Leucine dehydrogenase mutant and application thereof
A technology of leucine dehydrogenase and mutants, which is applied in the field of leucine dehydrogenase mutants and its applications, and can solve problems such as substrate intolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Expression of Leucine Dehydrogenase Wild Enzyme
[0036] (1) Synthesize and obtain the leucine dehydrogenase whose coding nucleotide sequence is shown in SEQ ID NO.1.
[0037] (2) Construction and transformation of gene expression vector
[0038] The leucine dehydrogenase and the pET-28a vector with the coding nucleotide sequence shown in SEQ ID NO.1 are double-digested with restriction endonucleases BamH I and Hind III, and the product after digestion is ligated with Solution I and The ligation product was transformed into Escherichia coli BL21(DE3), and four transformants were picked to extract plasmids BamH I and Hind III for enzyme digestion verification to obtain recombinant Escherichia coli BL21 / pET-28a-LeuDH.
Embodiment 2
[0039] Embodiment 2: the preparation of leucine dehydrogenase mutant
[0040] Specific steps are as follows:
[0041] (1) Preparation of Leucine Dehydrogenase Mutants
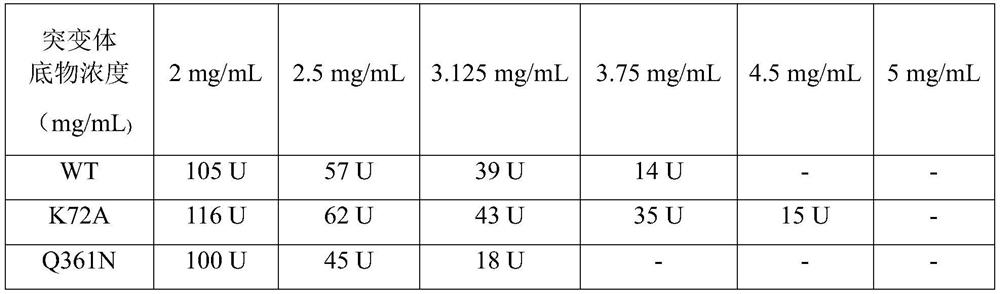
[0042] Using the whole plasmid reverse PCR method, the oligonucleotide fragment containing the mutation point was used as the homology arm to design the upstream and downstream primers, and the recombinant plasmid pET-28a-LeuDH obtained in Example 1 was used as the template to carry out site-directed mutation, and the gene carrying the gene encoding LeuDH was obtained. Recombinant plasmids pET-28a-LeuDH1~pET-28a-LeuDH4 of genes of amino acid dehydrogenase mutants K72A, Q361N, R69A, N84L;
[0043] Wherein, the mutation K72A is obtained by mutating the 72nd amino acid of the leucine dehydrogenase shown in SEQ ID NO.2 from lysine to asparagine, and the primers used are as follows:
[0044] K72A-F: 5'-CCGTCTGGCGGCGGGCATGACCTACAAGAATGCCGCGG-3' (SEQ ID NO.7)
[0045] K72A-R: 5'-TCATGCCCGCCGCCAGACGGAGCGCGTCAATCAGCGC-...
Embodiment 3
[0059] Example 3: Expression of Leucine Dehydrogenase Mutants
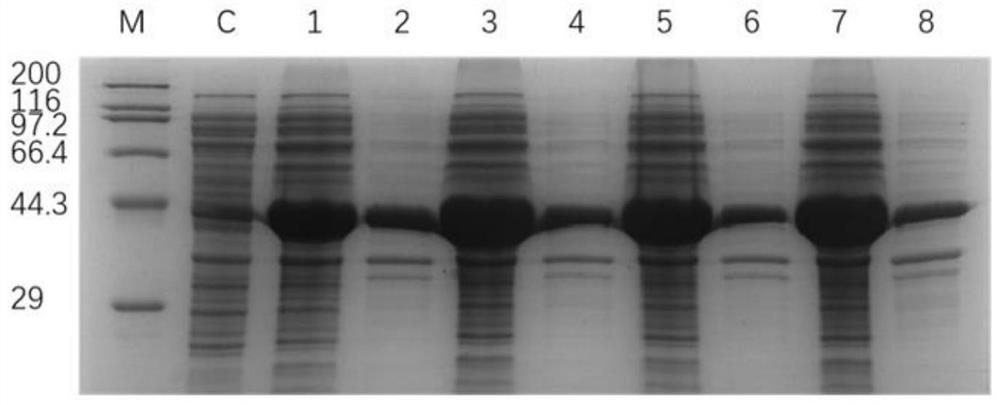
[0060] The recombinant Escherichia coli BL21 / pET-28a-LeuDH prepared in Example 1 and Example 2 and the recombinant Escherichia coli BL21 / pET-28a-LeuDH1~pET-28a-LeuDH4 were respectively inoculated in LB containing 50 μg / mL kanamycin In the liquid medium, after culturing overnight at 37°C and 180r / min, inoculate 1% of the inoculum into 50mL LB medium, culture at 37°C, rotate at 180r / min, and cultivate until OD600 reaches 0.5-0.8 Then add IPTG with a final concentration of 0.5mM for induction, and the induction temperature is lowered to 25°C. After induction for 8-10h, 4°C, 8000rpm centrifuge for 10min to collect the bacterial cells, take the collected wet bacterial cells, and use 5mL of 50mM pH 7.5 Wash twice with PBS buffer, resuspend in 5mL of 50mM PBS buffer with pH 7.5, oscillate and shake well, and then break under ultrasonic wave, break for 1s, stop for 3s, the total time is 15min. The cell lysate was centrif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com