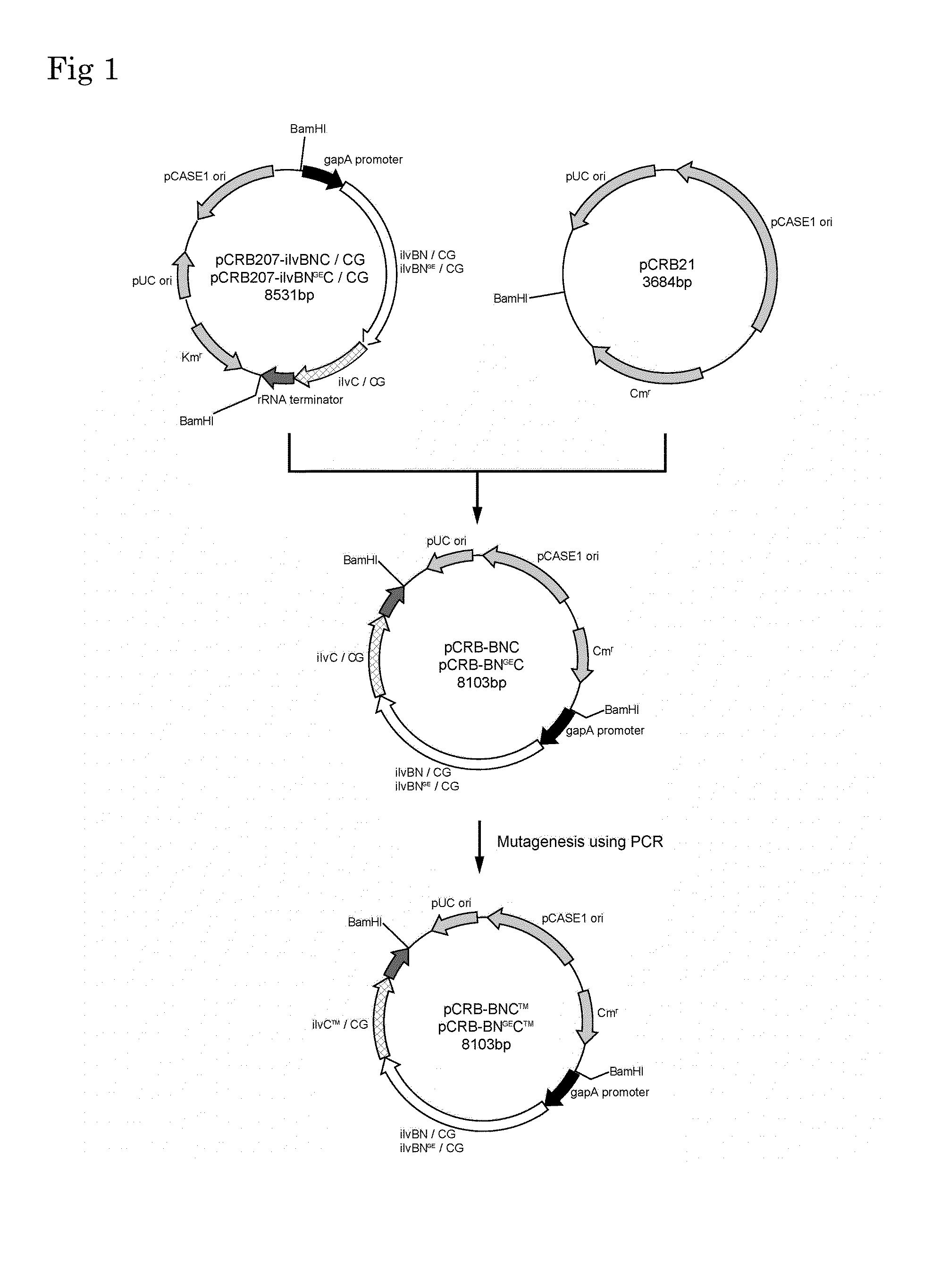
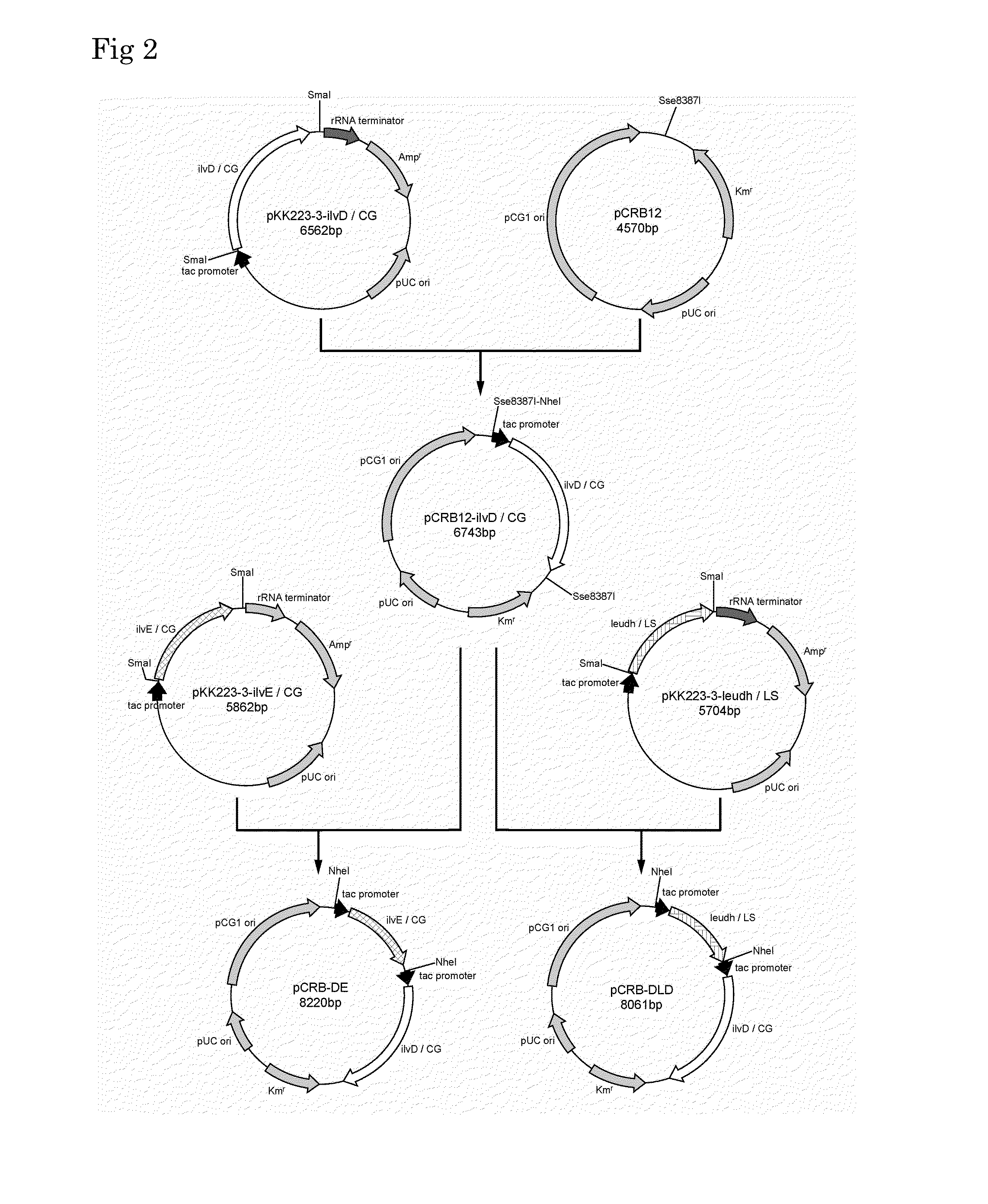
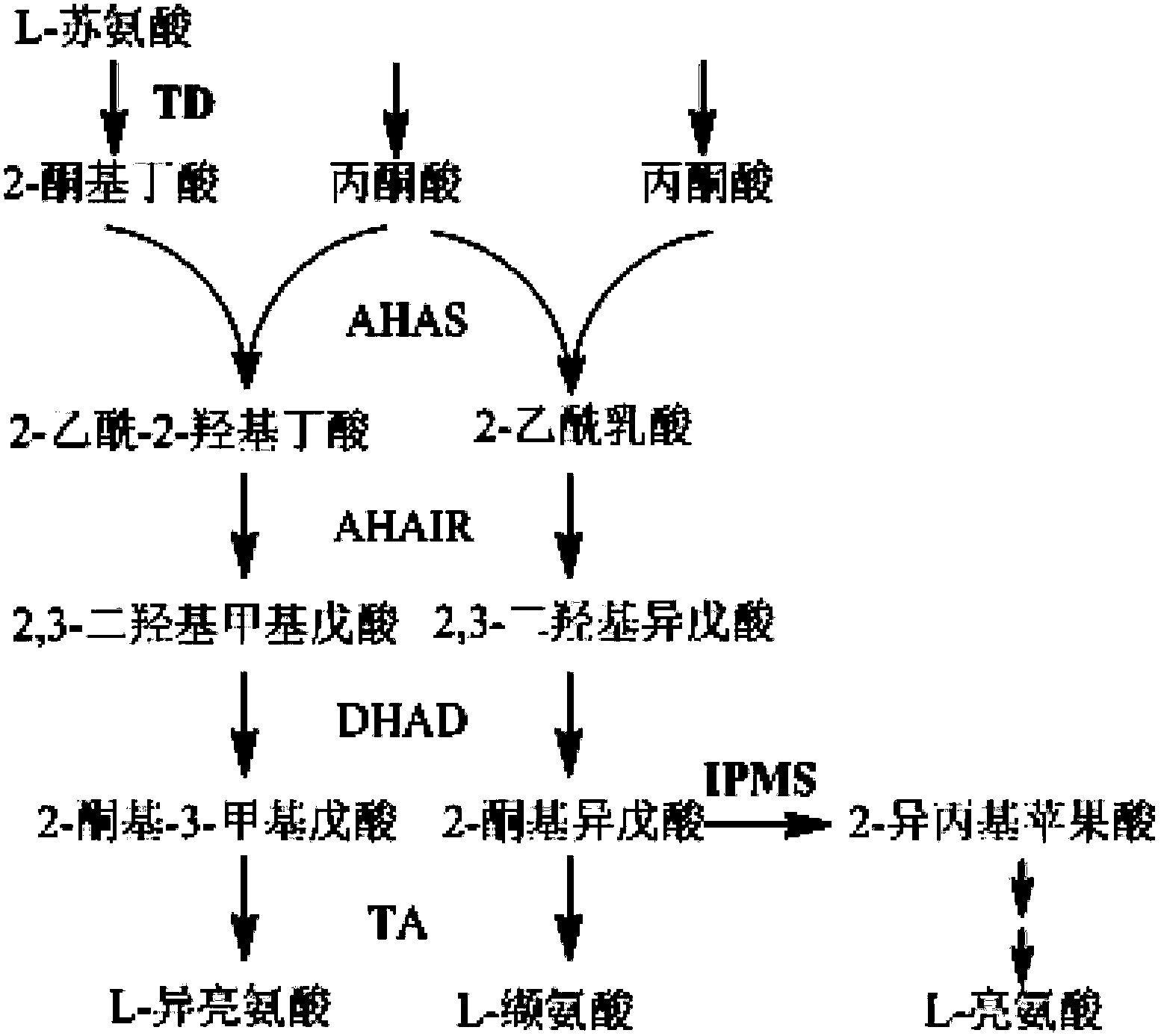
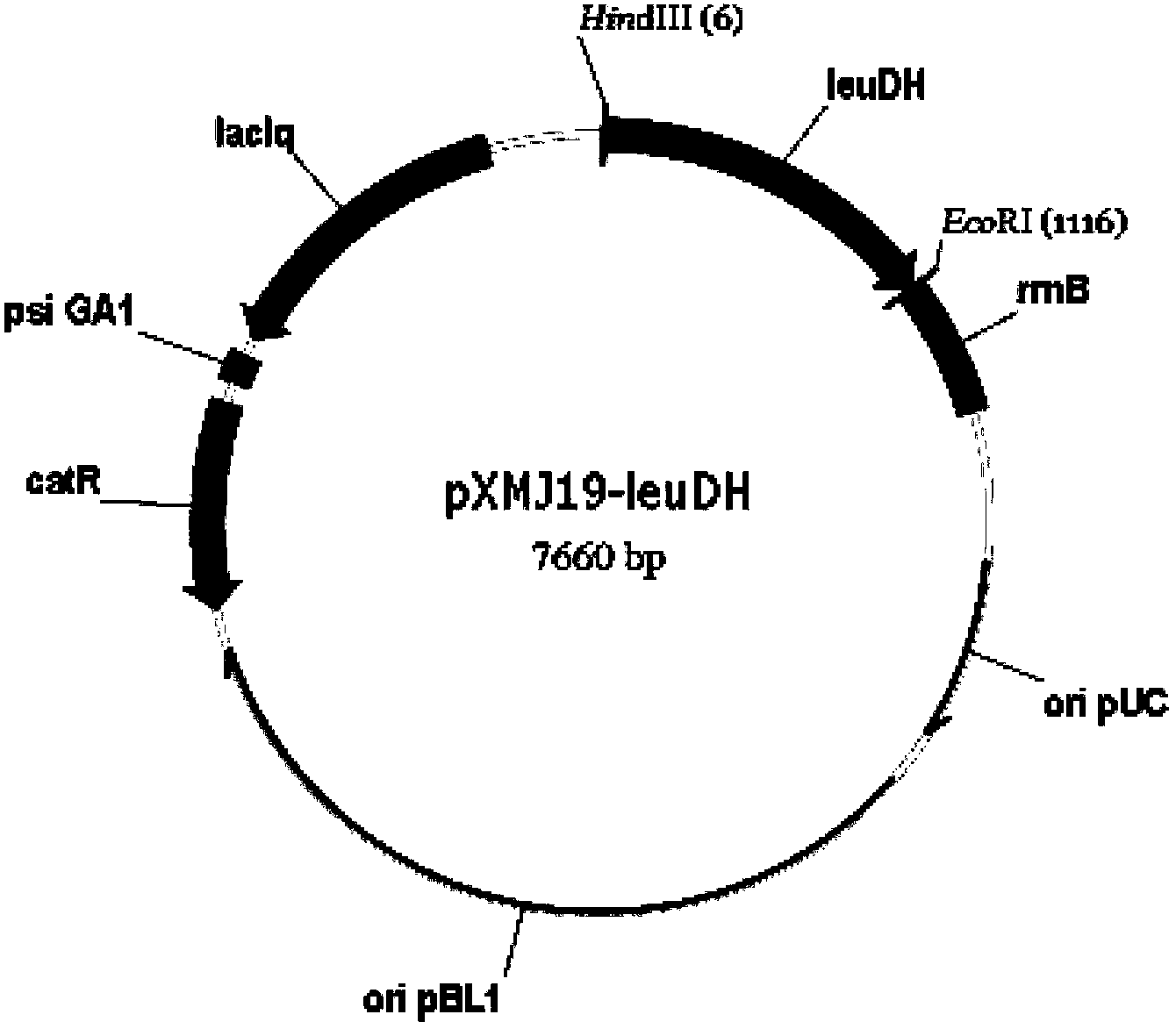
Patents
Literature
63 results about "Leucine dehydrogenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
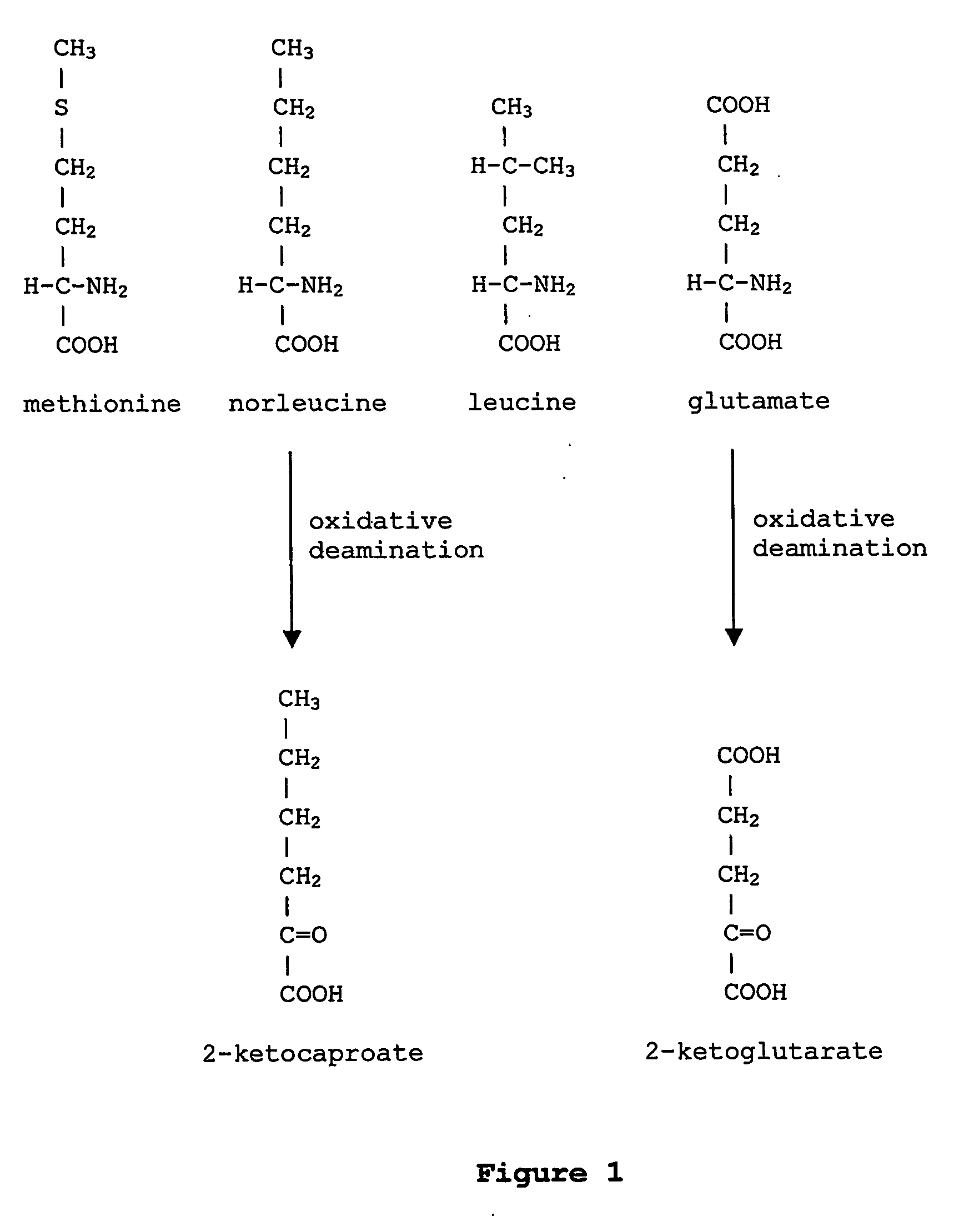
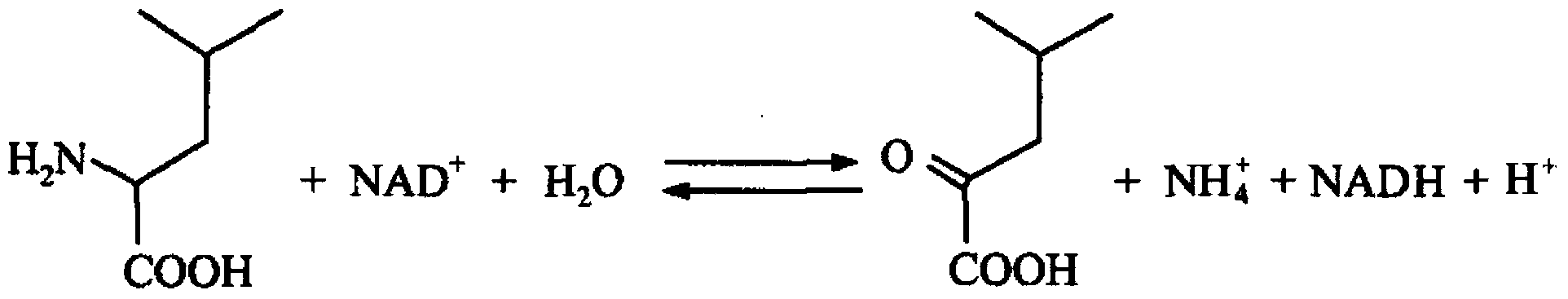
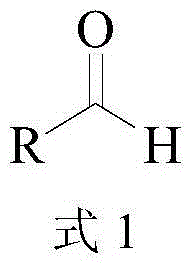
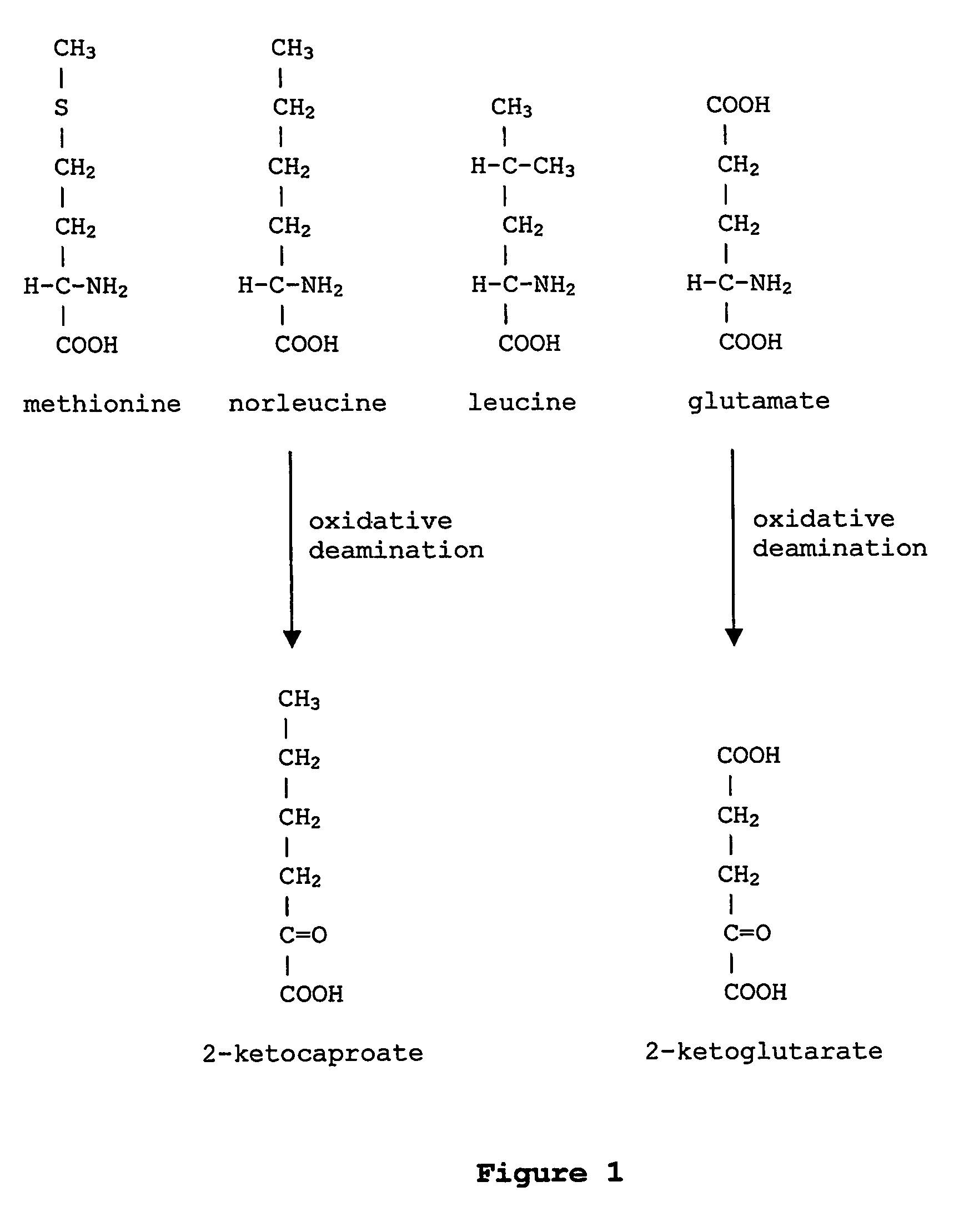
In enzymology, a leucine dehydrogenase (EC 1.4.1.9) is an enzyme that catalyzes the chemical reaction L-leucine + H₂O + NAD⁺ ⇌ 4-methyl-2-oxopentanoate + NH₃ + NADH + H⁺ The 3 substrates of this enzyme are L-leucine, H₂O, and NAD⁺, whereas its 4 products are 4-methyl-2-oxopentanoate, NH₃, NADH, and H⁺. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-NH2 group of donors with NAD+ or NADP+ as acceptor.
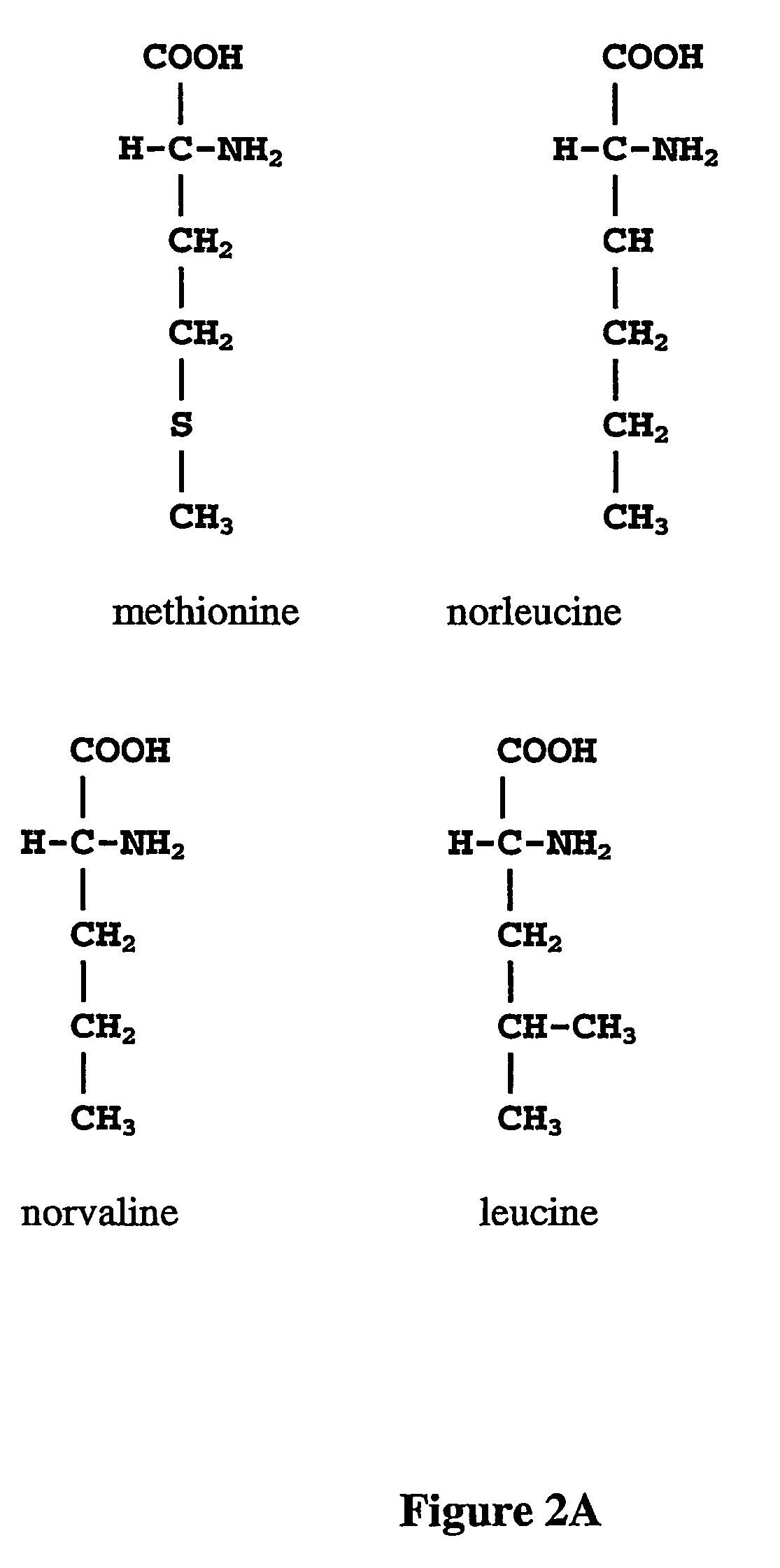
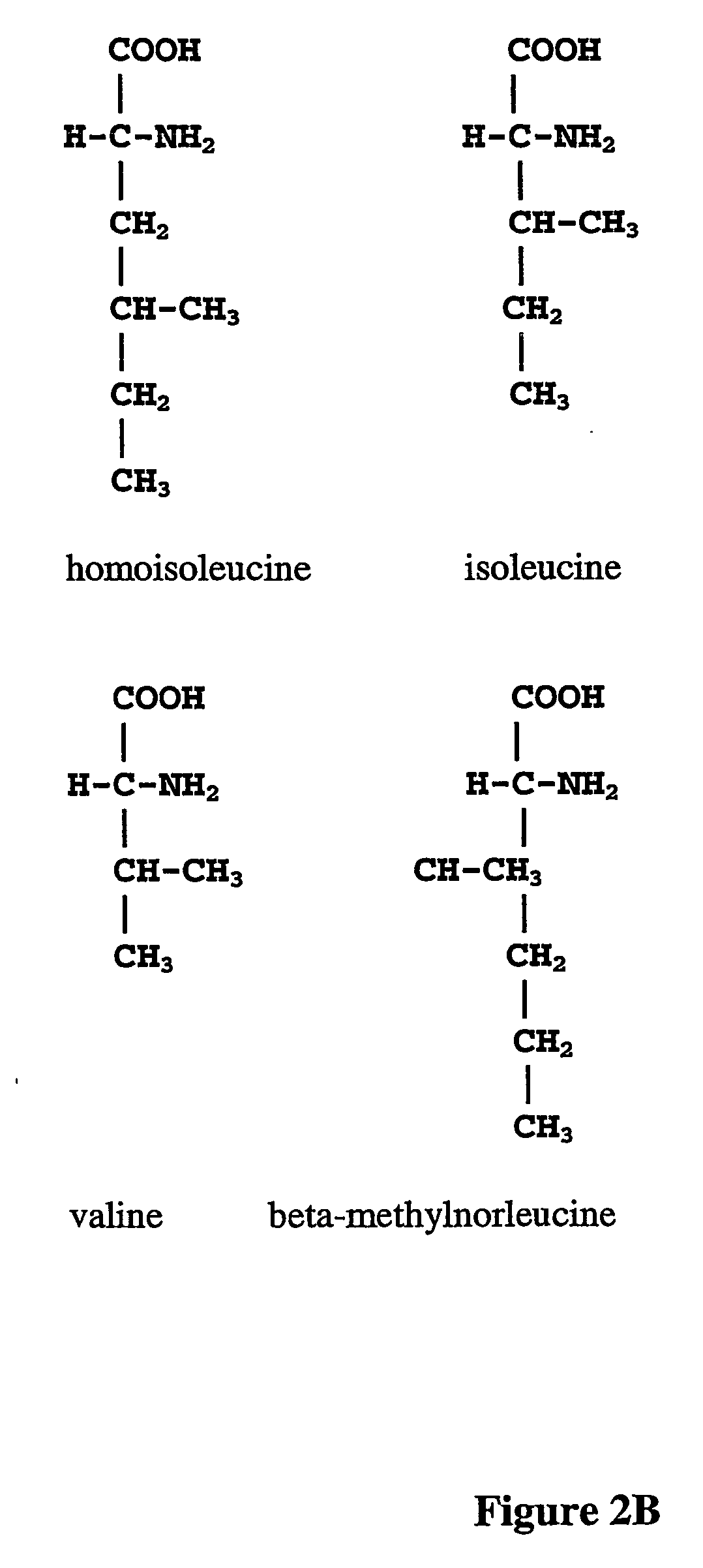
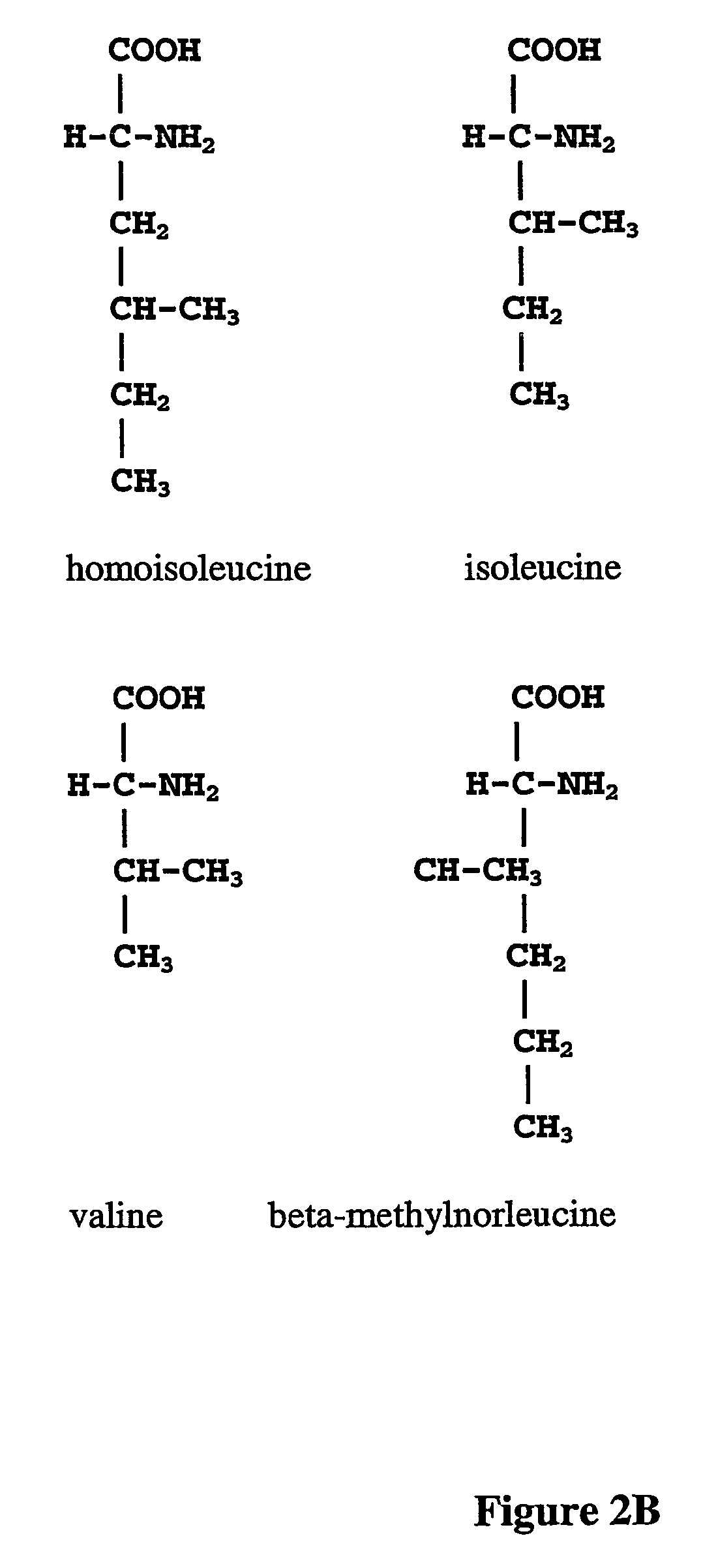
Prevention of incorporation of non-standard amino acids into protein
ActiveUS20070009995A1Cellular level is reducedReducing, or substantially eliminating, endogenous cellular levels of norleucineBacteriaPeptide/protein ingredientsBeta-methylnorleucinePhenylalanine dehydrogenase
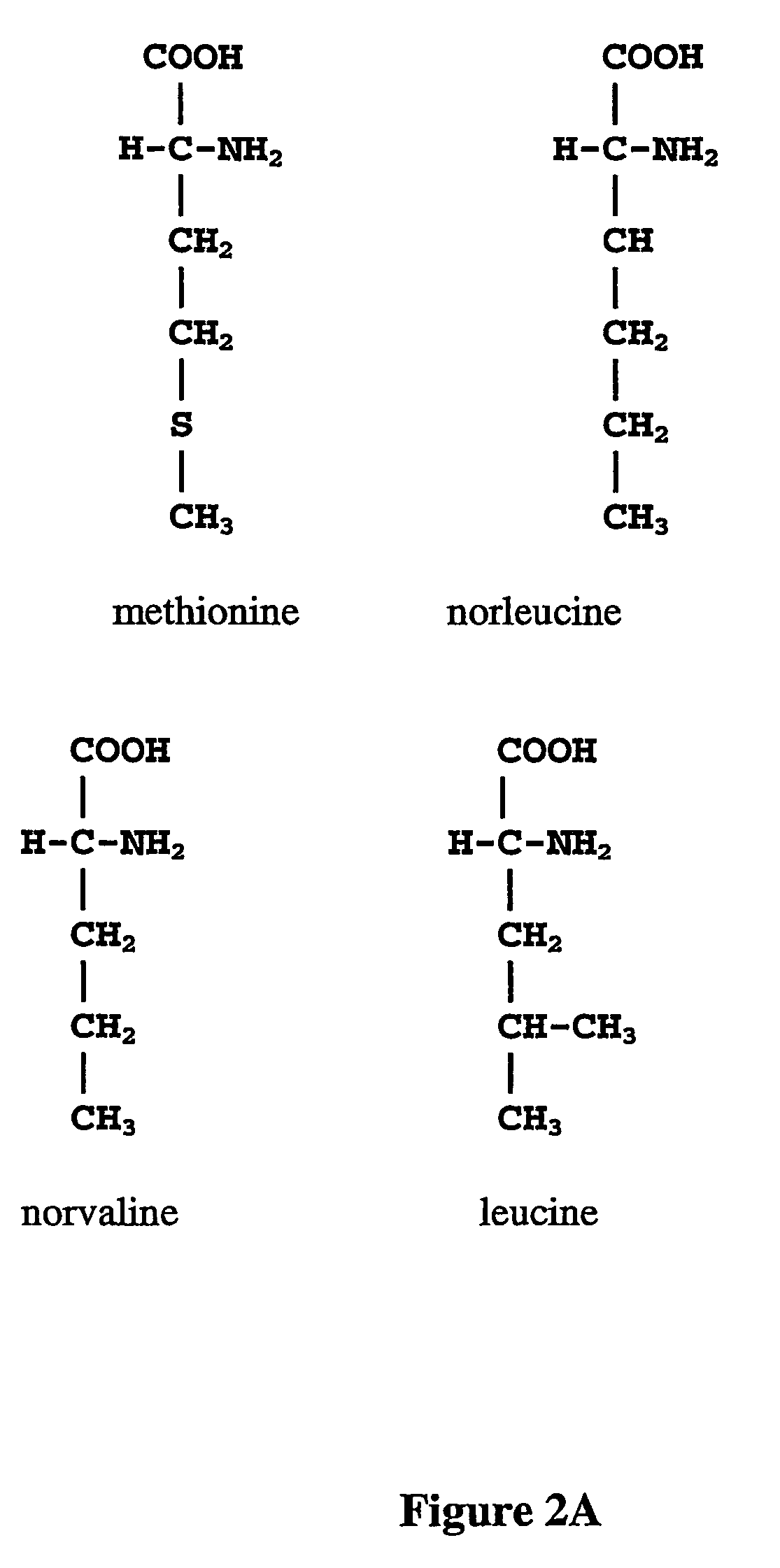
The instant invention is drawn to the methods and compositions necessary to provide recombinant proteins with a substantially reduced or eliminated content of norleucine or other non-standard amino acids. Various embodiments of the invention provide for the substantial elimination of the incorporation of non-standard amino acids into recombinant proteins by the co-expression or enhanced expression of a protein (or the enzymatically active portion thereof) capable of degrading norleucine or other non-standard amino acids, including norvaline, beta-methylnorleucine, and homoisoleucine. In certain particular embodiments of the invention, the norleucine is degraded by a glutamate dehydrogenase, a leucine dehydrogenase, a valine dehydrogenase, a phenylalanine dehydrogenase, a glutamate / leucine / phenylalanine / valine dehydrogenase, or an opine dehydrogenase. Also provided are the cells and DNA constructs for carrying out these methods.
Owner:MONSANTO TECH LLC
L-2-reanal biological preparation method
ActiveCN102605014ALow costMild transformation conditionsChemical recyclingFermentationSeparation technologyThreonine
The invention relates to an L-2-reanal biological preparation method, which includes: utilizing L-threonine as raw materials, stirring the L-threonine in water at the temperature of 15-50 DEG C under the catalytic action of L-threonine deaminase and whole cells with a leucine dehydogenase and coenzyme regenerating function, and obtaining L-2-reanal after reaction. In the L-2-reanal biological preparation method, water is used for substituting for buffer salt solution to form a water-phase reaction system, cost of raw materials is reduced, threonine deaminase and whole cells used for catalyzing can be produced in batch by fermentation of microorganisms, are low in cost and wide in sources. Biological enzymatic catalysis reaction is water-phase reaction, transformation conditions of the enzymic method are mild, raw materials are transformed thoroughly, post-treatment is simple, products are separated by isoelectric point crystallization technology and film evaporation separation technology jointly, and the L-2-reanal biological preparation method is capable of recovering water and ammonium formate is low in cost, free of discharge of waste water and waste residues, environment-friendly in process and applicable to industrialized production of the L-2-reanal.
Owner:ENZYMEWORKS
Genetically engineered bacterium for high-yielding L-valine and method for producing L-valine by fermentation
ActiveCN110607268AEasy to synthesizeReduce synthesisBacteriaHydrolasesLactate dehydrogenaseSaccharic acid
The invention provides a genetically engineered bacterium for high-yielding L-valine. A construction method of the genetically engineered bacterium comprises the steps that starting from an escherichia coli W3110, an acetolactate synthase gene alsS of a bacillus subtilis is integrated on a genome of the escherichia coli W3110 and subjected to high expression; an escherichia coli ppGpp 3'-pyrophosphoric acid hydrolytic enzyme mutant R290E / K292D gene spoT is integrated on the genome of the escherichia coli W3110 and subjected to high expression; genes of frdA, frdB, frdC and frdD of four subunits of a lactic dehydrogenase gene ldhA, a pyruvate formate lyase I gene pflB and fumaric reductase on the genome of the escherichia coli W3110 are knocked out; a branched chain amino acid transaminasegene ilvE of the escherichia coli is replaced with leucine dehydrogenase gene bcd of the bacillus subtilis; and an acetyl-hydroxyl acid isomerized reductase gene ilvC of the escherichia coli is replaced with an encoding gene of a mutant L67E / R68F / K75E. According to the genetically engineered bacterium for the high-yielding L-valine, an L-valine fermentation method is further modified. Double-phasedissolved oxygen control is adopted, and the L-valine yield and the saccharic acid conversion rate are improved.
Owner:TIANJIN UNIV OF SCI & TECH
Construction of leucine dehydrogenase mutants and application thereof
ActiveCN108559735AImprove hydrophobicityImprove rigidityOxidoreductasesFermentationGenetically engineeredGenetic engineering
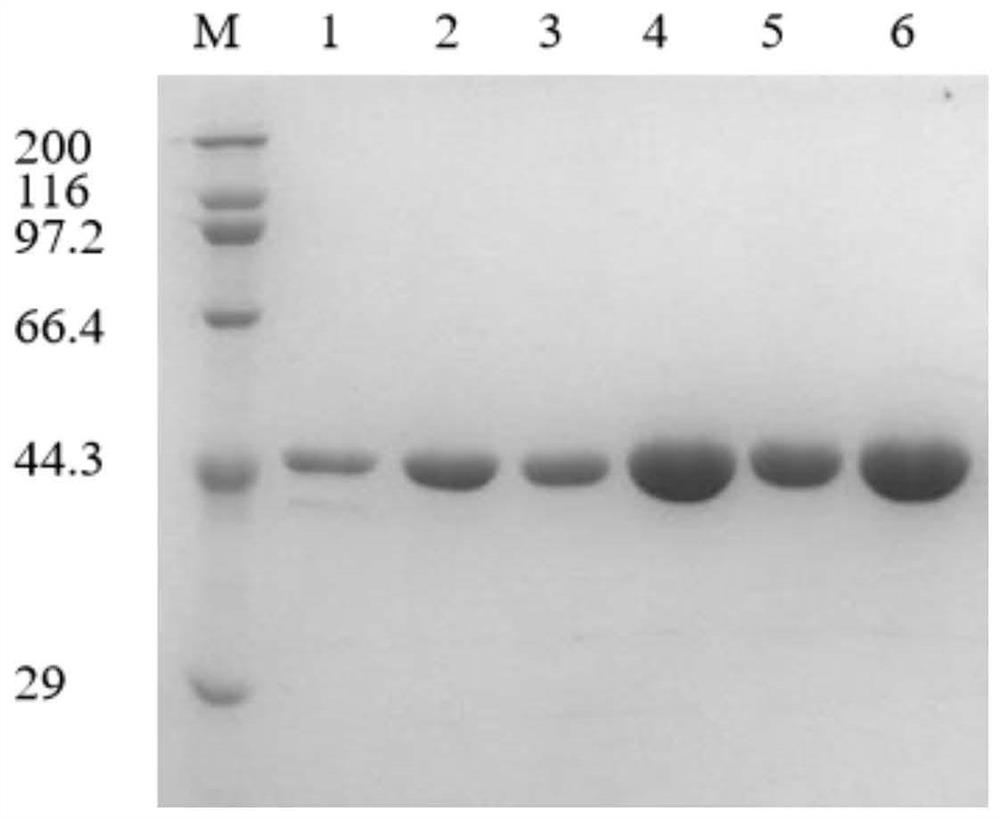
The invention provides construction of leucine dehydrogenase mutants and application thereof and belongs to the field of genetic engineering. The invention provides three leucine dehydrogenase mutantsof SEQ ID NO.4, SEQ ID NO.6 and SEQ ID NO.8, and application of the mutants and genetically engineered bacteria producing the mutants in preparation of optical pure chiral L-alpha-amino acid throughammoniation reduction of alpha-ketoacid. The invention has the advantages that the L-alpha-amino acid prepared by ammoniation reduction from the leucine dehydrogenase mutants or the genetically engineered bacteria has high catalytic activity and stability, high-optical purity L-alpha-amino acids can be synthesized (ee is greater than 99%), the conversion rate of L-phenylglycine catalyzed by mutantenzymes is improved by 2.62 times, and a practical and effective strategy is provided for industrial production.
Owner:JIANGNAN UNIV
Transformant of coryneform bacterium and method for producing valine by using same
This transformant is obtained by introducing, to a host coryneform bacterium, at least one of the DNAs (a), (b), and (c): (a) a DNA, or an analog thereof, obtained by introducing, to a DNA that encodes an acetohydroxy acid synthase derived from Corynebacterium glutamicum, a mutation that mutates the No. 156 glycine in the amino acid sequence encoded by this DNA to glutamic acid (G156E); (b) a DNA, or an analog thereof, obtained by introducing, to a DNA that encodes an acetohydroxy acid isomeroreductase derived from Corynebacterium glutamicum, mutations that mutate the No. 34 serine in the amino acid sequence encoded by this DNA to glycine (S34G), mutate the No. 48 leucine to glutamic acid (L48E), and mutate the No. 49 arginine to phenylalanine (R49F); and (c) a DNA, or an analog thereof, that encodes a leucine dehydrogenase derived from Lysinibacillus sphaericus.
Owner:RES INST OF INNOVATIVE TECH FOR THE EARTH
Leucine dehydrogenase mutant and application thereof
ActiveCN111676203AHigh catalytic activityBacteriaMicroorganism based processesBinding siteAcyl CoA dehydrogenase
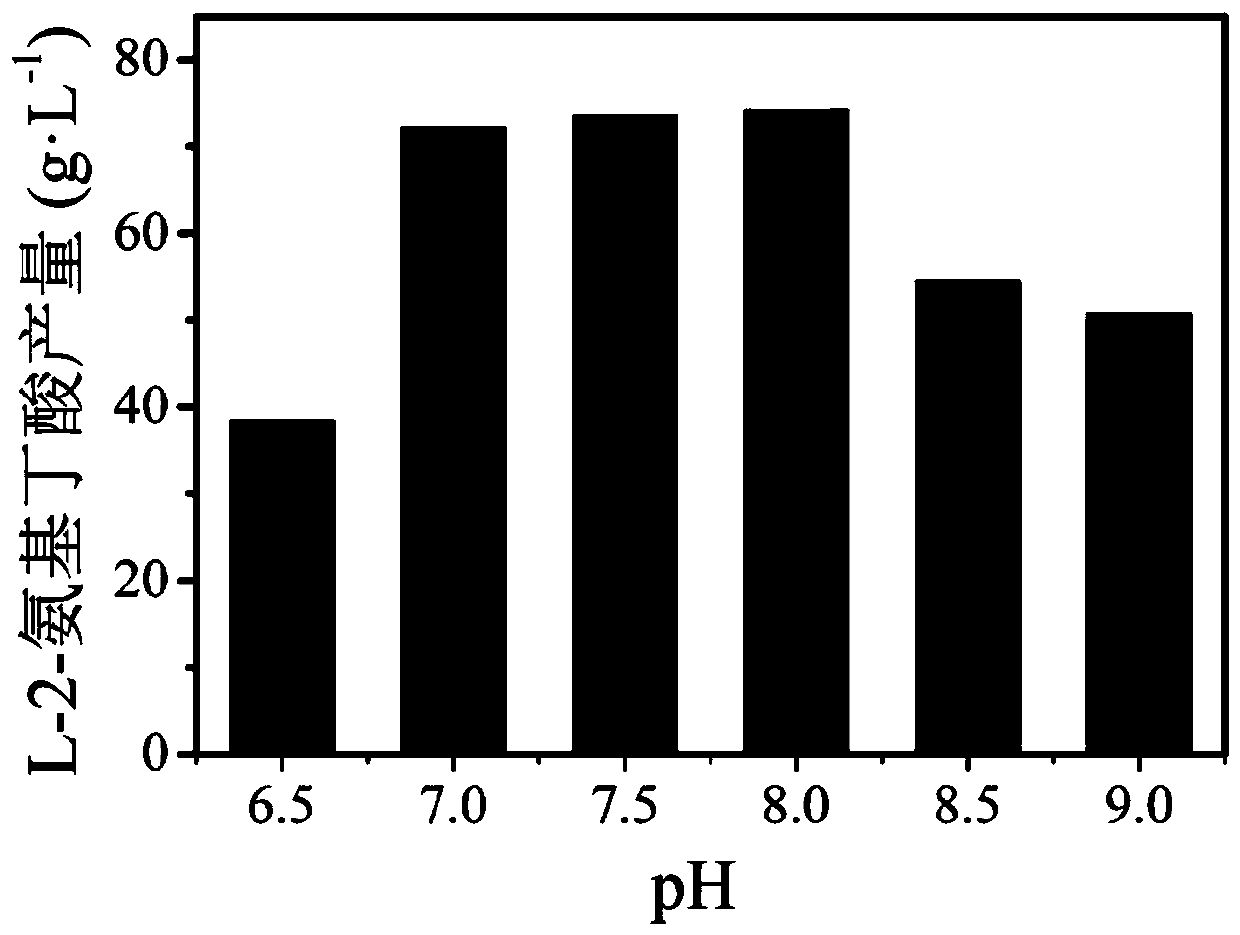
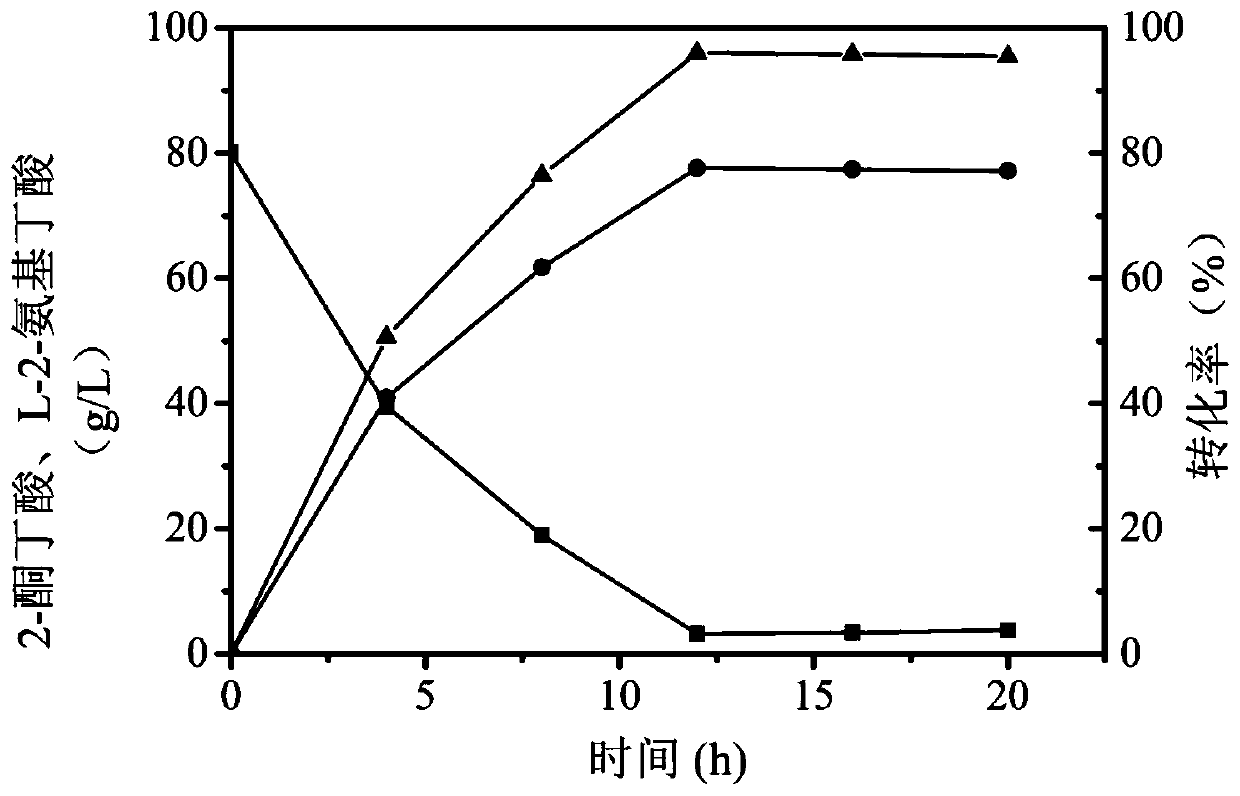
The invention discloses a leucine dehydrogenase mutant and application thereof, and belongs to the technical field of enzyme engineering and microbial engineering. Compared with leucine dehydrogenasewith the amino acid sequence shown as SEQ ID NO. 2, the leucine dehydrogenase mutant has the advantage that the 72 amino acid is mutated into alanine from lysine. The leucine dehydrogenase mutanthas the advantages that the site-specific mutagenesis is performed on alkaline amino acid residues in a leucine dehydrogenase substrate passage for the first time; the structure and the environment near the possible substrate binding site of the enzyme are modified; and the leucine dehydrogenase for more efficiently preparing L-2-aminobutyric acid is obtained. The leucine dehydrogenase mutant K72Awith the improved substrate tolerance provided by the invention can tolerate 4.5g / L 2-ketobutyric acid; the catalysis activity on the substrate 2-ketobutyric acid is improved by 15 percent.
Owner:JIANGNAN UNIV
Method for producing L-2-aminobutyric acid by double immobilized multi-enzyme systems
InactiveCN102517351AImprove recycling ratesImprove regeneration efficiencyChemical industryFermentationEnzyme systemOxidative enzyme
The invention discloses a method for producing L-2-aminobutyric acid by double immobilized multi-enzyme systems. The method provided by the invention comprises the following steps that 1, threonine dehydrogenase and leucine dehydrogenase are fixed on reversely-dissoluble pH-sensitive polymer carriers so that a co-immobilized multi-enzyme system is obtained; and 2, alcohol oxidase, formaldehyde dehydrogenase and formate dehydrogenlyase are fixed on reversely-dissoluble pH-sensitive polymer carriers so that a co-immobilized coenzyme regeneration system is obtained. The method for producing L-2-aminobutyric acid by the double immobilized multi-enzyme systems utilizes dissolution reversibility of co-immobilized enzymes, realizes effective separation of the co-immobilized enzymes and products, improves accessibility between the co-immobilized enzymes and reactants, improves recovery and utilization rates of threonine dehydrogenase, leucine dehydrogenase, alcohol oxidase, formaldehyde dehydrogenase and formate dehydrogenlyase, improves coenzyme regeneration efficiency, reduces follow-up separation purification processes, simplifies a process flow and reduces a production cost.
Owner:李鑫
Coryneform Bacterium Transformant and Process for Producing Valine Using the Same
A transformant obtainable by introducing one or more of the following DNAs (a), (b), and (c) into a coryneform bacterium as a host.(a) A DNA which encodes acetohydroxy acid synthase derived from Corynebacterium glutamicum and which has a mutation changing the glycine at position 156 to glutamic acid (G156E) in an amino acid sequence encoded by the DNA, or an analog thereof.(b) A DNA which encodes acetohydroxy acid isomeroreductase derived from Corynebacterium glutamicum and which has mutations changing the serine at position 34 to glycine (S34G), the leucine at position 48 to glutamic acid (L48E), and the arginine at position 49 to phenylalanine (R49F) in an amino acid sequence encoded by the DNA, or an analog thereof.(c) A DNA which encodes leucine dehydrogenase derived from Lysinibacillus sphaericus, or an analog thereof.
Owner:RES INST OF INNOVATIVE TECH FOR THE EARTH
Protein, DNA molecule, conversion host containing DNA and method for production of L-valine by utilization of conversion host
The invention relates to the biotechnology field, and especially relates to a protein, a DNA molecule, a conversion host containing the DNA and a method for production of L-valine by utilization of the conversion host. A coding gene leuDH (the nucleotide sequence is shown in SEQ ID No.2) of a leucine dehydrogenase with an auxiliary factor of NADH is converted and introduced into corynebacteria, and genetically engineered bacterium with changed redox equilibrium in cells are obtained. The bacterial strain is cultured and can be used for production of L-valine, and the yield of L-valine is raised remarkably.
Owner:MEIHUA BIOTECH LANGFANG CO LTD
Leucine dehydrogenase as well as preparation method and application thereof
ActiveCN104774813AIncrease productivityWide range of pH toleranceBacteriaOxidoreductasesHalf-lifeNucleotide
The invention discloses a leucine dehydrogenase as well as a preparation method and application thereof. The leucine dehydrogenase is encoded by taking Laceyella sacchari genome as a basis and cloning the leucine dehydrogenase gene LeuDH thereof, wherein the amino acid sequence is shown in SEQ ID NO. 1. The invention further discloses a gene for encoding the leucine dehydrogenase, and the nucleotide sequence is shown in SEQ ID NO. 2. The invention provides a novel dehydrogenase different from the present existing leucine dehydrogenase in sources. The leucine dehydrogenase is good in heat-resistant property, wide in pH application range, and capable of effectively prolonging a half-life period and reducing the production cost in industrial production.
Owner:NANJING UNIV OF TECH
Leucine dehydrogenase mutant and application thereof
ActiveCN109777788AIncrease production intensityEasy downstream purificationBacteriaMicroorganism based processesButyrateBio engineering
The invention discloses a leucine dehydrogenase mutant and application thereof and belongs to the technical field of biological engineering. On the basis of the amino acid sequence, shown in SEQ ID NO.1, of the leucine dehydrogenase, an amino acid residue M at the 47th site is mutated into V, an amino acid residue N at the 109th site is mutated into I, and correspondingly the leucine dehydrogenasemutant with mutational sites M47V and N109I is obtained, wherein the amino acid sequence of the leucine dehydrogenase mutant is shown in SEQ ID NO.3, and the leucine dehydrogenase enzyme activity ofa unit strain of recombinant bacteria E.coli BL21-pET28a-BtLDH007 expressing the mutant reaches 170.9 U / g. 2-keto-butyric acid is adopted as a substrate for producing L-2-aminobutyric acid, wherein the highest yield of the L-2-aminobutyric acid reaches 77.6 g / L, and the conversion rate is 96.0%.
Owner:JIANGNAN UNIV
Synthesis method of chiral tert-leucine and final product obtained in method
InactiveCN102250976ALow priceMeet the needs of large-scale productionOrganic chemistryFermentationSynthesis methodsReaction temperature
The invention provides a synthesis method of chiral tert-leucine, and the synthesis method is characterized by comprising the following steps: adding raw materials and water to a reaction kettle; adding ammonium formate solid and adding a pH regulator to regulate the pH value after completely dissolving; then sequentially adding an ammonium formate buffer of catalyst oxidized coenzyme, a buffer solution of formic dehydrogenase and a buffer solution of chiral leucine dehydrogenase; stirring to start reaction while controlling the reaction temperature at 10-40 DEG C and the pH value of the reaction system at 6.0-10.0; and after the reaction is finished, acquiring a final product, namely deuterated chiral tertleucine. The synthesis method provided by the invention has the advantages that: the used raw materials are easily available and inexpensive; the used raw materials are commercial raw materials or easily prepared raw materials, and can meet the needs of large-scale production; and the product is obtained by one-step reaction, water is used as the solvent, enzyme protein is used as the catalyst, and the product has a yield more than 80% and both chemical purity and chiral purity greater than 99%.
Owner:ASYMCHEM LAB TIANJIN +4
Single cell factory capable of efficiently synthesizing L-phenylglycine as well as construction and application of single cell factory
ActiveCN108103038AOptimize regeneration rateReduce the cost of trainingMicroorganism based processesOxidoreductasesEscherichia coliCell factory
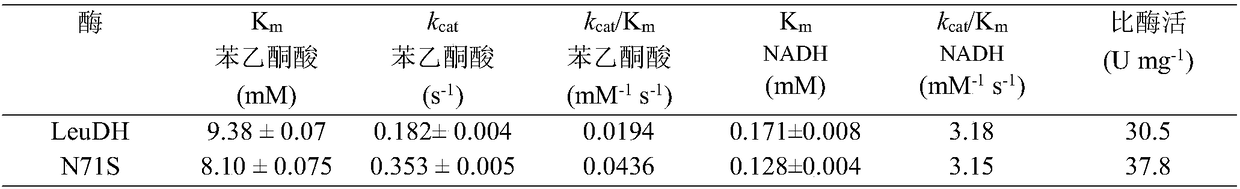
The invention discloses a single cell factory capable of efficiently synthesizing L-phenylglycine as well as construction and application of the single cell factory and belongs to the technical fieldof microorganisms. Firstly, efficient expression of leucine dehydrogenase obtained from Bacillus cereus in escherichia coli is realized, and site-directed mutation is carried out to obtain a mutant N71S with a remarkably improved reduction property; a mutant enzyme and a formate dehydrogenase mutant are co-expressed in the escherichia coli to form an intracellular in-situ co-factor NADH (Nicotinamide Adenine Dinucleotide) circulating system; the expression amount of the formate dehydrogenase mutant is optimized and controlled through a promoter and an RBS (Ribosomal Binding Site) sequence to successfully construct a recombinant escherichia coli single cell factory; the single cell factory is subjected to whole-cell conversion to prepare the L-phenylglycine. The method disclosed by the invention has the advantages of simple and rapid conversion process, low cost, no byproduct and easiness for separation and purification; when conversion is carried out in a 5L fermentation tank for 4h, the yield of the L-phenylglycine can reach 105.7g / l, the conversion rate is 93.3 percent and the space-time yield of the L-phenylglycine is 26.3g / L; an actually practical and effective strategy is provided for industrial production of the L-phenylglycine.
Owner:JIANGNAN UNIV
Coenzyme regeneration system and establishment method thereof
ActiveCN105132487ACoenzyme regeneration efficiency is highImprove regeneration efficiencyFermentationVector-based foreign material introductionHeterologousBiochemistry
The invention provides a coenzyme regeneration system which comprises formate dehydrogenase (FDH), leucine dehydrogenase (LDH) and ammonium formate. The invention further provides an establishment method of the coenzyme regeneration system. The establishment method mainly includes the steps of 1), heterogeneous expression of formate dhydrogenase and leucine dehydrogenase and 2), establishment of the coenzyme regeneration system. The coenzyme regeneration system is high in coenzyme regeneration efficiency and suitable for large-scale industrialized application, the establishment method is simple and convenient, and cost of coenzyme reaction is lowered greatly.
Owner:ABA CHEM CORP
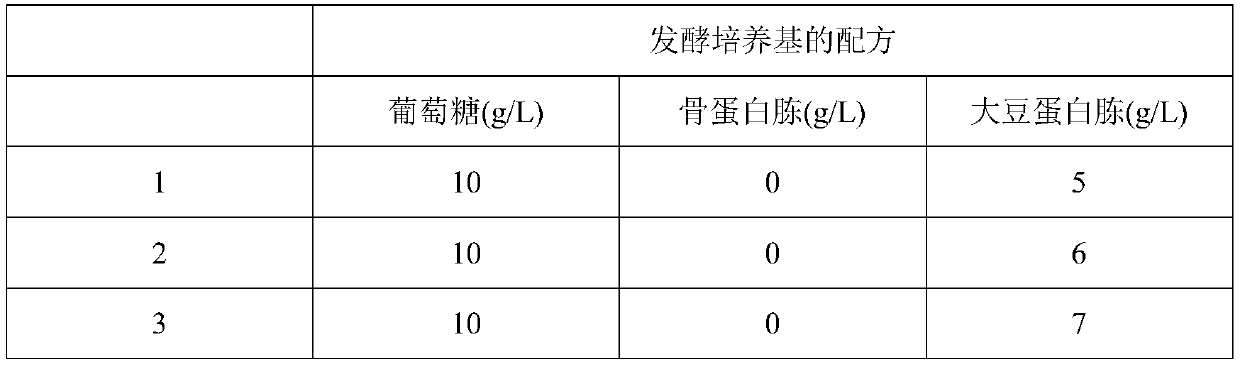
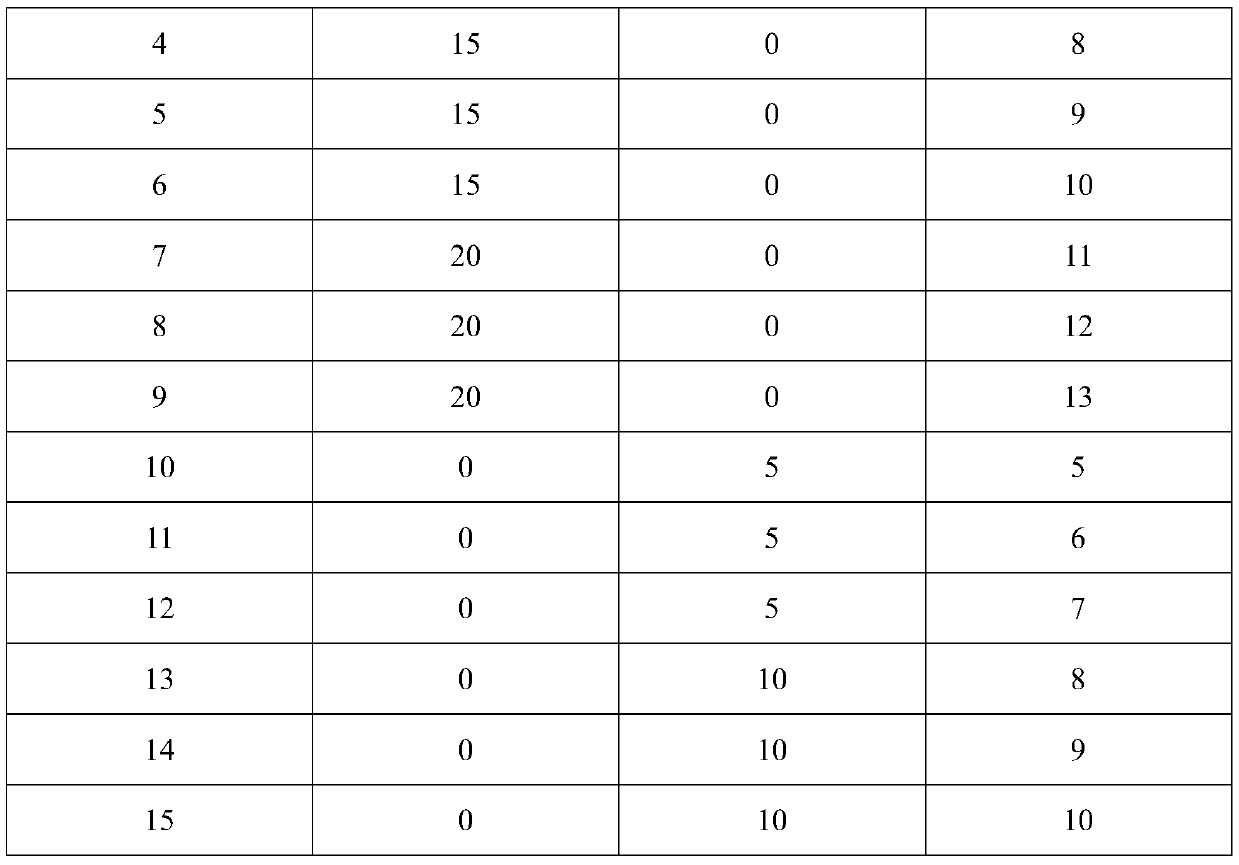
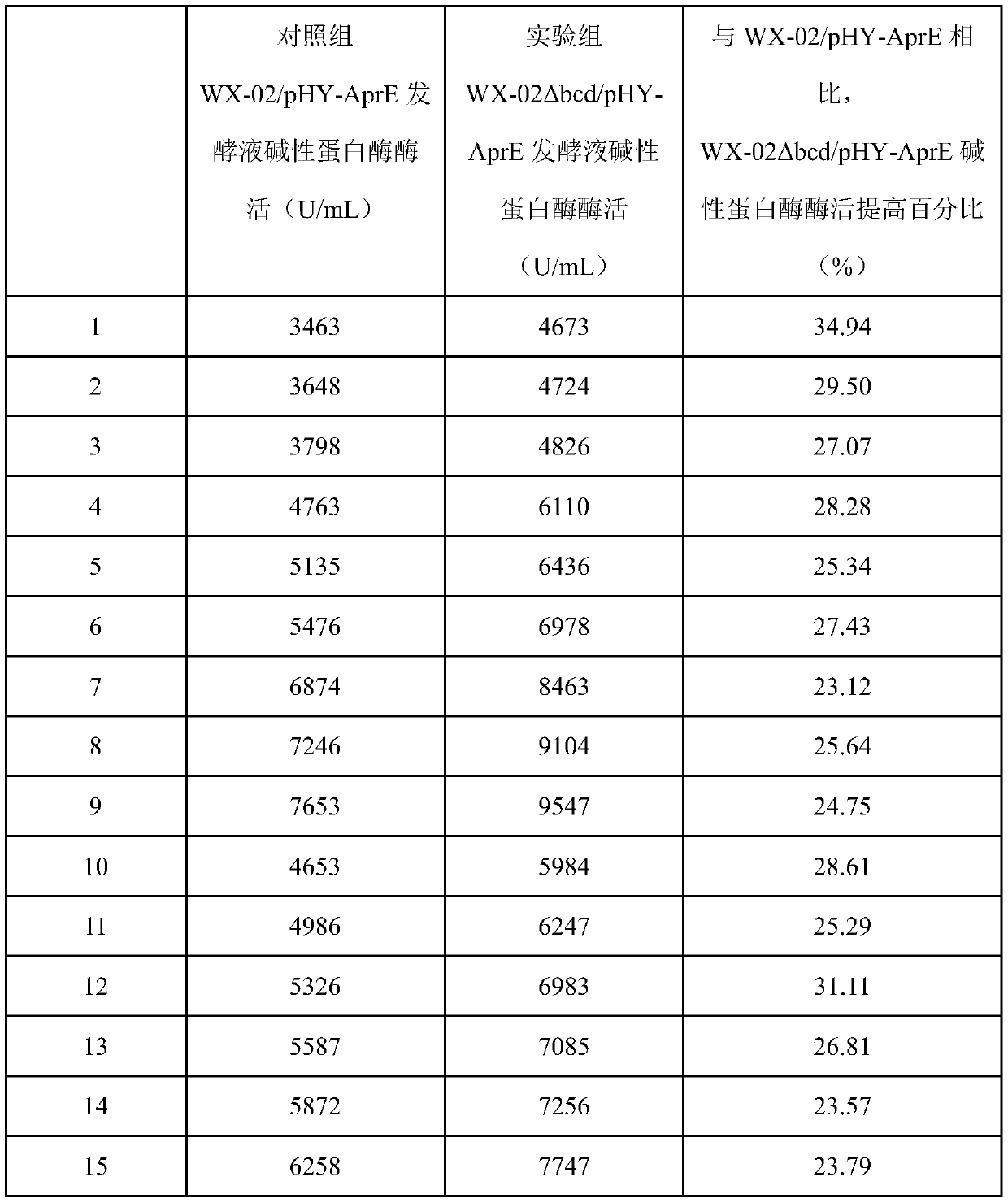
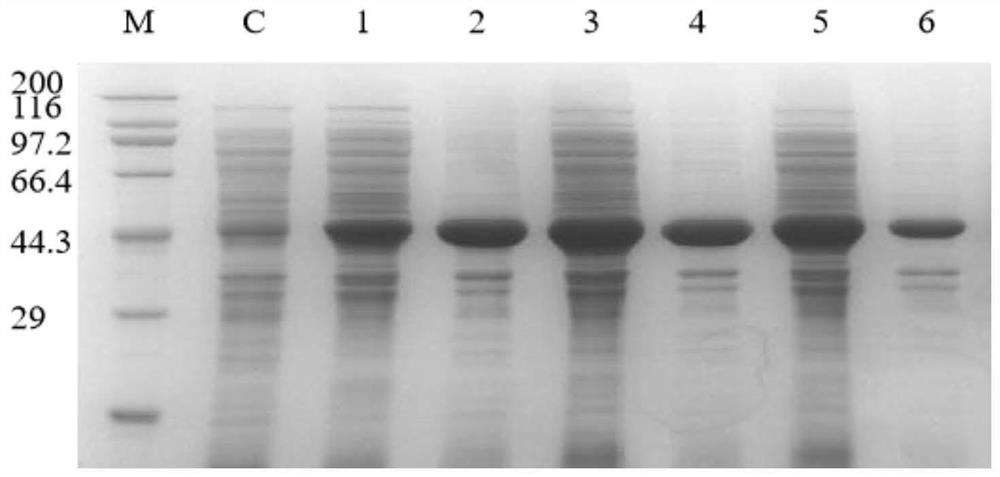
Application of bacillus licheniformis without leucine dehydrogenase gene in heterologous protein production
ActiveCN110904174AIncrease productionImprove expression levelBacteriaHydrolasesBacillus licheniformisHeterologous
The invention, which relates to the field of bacillus licheniformis gene engineering modification and protein efficient expression, discloses application of bacillus licheniformis without a bcd gene in protein production. According to the invention, the leucine dehydrogenase gene bcd in bacillus licheniformis WX-02 is knocked out through a genetic engineering method and thus the bacillus licheniformis WX-02 delta bcd lacking the gene bcd is successfully obtained; and alkaline protease AprE free expression plasmids pHY-AprE, a keratinase free expression vector pHY-Ker and a neutral protease free expression vector pHY-NprE are transferred. Compared with a control strain, the constructed engineering strain has a remarkable effect in the aspect of improving the enzyme activity of the protease,and the enzyme activity is improved by over 23%.
Owner:HUBEI UNIV
Leucine dehydrogenase mutant with improved catalytic activity and application of leucine dehydrogenase mutant
ActiveCN111826360AHigh catalytic activityEfficient preparationBacteriaMicroorganism based processesGlycineSpecific enzyme
The invention discloses a leucine dehydrogenase mutant with improved catalytic activity and application of the leucine dehydrogenase mutant, and belongs to the technical field of enzyme engineering and microbial engineering. The invention provides a leucine dehydrogenase mutant, which is subjected to C-terminal modification compared with leucine dehydrogenase with an amino acid sequence shown as SEQ ID NO.2, 364-bit arginine to 374-bit glycine of a C-terminal residue are cut off. A leucine dehydrogenase substrate access channel is modified for the first time, the access channel of the substrate is possibly expanded by cutting off the mutant at the C-terminal, so that the substrate is more easily combined with protein, and the leucine dehydrogenase capable of preparing L-2-aminobutyric acidmore efficiently is obtained. The specific enzyme activity of the leucine dehydrogenase mutant [Delta]364-374 provided by the invention is 201U / mg, and is improved by 20% compared with that of wild enzyme.
Owner:JIANGNAN UNIV
Method for preparing optical pure L-tertiary leucine from active inclusion body
ActiveCN107858384AReduce manufacturing costLow costAntibody mimetics/scaffoldsOxidoreductasesActive componentTert-leucine
The invention discloses a method for preparing optical pure L-tertiary leucine from an active inclusion body. The method comprises the following steps: (1) preparing a difunctional enzymatic activityinclusion body, wherein the difunctional enzymatic activity inclusion body comprises an active component, namely a fused difunctional enzyme; the fused difunctional enzyme comprises leucine dehydrogenase, LeuDH part connected and a polymerase part which are connected through a connecting peptide; the polymerase part is used for coenzyme NAD+ (Nicotinamide Adenine Dinucleotide+) regeneration; (2) putting the difunctional enzymatic activity inclusion body into a reaction mixed liquid with the pH value of 6.0-10.0, re-suspending, performing a reaction at 20-40 DEG C, and controlling the pH valueto be 6.0-10.0 in the reaction period, wherein the reaction mixed liquid comprises 50-1000mM of trimethyl pyroracemic acid, 50-1000mM of ammonium formate and 0.05-5mM of coenzyme NAD+.
Owner:XIAMEN UNIV
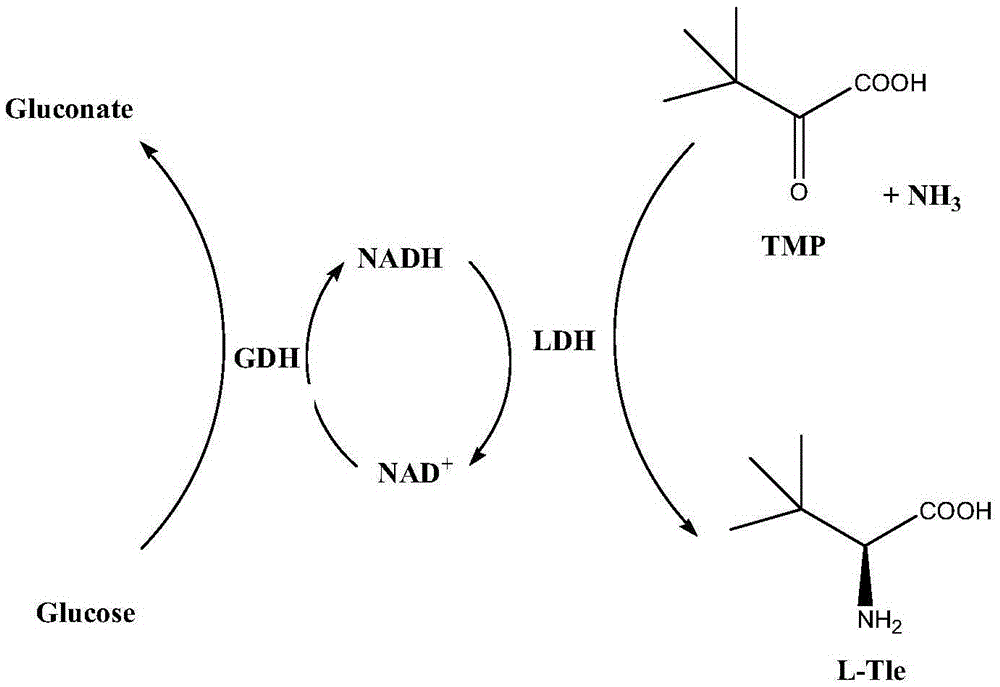
Efficient co-production strategy of L-phenylglycine and gluconic acid
ActiveCN106119272AGood market demandThe conversion process is fast and efficientOxidoreductasesFermentationEscherichia coliGluconic acid
The invention provides a method for co-producing L-phenylglycine and gluconate through single expression and co-expression of glucose dehydrogenase and L-leucine dehydrogenase in escherichia coli through utilizing a recombinant escherichia coli enzyme method and a whole cell method. The method is as follows: a glucose dehydrogenase gene and an L-leucine dehydrogenase gene are used for constructing recombinant single expression and co-expression carriers and are transformed into a gene engineering bacterium, namely the escherichia coli. The circulation of cofactors in a transformation system can be promoted through utilizing a recombinant bacterium enzyme method and the whole cell method; only a few of exogenous cofactors are added or the exogenous cofactors do not need to be used, and the L-phenylglycine and gluconic acid, which have high additional value, are co-produced by substrates including benzoylformic acid and glucose through utilizing a cofactor cyclic regeneration system; a transformation process is simple and rapid and low in cost. When transformation is carried out in a 5L fermentation tank for 2h to 4h, the yields of the L-phenylglycine and the gluconic acid, obtained by the method, can respectively reach 58.8g / L and 75.6g / L, and an actual and effective strategy is provided for industrial production.
Owner:JIANGNAN UNIV
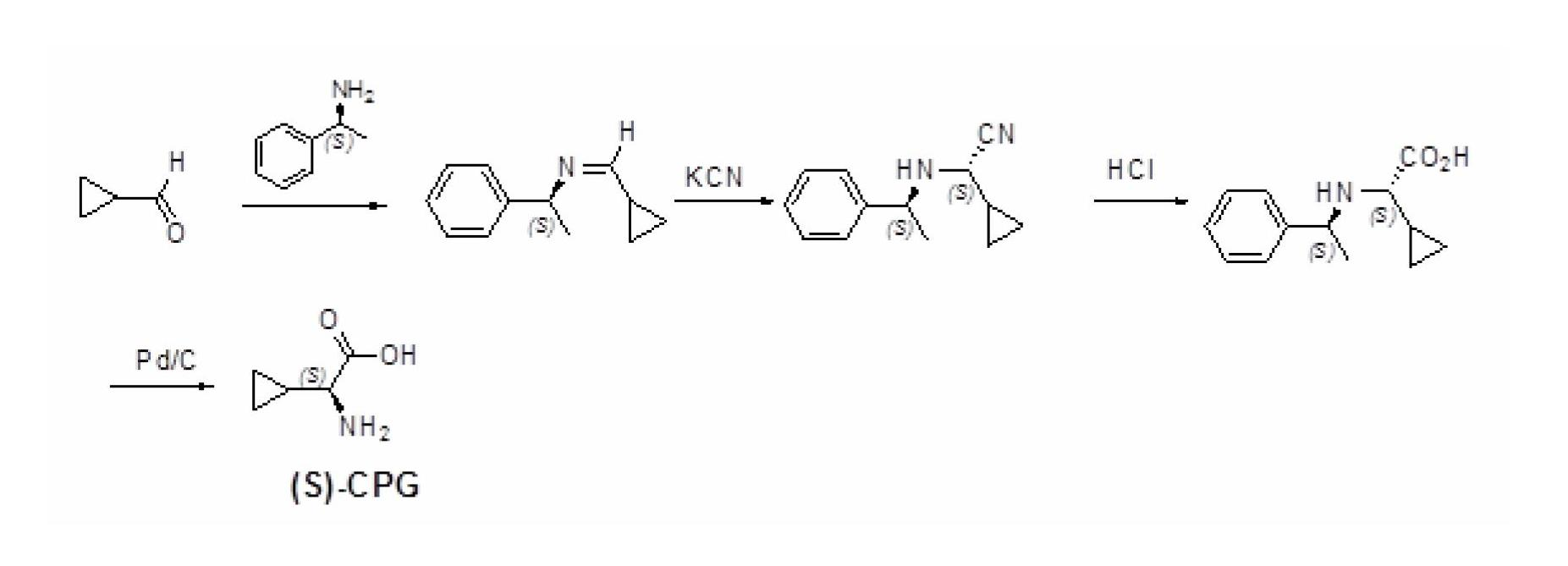
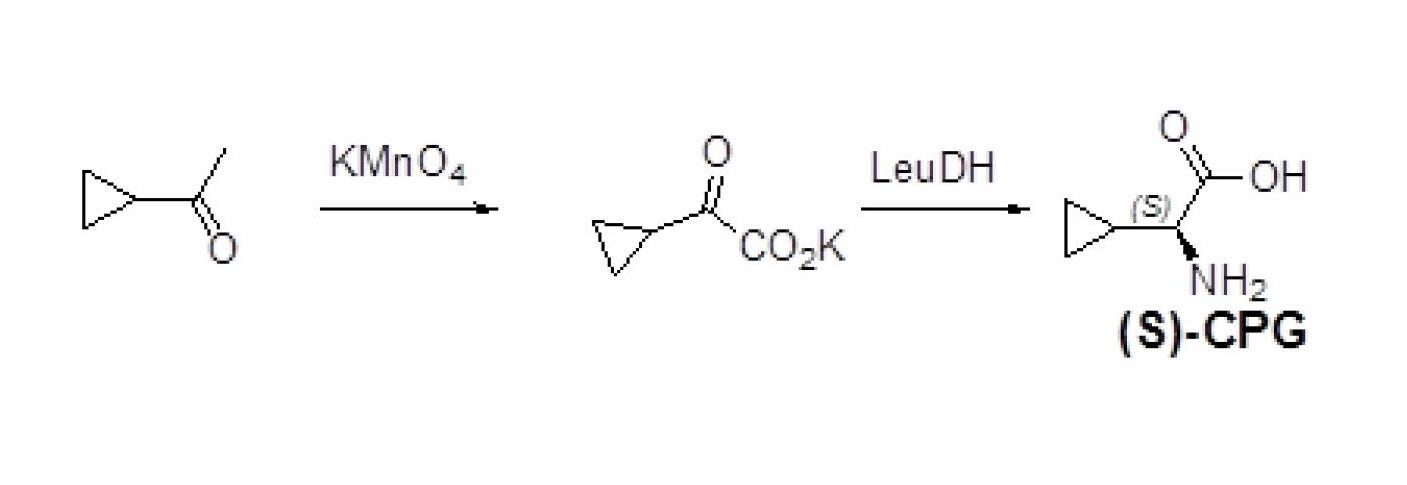
Method for preparing (S)-2-aminocyclopropylacetic acid
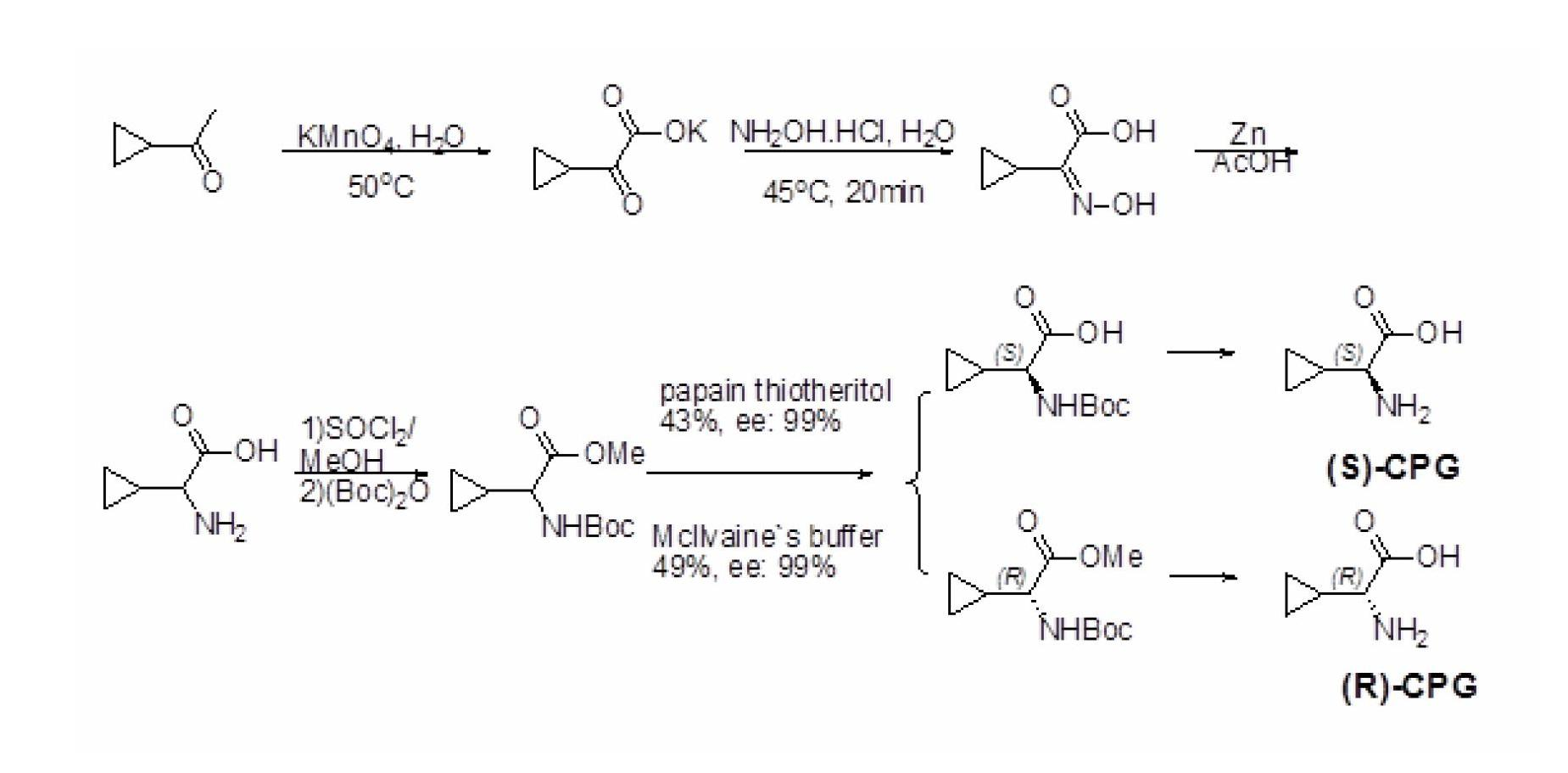
InactiveCN102676599ASimple and optimized synthetic process routeLow costFermentationKetonic acidsDehydrogenation
The invention relates to a method for preparing a (S)-2-aminocyclopropylacetic acid. The method includes two sequentially processed steps that step one, cyclopropyl methyl ketone which serves as a raw material is subjected to an oxidation reaction to generate 2-cyclopropyl ketonic acid and / or 2-cyclopropyl keto acid salts; and step two, an obtained product from the step one is subjected to a dehydrogenation reaction with a solution containing ammonium ions and formate ions under the action of leucine dehydrogenase to generate the (S)-2-aminocyclopropylacetic acid. Compared with methods in prior art, cheap cyclopropyl methyl ketone is utilized by the technical scheme as the raw material, two steps of a chemical method and an enzyme method are combined to prepare the (S)-2-aminocyclopropylacetic acid, the synthesis process route is concise and optimized, the cost is low, and the method is high efficient and suitable for industrial production.
Owner:SYNCOZYMES SHANGHAI
Biosynthetic method of high purity L-alpha-amino acid
The invention discloses a biosynthetic method of high purity L-alpha-amino acid. The method comprises the following steps: in a reaction liquid, carrying out enzymic catalytic reaction on an aldehyde compound and a glycine raw material under the catalytic effect of L-threonine aldolase and L-threonine dehydratase compound enzymes to obtain a 2-ketonic acid product; and carrying out enzymic catalytic reaction on the 2-ketonic acid product under catalysis of a coenzyme consisting of leucine dehydrogenase and hydrogenlyase or glucose dehydrogenase, and sequentially carrying out electrodialysis desalting, active carbon decoloring, concentration and crystallization and vacuum drying on the reaction product so as to obtain high purity L-alpha-amino acid crystals with a high yield. The method which uses an aqueous phase as a reaction system is environment-friendly and cheap, low in equipment requirement and low in cost, and the enzyme can be repeatedly used, so that the method can be used for satisfying industrial production.
Owner:HUNAN BAOLISHI BIOTECH
Leucine dehydrogenase mutant and construction method and application thereof
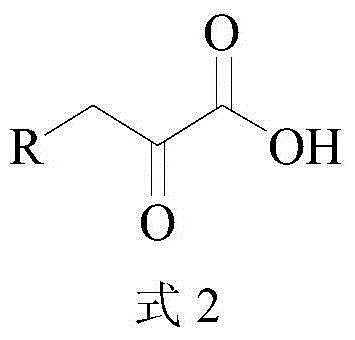
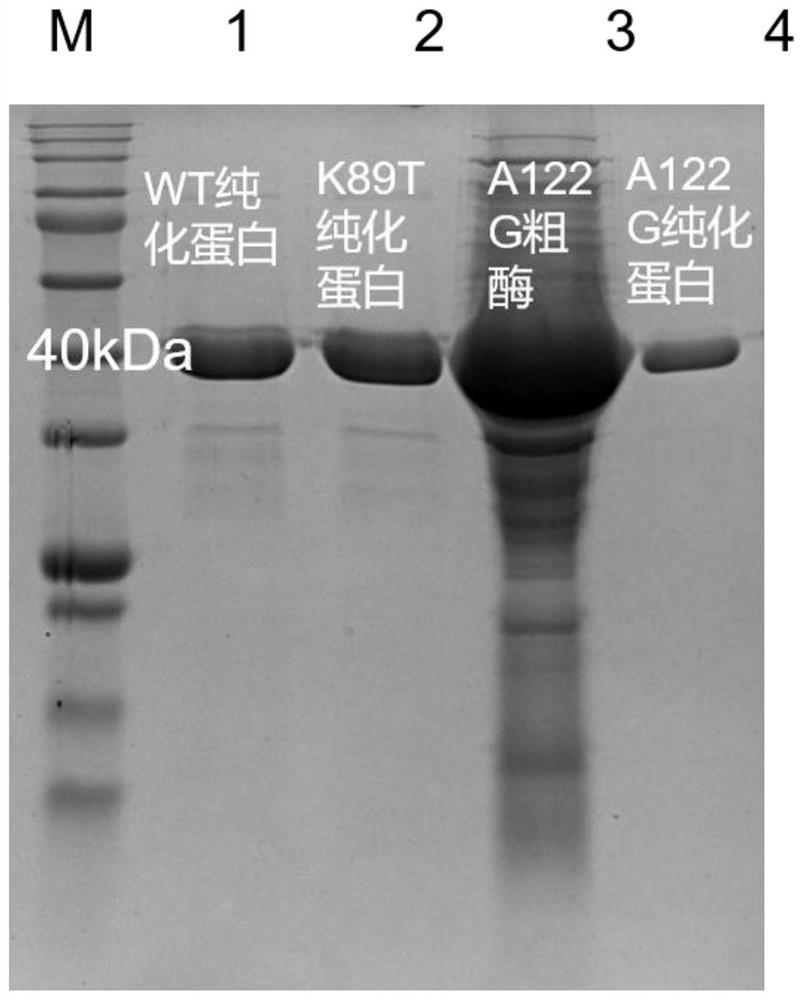
The invention provides a leucine dehydrogenase mutant and a construction method and application thereof, and belongs to the technical field of gene engineering. The mutant disclosed by the invention is obtained by mutating an amino acid sequence of wild leucine dehydrogenase; the 89th lysine of the amino acid sequence of the wild type leucine dehydrogenase is subjected to saturated mutation to form NNN or the 122th alanine is subjected to site-specific mutagenesis to form glycine, and the amino acid sequence of the wild type leucine dehydrogenase is shown as SEQ ID NO. 1. Experimental resultsshow that the K89T leucine dehydrogenase has certain activity on Asn and Thr, the A122G leucine dehydrogenase has certain activity on Phe, His and Met, and the wild leucine dehydrogenase has no activity on the amino acids completely; in addition, the enzyme activity of the K89T leucine dehydrogenase on Asp oxidative deamination reaction is twice that of wild leucine dehydrogenase.
Owner:XIAMEN UNIV
Modified Leucine Dehydrogenase
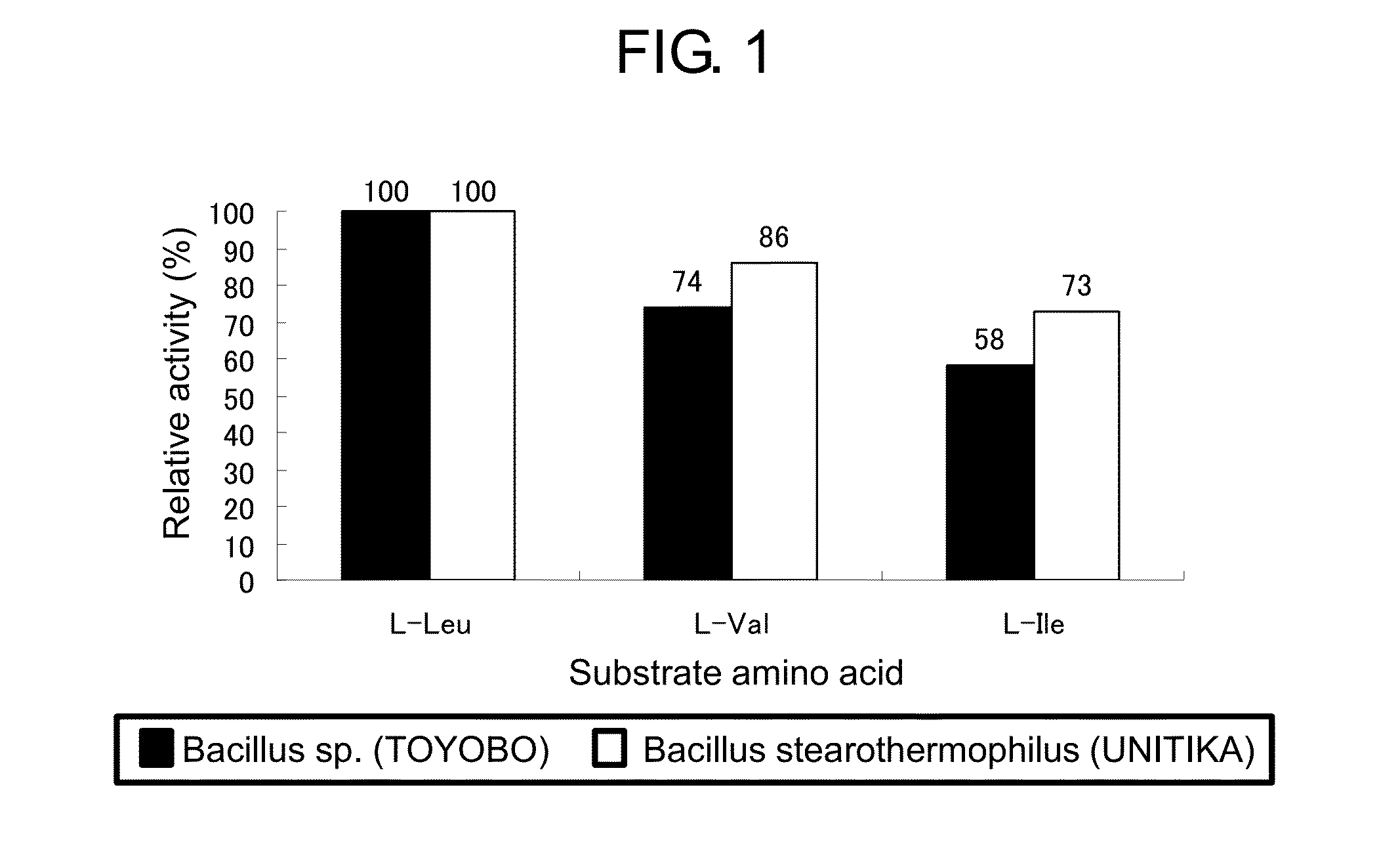
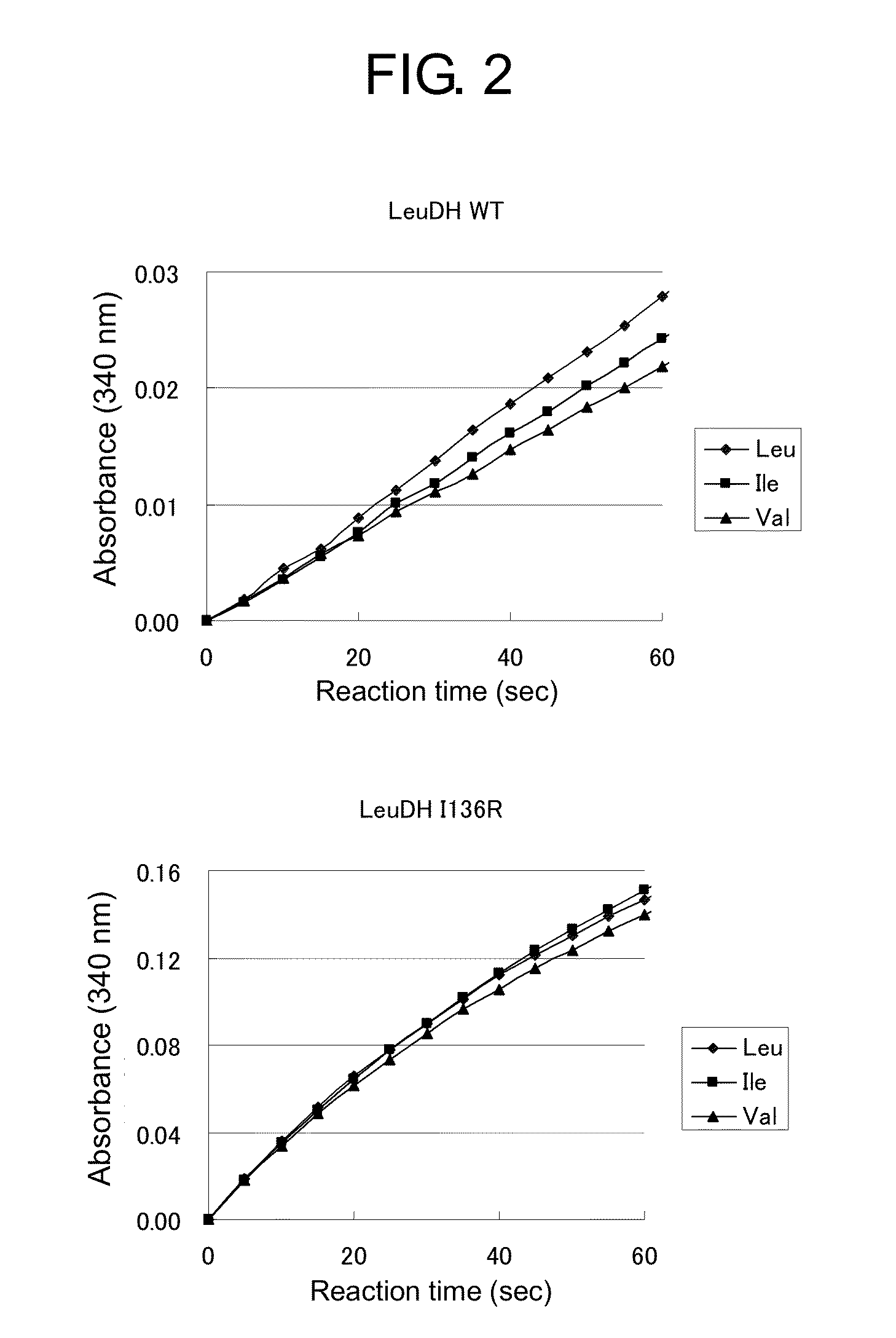
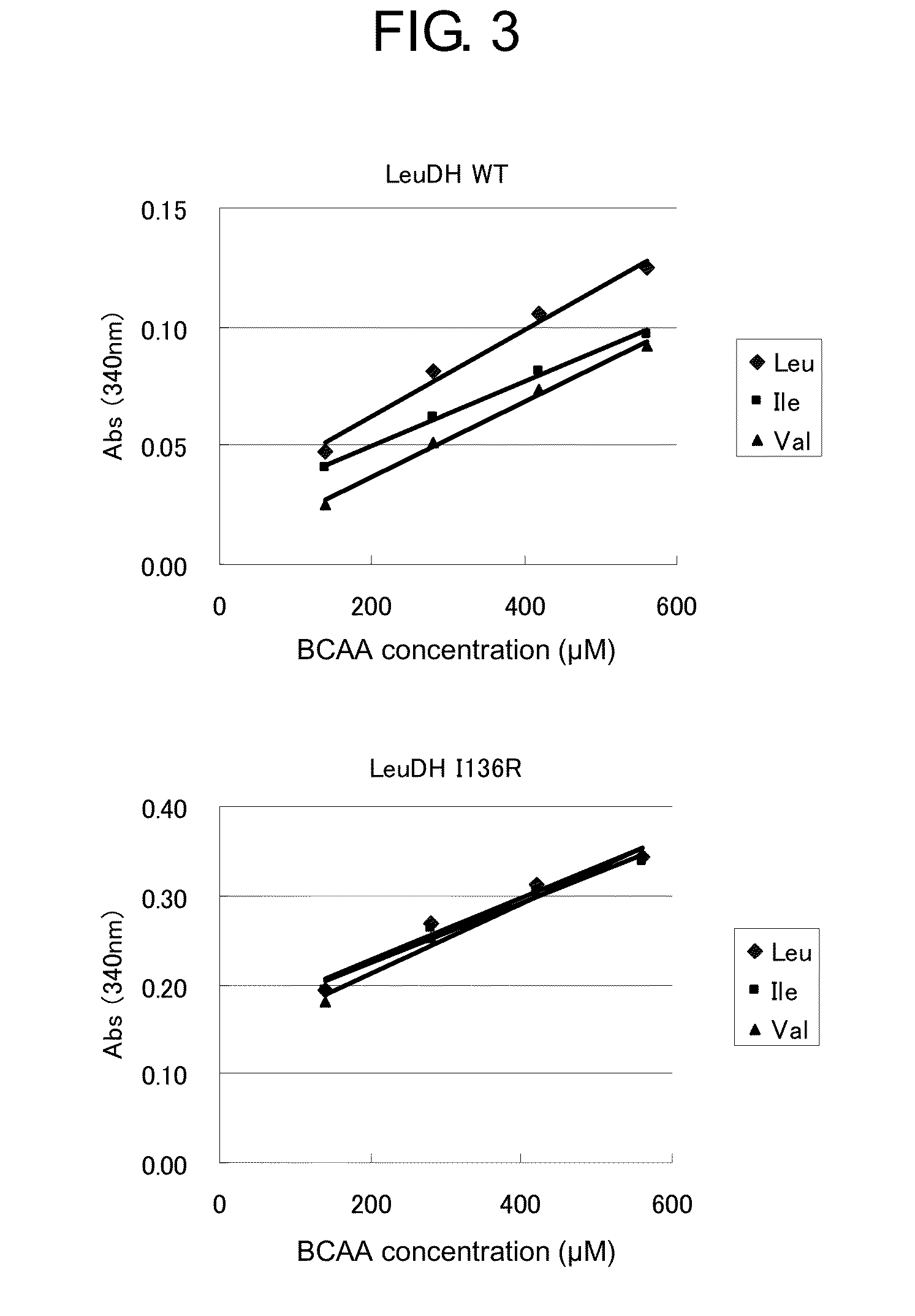
ActiveUS20150010936A1Rapid measurement of concentrationIncreased substrate specificityPolypeptide with localisation/targeting motifBacteriaTest sampleEnzyme measurement
The present invention provides a means and method useful for measurement of a total branched-chain amino acid concentration. Specifically, the present invention provides a modified enzyme in which at least one amino acid residue is mutated so as to improve a property of a leucine dehydrogenase which is associated with the measurement of the total branched-chain amino acids, such as, for example, substrate specificities of leucine dehydrogenase for total branched-chain amino acids, activity of leucine dehydrogenase for any branched-chain amino acids, and thermal stability of leucine dehydrogenase; and a method of analyzing the total branched-chain amino acids, comprising measuring the total branched-chain amino acids contained in a test sample using the modified enzyme.
Owner:AJINOMOTO CO INC
Leucine dehydrogenase mutant and application thereof to synthesis of aromatic chiral amine
ActiveCN110656095AGain catalytic activityHigh catalytic activityBacteriaMicroorganism based processesThreonineKetone
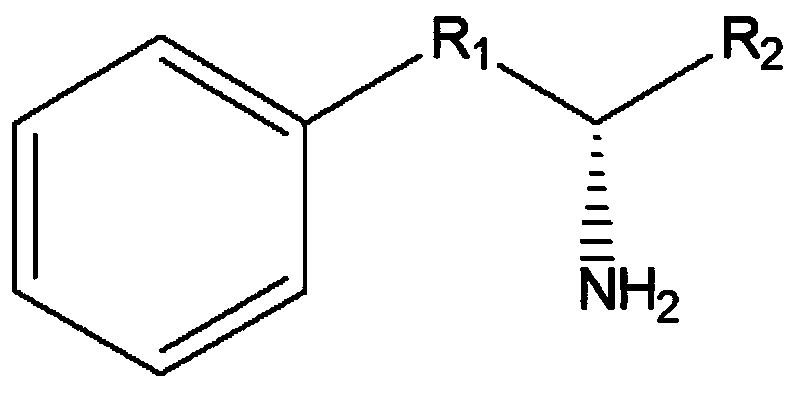
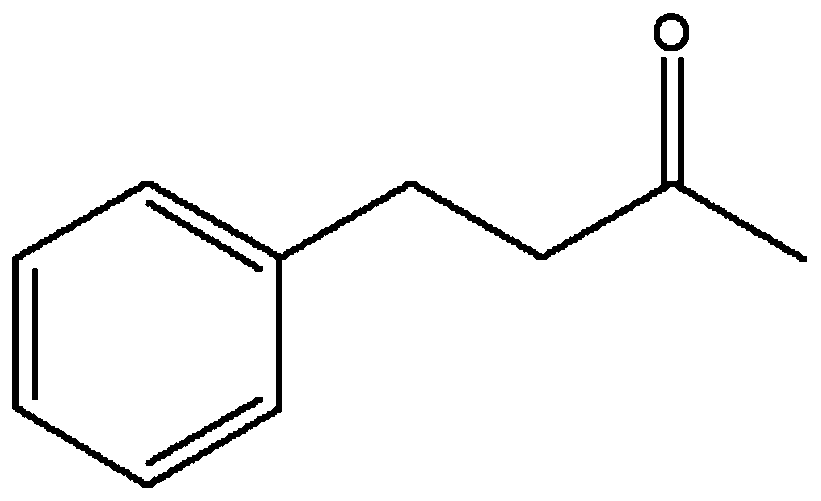
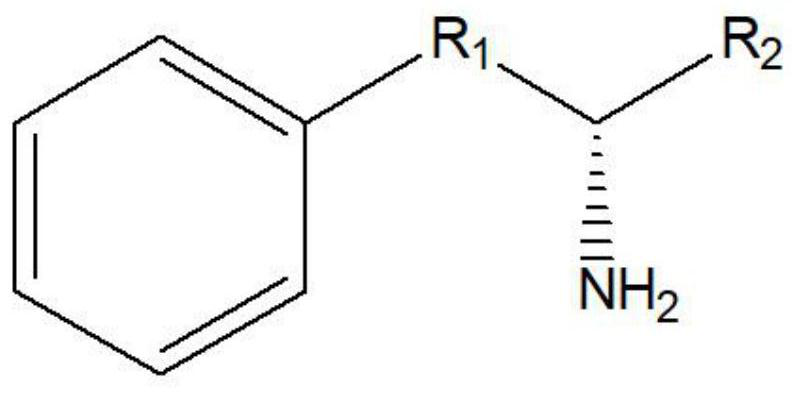
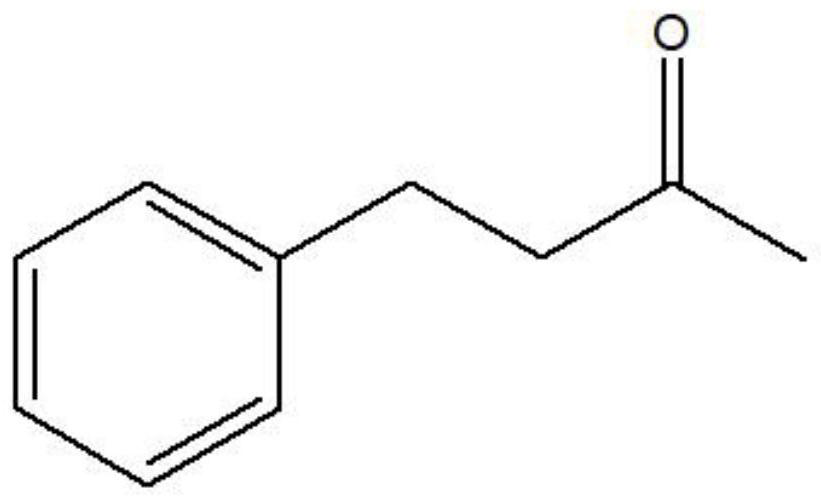
The invention discloses a leucine dehydrogenase mutant and an application thereof to synthesis of aromatic chiral amine, and belongs to the technical field of enzyme engineering and microorganism engineering. Mutation is performed on the 70th lysine and the 263rd asparagine, and the 115th alanine, the 136th threonine and / or the 42rd leucine of leucine dehydrogenase (Leucine Dehydrogenase, LeuDH, EC 1.4.1.9 ) as shown in SEQID NO.1 of an amino acid sequence, so that the leucine dehydrogenase obtains catalytic activity to aromatic chiral ketone of benzylacetone, phenylacetone and the like.
Owner:JIANGNAN UNIV
Prevention of incorporation of non-standard amino acids into protein
ActiveUS8603781B2Reducing, or substantially eliminating, endogenous cellular levels of norleucineLower Level RequirementsBacteriaPeptide/protein ingredientsBeta-methylnorleucinePhenylalanine dehydrogenase
The instant invention is drawn to the methods and compositions necessary to provide recombinant proteins with a substantially reduced or eliminated content of norleucine or other non-standard amino acids. Various embodiments of the invention provide for the substantial elimination of the incorporation of non-standard amino acids into recombinant proteins by the co-expression or enhanced expression of a protein (or the enzymatically active portion thereof) capable of degrading norleucine or other non-standard amino acids, including norvaline, beta-methylnorleucine, and homoisoleucine. In certain particular embodiments of the invention, the norleucine is degraded by a glutamate dehydrogenase, a leucine dehydrogenase, a valine dehydrogenase, a phenylalanine dehydrogenase, a glutamate / leucine / phenylalanine / valine dehydrogenase, or an opine dehydrogenase. Also provided are the cells and DNA constructs for carrying out these methods.
Owner:MONSANTO TECH LLC
Method for preparing L-tert-leucine by coupling leucine dehydrogenase with glucose dehydrogenase
ActiveCN105603016AAchieve recyclingAchieve zero additionOrganic chemistryFermentationHigh concentrationNiacin
The invention discloses a method for preparing L-tert-leucine by coupling leucine dehydrogenase with glucose dehydrogenase, belonging to the technical field of enzyme engineering and chiral medical intermediate preparation. The method comprises the following steps: adding substrates trimethylpyruvic acid and glucose into a reaction system; adjusting the pH value of the system to 8.0-9.0; and adding a crude enzyme (containing leucine dehydrogenase, glucose dehydrogenase and coenzyme) with broken co-expressed strain and cultured by a culture medium added with substances such as niacin, and then starting reactions while controlling the temperature at 25-30 DEG C and pH value at 8.0-9.0 in the reaction process to generate L-tert-leucine. In the invention, a system in which leucine dehydrogenase is coupled with glucose dehydrogenase is adopted, and the L-tert-leucine is prepared by use of the coenzyme contained in the thalli; and the method has the characteristics of high concentration of single-batch reaction substrates, high reaction efficiency, no coenzyme addition and the like.
Owner:JIANGNAN UNIV
Amino acid dehydrogenase mutant and application thereof
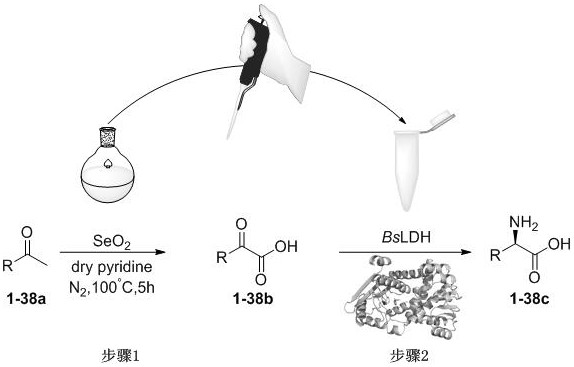
PendingCN113583988AHigh activityEasy to operateOrganic compound preparationOxidoreductasesOrganic synthesisAcyl CoA dehydrogenase
The invention belongs to the crossing field of chemical catalysis and enzyme catalysis, relates to an amino acid dehydrogenase mutant and application thereof, and in particular relates to organic synthesis, mutation modification of bacillus subtilis leucine dehydrogenase BsLDH, asymmetric reductive ammoniation of a substrate [alpha]-keto acid and a derivative thereof, and application of the amino acid dehydrogenase mutant to preparation of an amino acid dehydrogenase mutant, and a chemical-enzyme method continuous reaction preparation process of the chiral non-natural amino acid. The mutant disclosed by the invention can catalyze stereoselective reductive ammoniation of various [alpha]-keto acid compounds to synthesize non-natural amino acids with various structures. The mutant gene is expressed in E.coli Rosseta (DE3) after being recombined and transformed, and the mutant gene and glucose dehydrogenase (GDH) form a coenzyme circulation system for catalyzing [alpha]-keto acid and derivatives thereof to obtain chiral non-natural amino acid. Meanwhile, a methyl ketone compound is used as a starting raw material, and a chemical enzyme method reaction process for stereoselectively preparing chiral non-natural amino acid is constructed.
Owner:SHENYANG PHARMA UNIVERSITY
Engineering bacterium for converting branched chain amino acid and application of engineering bacterium in preparation of product for treating maple sugar urine
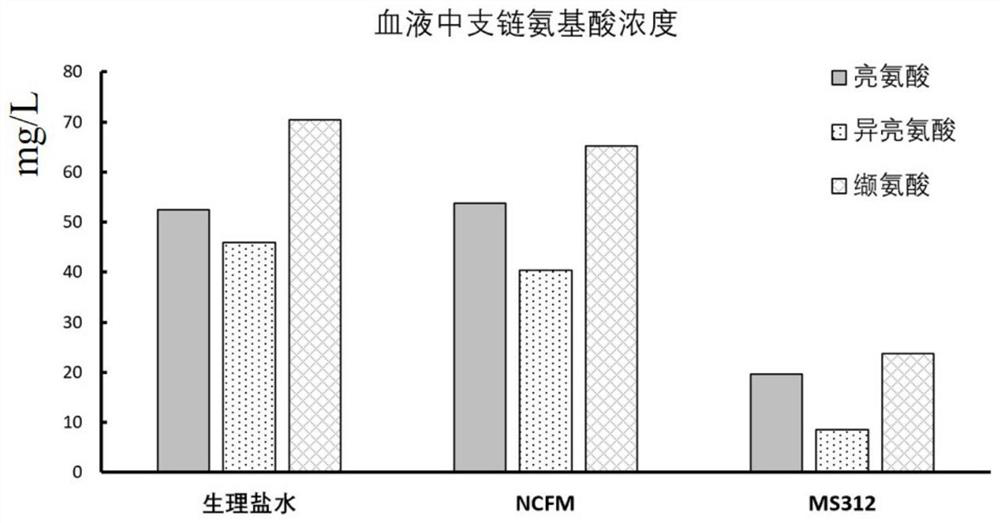
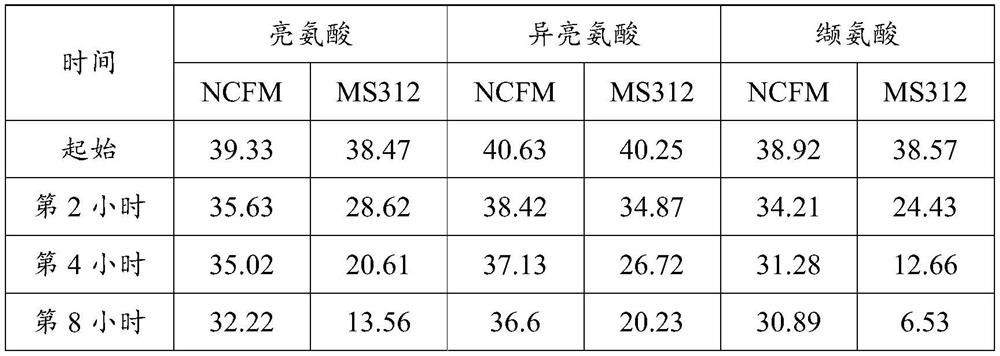
ActiveCN114561333AEfficient degradationEfficient conversionBacteriaConfectioneryDiseaseAcyl CoA dehydrogenase
The invention relates to an engineering bacterium for converting branched chain amino acid and application of the engineering bacterium in preparation of a product for treating maple sugar urine. According to the invention, a carrier containing a gene LeuDH and a gene MorA is constructed, and the carrier is transferred into lactobacillus in a homologous recombination mode. Through verification, the recombinant strain can effectively express leucine dehydrogenase and alpha-hydroxy acid dehydrogenase and realize the degradation effect on branched chain amino acid, is expected to be used as an engineering bacterium to be applied to preparation of drugs for treating maple sugar urine diseases, and has important significance on clinical treatment of maple sugar urine diseases.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
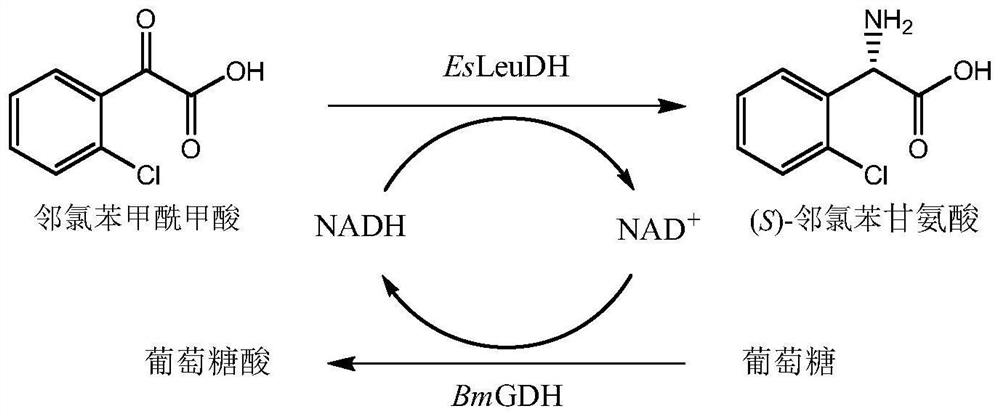
Leucine dehydrogenase mutant and application thereof in synthesis of (S)-2-chlorophenylglycine
ActiveCN114507650AHigh e.e valueIncrease catalytic rateBacteriaMicroorganism based processesGlycineChlorobenzene
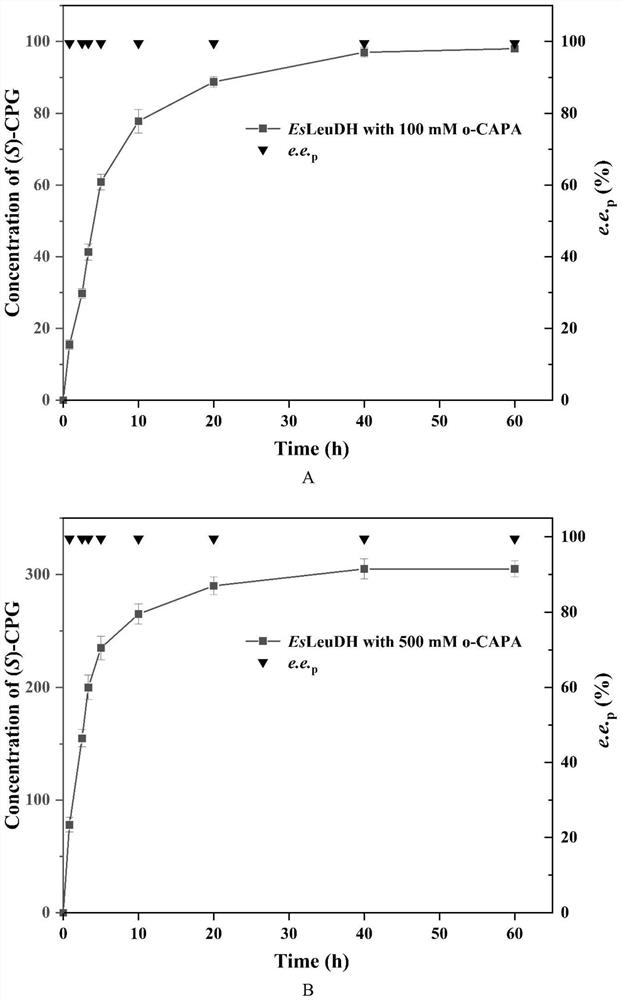
The invention discloses a leucine dehydrogenase mutant and application of the leucine dehydrogenase mutant in synthesis of (S)-2-chlorophenylglycine. The leucine dehydrogenase mutant is obtained by performing site-specific saturation mutation on the 362th site of an amino acid sequence shown in SEQ ID NO.2. The invention further discloses a preparation method of the leucine dehydrogenase mutant. The activity of the constructed leucine dehydrogenase mutant EsLeuDH-F362L is increased by 32 times compared with that of wild leucine dehydrogenase, the feeding amount of the substrate o-chlorobenzoyl formic acid of the mutant EsLeuDH-F362L can reach 500 mM, the product concentration is gradually increased along with time, the reaction can be completed within 4.0 h, the substrate conversion rate is larger than 99%, and the e.e value of the product is always kept to be 99.5% or above. Therefore, the bright group acid dehydrogenase mutant EsLeuDH-F362L has an industrial application prospect.
Owner:ZHEJIANG UNIV OF TECH
Preparation of high-purity l-tert-leucine by biological method
ActiveCN103966275BHigh selectivityMild conditionsMicroorganism based processesFermentationChemical synthesisTert-leucine
Owner:山东斯递尔化工科技有限公司
Leucine dehydrogenase mutants and their application in the synthesis of aromatic chiral amines
ActiveCN110656095BGain catalytic activityHigh catalytic activityBacteriaMicroorganism based processesThreonineKetone
The invention discloses a leucine dehydrogenase mutant and its application in the synthesis of aromatic chiral amines, belonging to the technical fields of enzyme engineering and microorganism engineering. The present invention adopts the 70th lysine and the 263rd asparagine of the leucine dehydrogenase (Leucine Dehydrogenase, LeuDH, EC 1.4.1.9) shown in the amino acid sequence as SEQ ID NO.1, and the 263rd asparagine The 115th alanine, the 136th threonine and / or the 42nd leucine are mutated, so that the leucine dehydrogenase obtains aromatic compounds such as 4-phenyl-2-butanone and phenylacetone. Catalytic activity of chiral ketones.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com