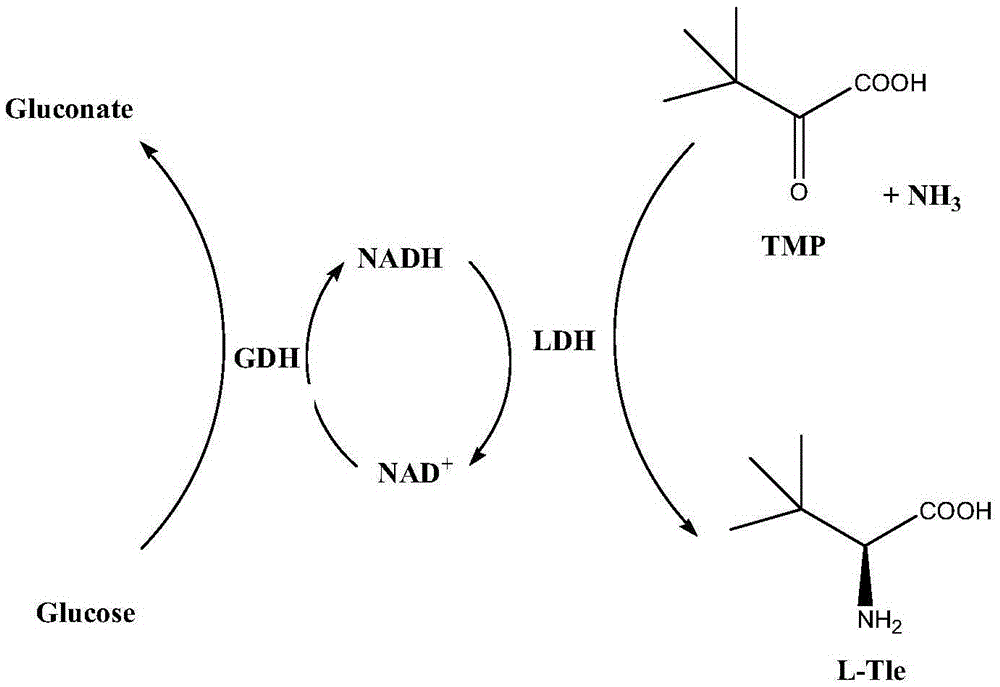
Method for preparing L-tert-leucine by coupling leucine dehydrogenase with glucose dehydrogenase
A technology of leucine dehydrogenase and glucose dehydrogenase, which is applied in organic chemistry, fermentation and other directions, can solve the problems of poor economy, low reaction efficiency, low activity of formate dehydrogenase, etc. The effect of simple process and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]Example 1: Construction of Escherichia coli recombinant strains co-expressing leucine dehydrogenase and glucose dehydrogenase
[0031] Use the gene sequence in the NCBI database to design primers, transfer the two enzyme genes of leucine dehydrogenase and glucose dehydrogenase from Bacilluscereus and Bacillussp, respectively, and use restriction enzymes XhoI and NheI to pair the target gene with the expression plasmid pET28a was subjected to double digestion, and the target gene was ligated with the vector to obtain recombinant plasmids pET28a-LDH and pET28a-GDH. Starting from the expression plasmids of the two genes, an SD-AS sequence (GGAGATATACC) was added between the two genes by using the overlap extension PCR technique. Primers containing the SD-AS sequence were designed, the gene GDH-SD-AS-LDH was obtained after two rounds of PCR, the gene was connected to the expression vector pET28a, and transformed into the E. coli host BL21 to obtain the recombinant strain E.c...
Embodiment 2
[0032] Embodiment 2: LB medium induces the expression of genetically engineered strains
[0033] The recombinant bacteria were fermented and cultured using LB medium. The components of LB medium were peptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH 7.0, prepared with deionized water, and kanamycin sulfate 50μg / mL was added before use. The culture conditions are as follows: the initial temperature is 37°C, the rotation speed of the shaker is 200r / min, when the cell OD 600 After reaching 0.6-2.0, add IPTG with a final concentration of 0.1 mM, and culture at 18°C for 17h.
Embodiment 3
[0034] Example 3: LB medium added with niacin and other substances induces expression of genetically engineered strains
[0035] Add 1 g / L nicotinic acid (or precursor substances in the coenzyme synthesis pathway such as nicotinamide, L-tryptophan, L-aspartic acid, etc.) to the LB medium. The components of LB medium were peptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH 7.0, prepared with deionized water, and kanamycin sulfate 50μg / mL was added before use. The culture conditions are as follows: the initial temperature is 37°C, the rotation speed of the shaker is 200r / min, when the cell OD 600 After reaching 0.6-2.0, add IPTG with a final concentration of 0.1 mM, and culture at 18°C for 17h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com