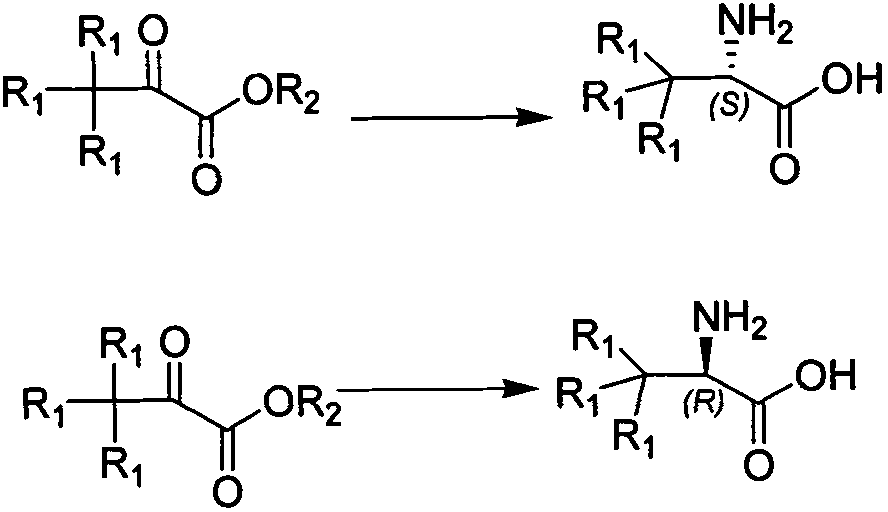
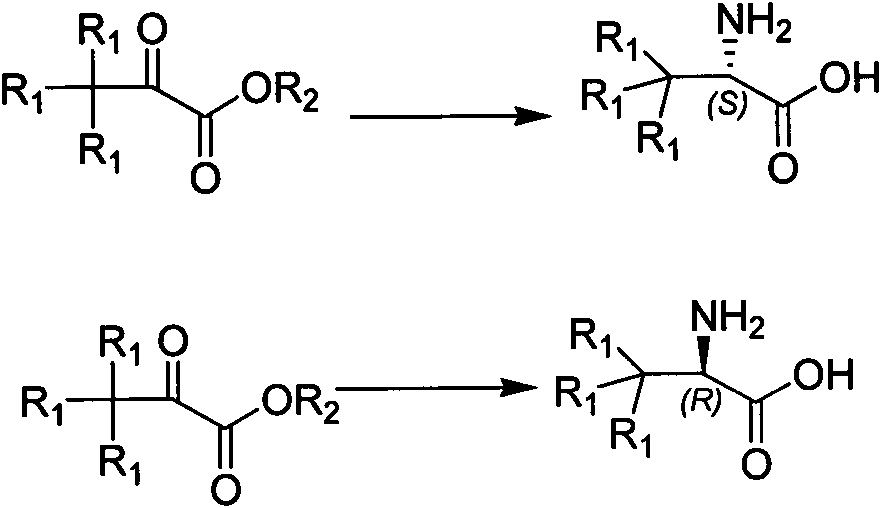
Synthesis method of chiral tert-leucine and final product obtained in method
A technology for the synthesis of tertiary leucine and its synthesis method, which is applied in the field of chiral tertiary leucine synthesis and its final products, can solve the problems of poor operation stability and expensive chiral catalyst, and achieve mild chemical reaction conditions and environmental protection. Friendly, Inexpensive Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of deuterated L-tert-leucine,
[0031] Add 20kg raw material deuterated trimethylpyruvate in 200L reactor 60L of pure water, add 36kg of ammonium formate solid, after all dissolved, adjust the pH to 6.5-8.5 with saturated ammonia water. After the adjustment, 200g of ammonium formate buffer solution of oxidized coenzyme, 40g of formate dehydrogenase buffer solution and 18g of L-leucine dehydrogenase buffer solution were added successively, stirred for 0.5h, and the temperature of the system was raised to 20±2°C. Control the temperature to start the reaction, 2D-NMR tracking to the end of the reaction, lower the temperature, concentrate the system, and dry it by centrifugation to obtain the product deuterated L-tert-leucine. 17.6kg, yield 87%, HPLC:>99%, ee 1 H-NMR: 3.4ppm, s, 1H; 2 D-NMR: 1.0ppm, s, 9D;
Embodiment 2
[0033] To prepare L-tert-leucine,
[0034] Add 15kg raw material trimethylpyruvate in 200L reactor Add 37.5kg of solid ammonium formate to 75L of purified water, and after all of it is dissolved, adjust the pH to 6.5-8.5 with 0.1M disodium hydrogen phosphate / sodium dihydrogen phosphate. After the adjustment, add the ammonium formate buffer solution of 120g of oxidized coenzyme, the buffer solution of 25.5g of formate dehydrogenase and the buffer solution of 10.5g of L-leucine dehydrogenase successively, stir for 0.5h, and the temperature of the system is raised to 38±2 ℃, temperature control to start the reaction, 2D-NMR tracking to the end of the reaction, cooling down, concentrating the system, centrifuging and drying to obtain the product preparation L-tert-leucine, 13.6kg, yield 90%, HPLC:>99%, ee
[0035] ( 1 H-NMR: 3.4ppm, s, 1H, 1.0ppm, s, 9H ;)
Embodiment 3
[0037] Preparation of deuterated D-tert-leucine,
[0038] Add 15kg raw material deuterated trimethylpyruvate in 200L reactor 60L of pure water, add 30kg of ammonium formate solid, after all dissolved, adjust the pH to 6.5-8.5 with 0.2M dipotassium hydrogen phosphate / potassium dihydrogen phosphate. After the adjustment, add the ammonium formate buffer solution of 180g of oxidized coenzyme, the buffer solution of 34.5g of formate dehydrogenase and the buffer solution of 16.5g of D-leucine dehydrogenase successively, stir for 0.5h, and the temperature of the system is raised to 20±2 ℃, temperature control to start the reaction, 2D-NMR tracking to the end of the reaction, cooling down, concentrating the system, centrifuging and drying to obtain the product deuterated D-tert-leucine, 13.4kg, yield 89%, HPLC:>99%, ee 1 H-NMR: 3.4ppm, s, 1H; 2 D-NMR: 1.0ppm, s, 9D;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com