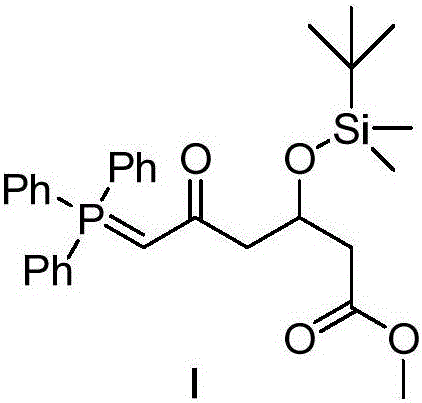
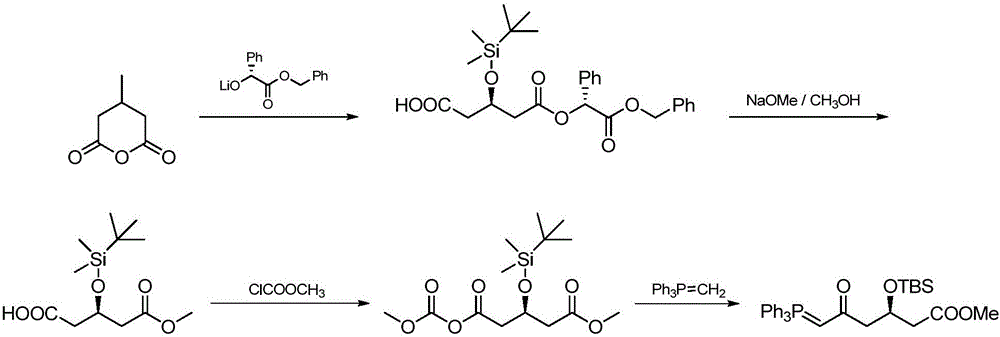
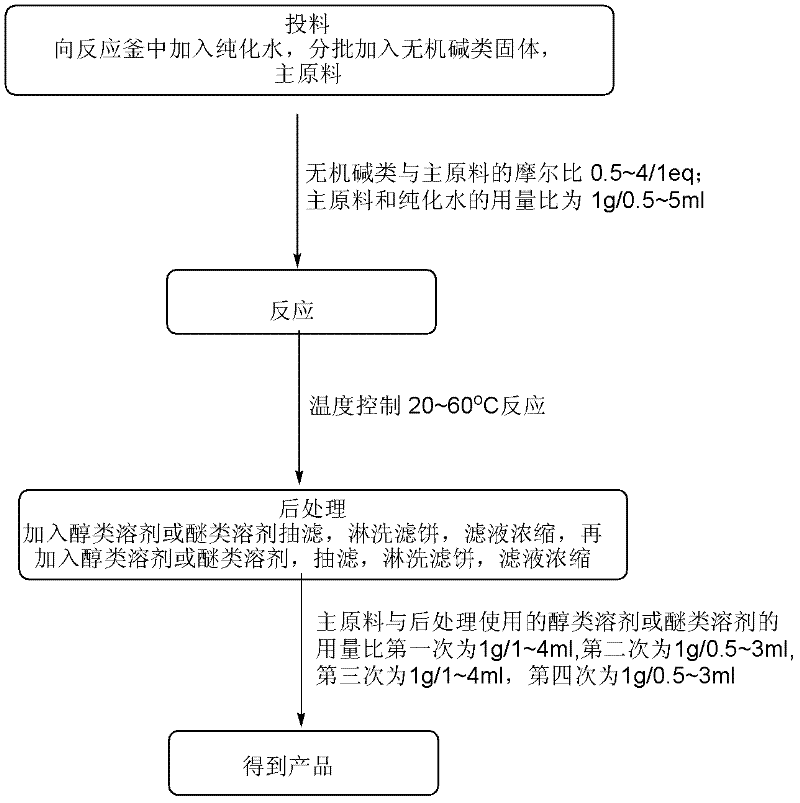
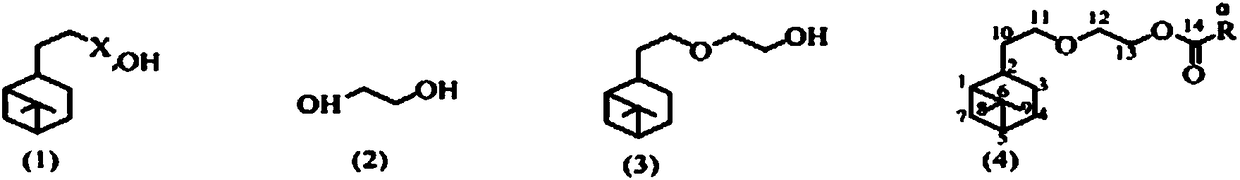
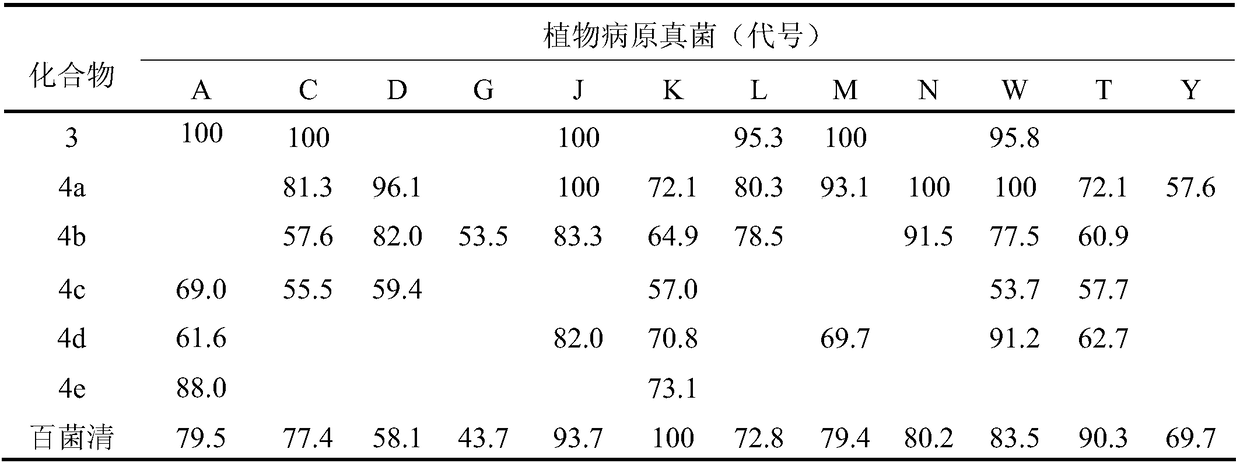
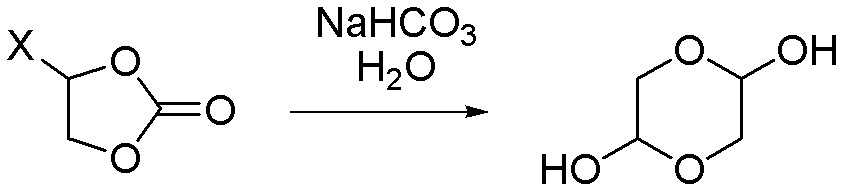Patents
Literature
30results about How to "Mild chemical reaction conditions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
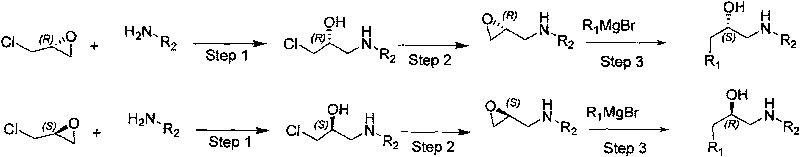
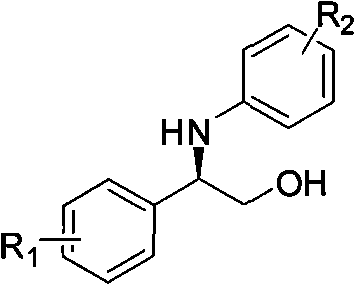
Synthesizing method, partial intermediate products and final products of chiral beta-alkamine derivative
ActiveCN101717341ALow priceMeet the needs of large-scale productionOrganic compound preparationAmino-hyroxy compound preparationArylChemical reaction
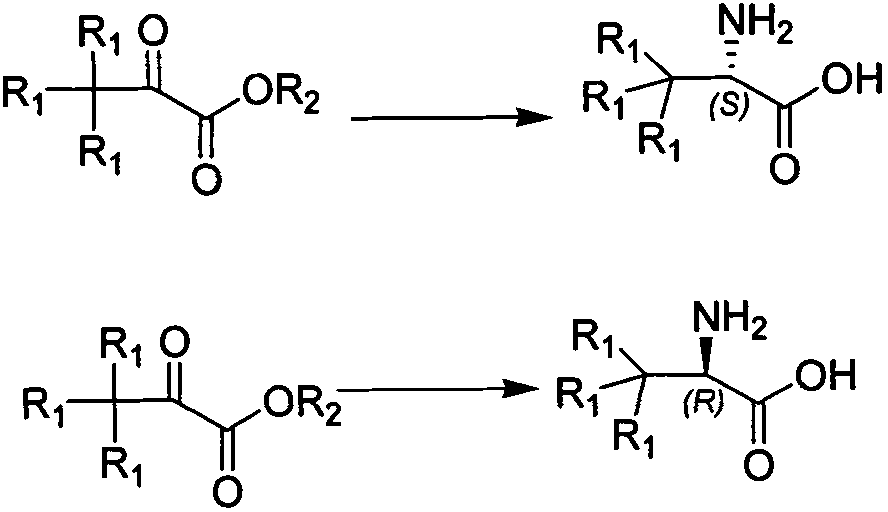
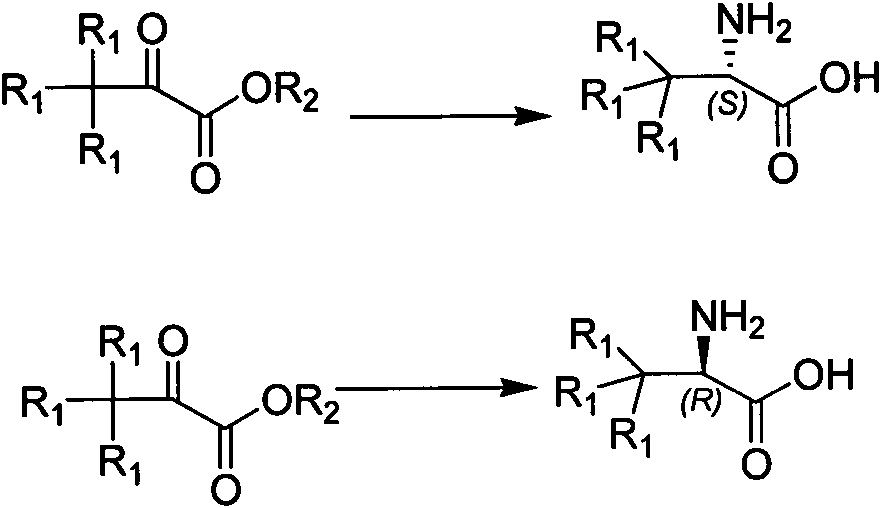
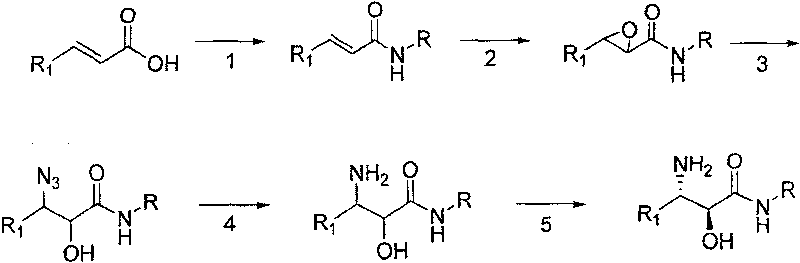
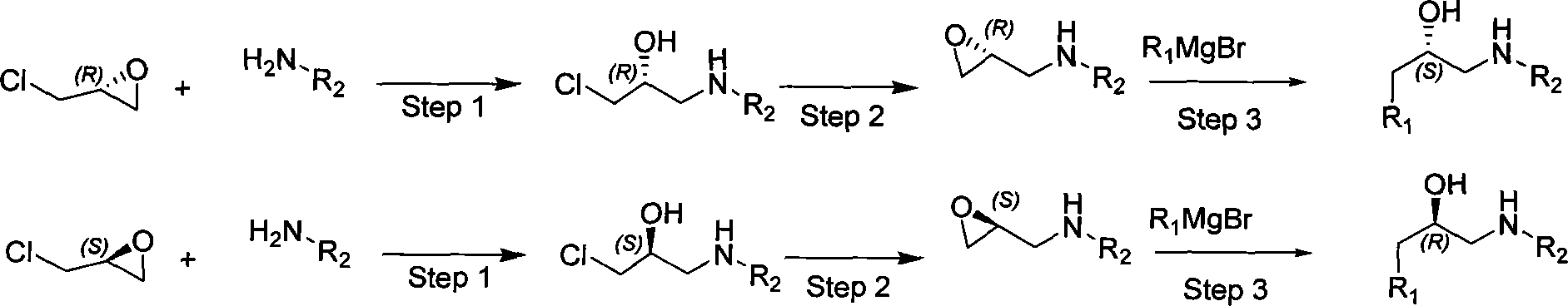
The invention relates to a synthesizing method, partial intermediate products and final products of a chiral beta-alkamine derivative. The synthesizing method of the chiral beta-alkamine derivative is characterized by comprising the steps of: selecting commercialized materials in the market and NH2R2 as initial materials, wherein R2 is a Cl-C6 alkyl group, a C3-C6 naphthenic base and an aryl group or an aryloxy; obtaining the intermediate products and the final products through a chemical reaction process with moderate conditions, wherein R1 and R2 are the Cl-C6 alkyl group, the C3-C6 naphthenic base and the aryl group or the aryloxy, and a chiral center is in an S or R shape. The invention has the advantages that the adopted materials are easy to obtain and at low price and can meet the requirements of large-scale production, chiral compounds are used as the initial materials, optical purity is retained in consequent reaction without finding racemization, and committed steps accord with the requirements of the current green chemistry; in addition, the invention has the advantages of simple synthesizing method, good selectivity, high yield coefficient, easy operation and good market benefits.
Owner:ASYMCHEM LIFE SCI TIANJIN
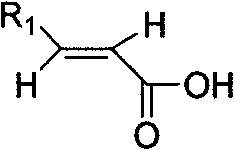
Method for synthesizing derivative of beta-amino acid and intermediate product thereof
ActiveCN101186587ALow priceMeet the needs of large-scale productionOrganic chemistryChemical reactionSynthesis methods
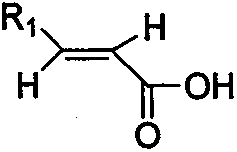
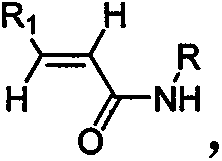
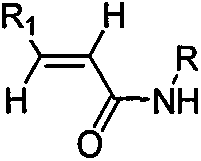
The invention provides a synthesis method of beta-amino acid derivative, and relative intermediate product, in particular to a synthesis method of formula (I) and relative intermediate product. The invention is characterized in that the invention uses commercialized material as formula (II) (trans-, E-type) as initial material, to obtain final product which formula is (III) via chemical reaction with mild condition. The invention has easily accessible starting material, stable technological condition and support for scaled industrial production.
Owner:ASYMCHEM LAB FUXIN
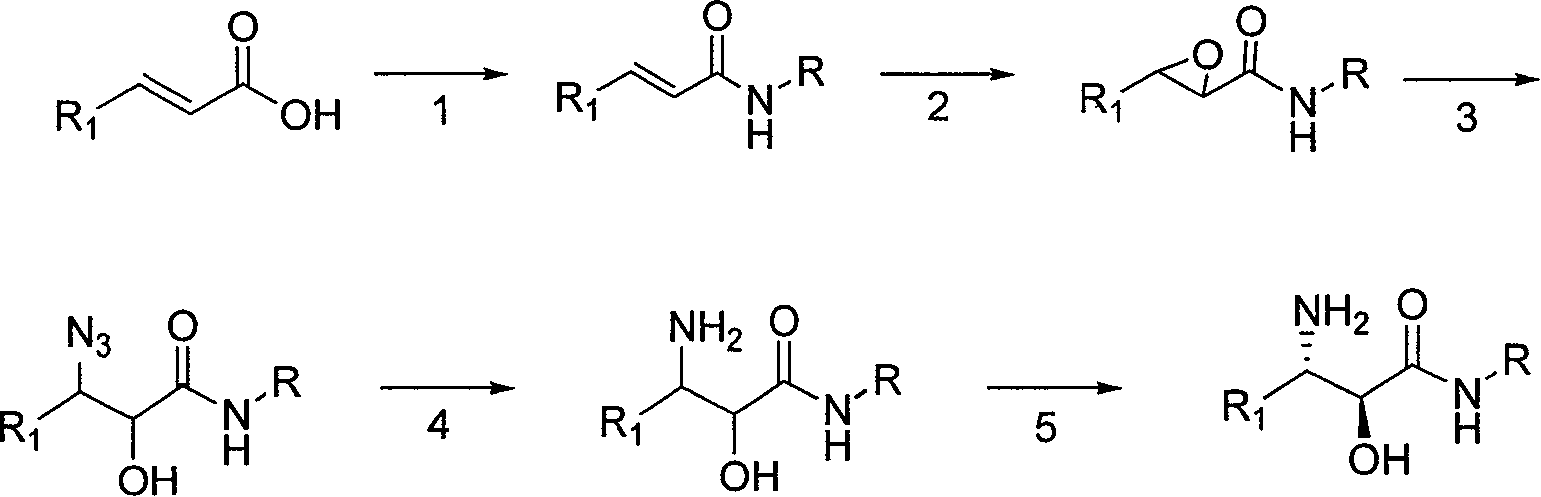
Synthesis method of chiral tert-leucine and final product obtained in method
InactiveCN102250976ALow priceMeet the needs of large-scale productionOrganic chemistryFermentationSynthesis methodsReaction temperature
The invention provides a synthesis method of chiral tert-leucine, and the synthesis method is characterized by comprising the following steps: adding raw materials and water to a reaction kettle; adding ammonium formate solid and adding a pH regulator to regulate the pH value after completely dissolving; then sequentially adding an ammonium formate buffer of catalyst oxidized coenzyme, a buffer solution of formic dehydrogenase and a buffer solution of chiral leucine dehydrogenase; stirring to start reaction while controlling the reaction temperature at 10-40 DEG C and the pH value of the reaction system at 6.0-10.0; and after the reaction is finished, acquiring a final product, namely deuterated chiral tertleucine. The synthesis method provided by the invention has the advantages that: the used raw materials are easily available and inexpensive; the used raw materials are commercial raw materials or easily prepared raw materials, and can meet the needs of large-scale production; and the product is obtained by one-step reaction, water is used as the solvent, enzyme protein is used as the catalyst, and the product has a yield more than 80% and both chemical purity and chiral purity greater than 99%.
Owner:ASYMCHEM LAB TIANJIN +4
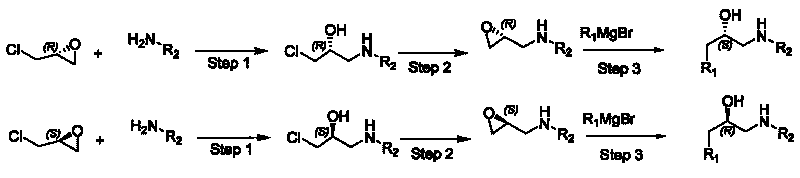
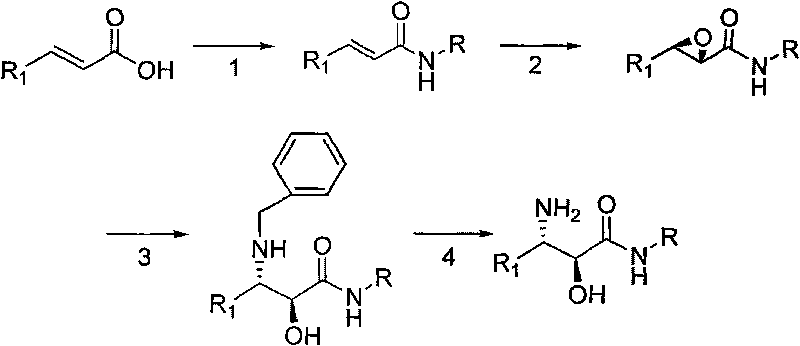
Synthesis method of derivative of chiral Beta-amino-alcohol and part of final products thereof
InactiveCN101747211AMeet the needs of large-scale productionNo racemization was foundPhysical/chemical process catalystsOrganic compound preparationAlcoholStereochemistry
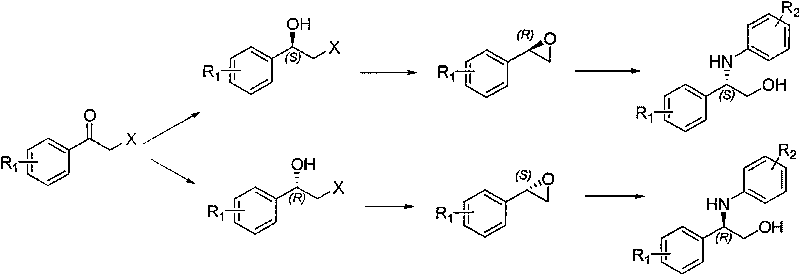
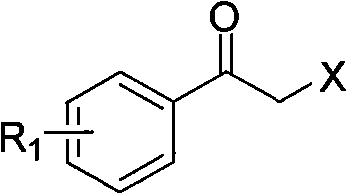
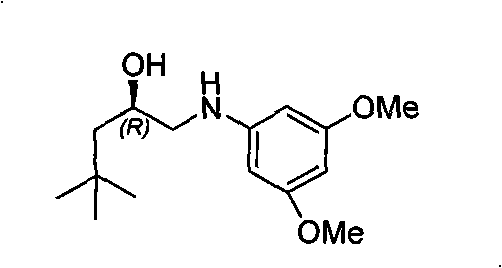
The invention relates to a synthesis method of a derivative of chiral Beta-amino-alcohol and part of intermediate products and final products thereof. The method selects a raw material which is commercialized on a market or a raw material of Alpha-halogeno ketone with easy preparation as an initial raw material, wherein X is Br or Cl, the final product with the chemical formula as the accompanying drawing is obtained by reaction or wherein R1=H, 2-Cl, 3-Cl, 4-Cl, 2, 4-Cl, 4-Br, 4-F, 4-CF3 and 4-NO2; R2=H, 2-OMe, 3-OMe, 4-OMe, 4-F, 4-Cl, 4-Br, 3-Br, 3-F, 3-Cl, 2-Br, 2-Cl, 2F, 4-Me, 3-Me and 2-Me, and the chiral center is S or R; the intermediate product with the chemical formula is shown in the accompanying drawing and the chiral center is in S configuration or R configuration; and the final product with the chemical formula is shown in the accompanying drawing and the chiral center is in S configuration or R configuration.
Owner:ASYMCHEM LIFE SCI TIANJIN
Synthesis method of chiral epoxy compound and intermediate products and final product
ActiveCN101691338ALow priceReduce generationOrganic compound preparationCarboxylic acid amides preparationEpoxyChemical reaction
The invention discloses a synthesis method of a chiral epoxy compound and intermediate products and a final product beta-amino acid derivative. The chiral epoxy compound is obtained by utilizing commercialized raw materials in markets as initial raw materials and then obtaining a chiral intermediate in the process of chemical reaction under mild conditions. In the method, the novel chiral catalyst method is utilized, a great amount of novel chiral catalyst ligand is synthesized by adopting the method in documents, epoxidation is carried out on olefin and then olefin is applied to the synthesis of the beta-amino acid derivative, thereby improving the use ratio of raw materials and optimizing the process from the origin. Epoxidation is carried out on unsaturated amide by the novel chiral ligand, and then open-loop deprotection is carried out to obtain the product. The value of product obtained by oxidation reaction is 80-99%, the yield coefficient thereof is 80-95%, and the chemical purity thereof is 90-99%, therefore, the chiral epoxy compound can be used for mass production and has very good industrial value.
Owner:ASYMCHEM LIFE SCI TIANJIN
Reducing response magnetic drug-loaded nanoparticles with synergetic anti-cancer interaction and preparation method of reducing response magnetic drug-loaded nanoparticles
InactiveCN106822902AReductively responsiveSuperparamagnetic responsivenessOrganic active ingredientsPharmaceutical non-active ingredientsSide effectSuperparamagnetism
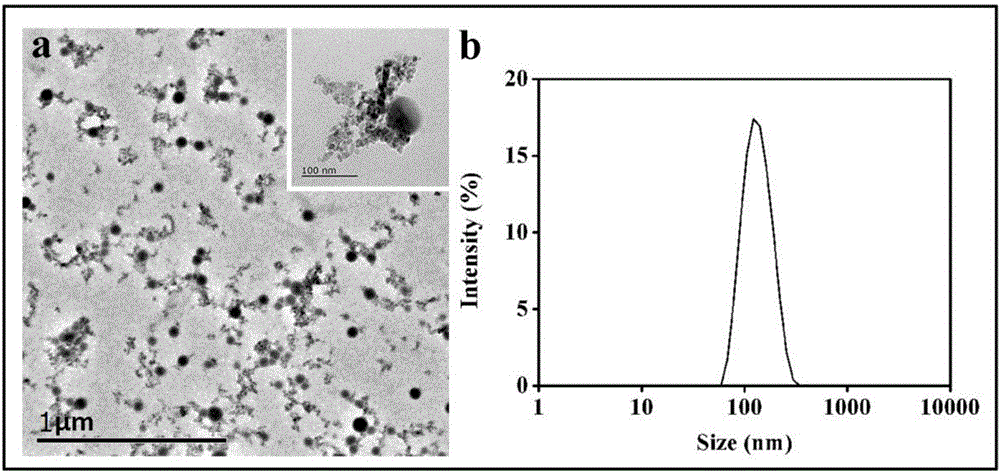
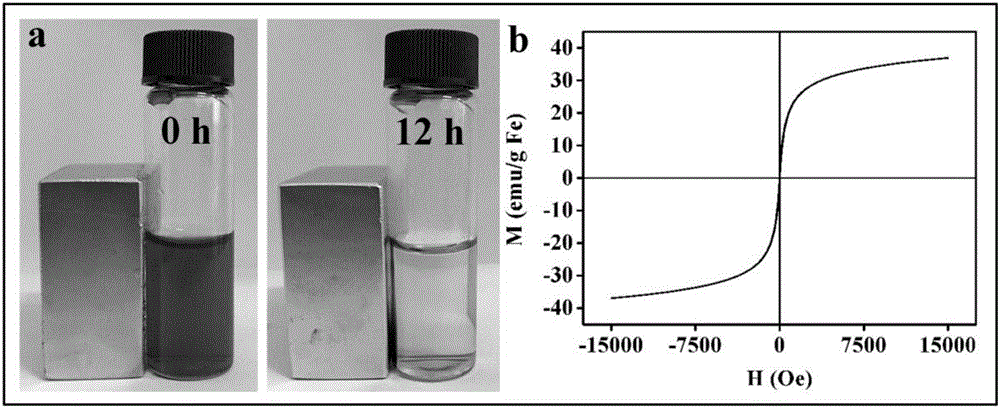
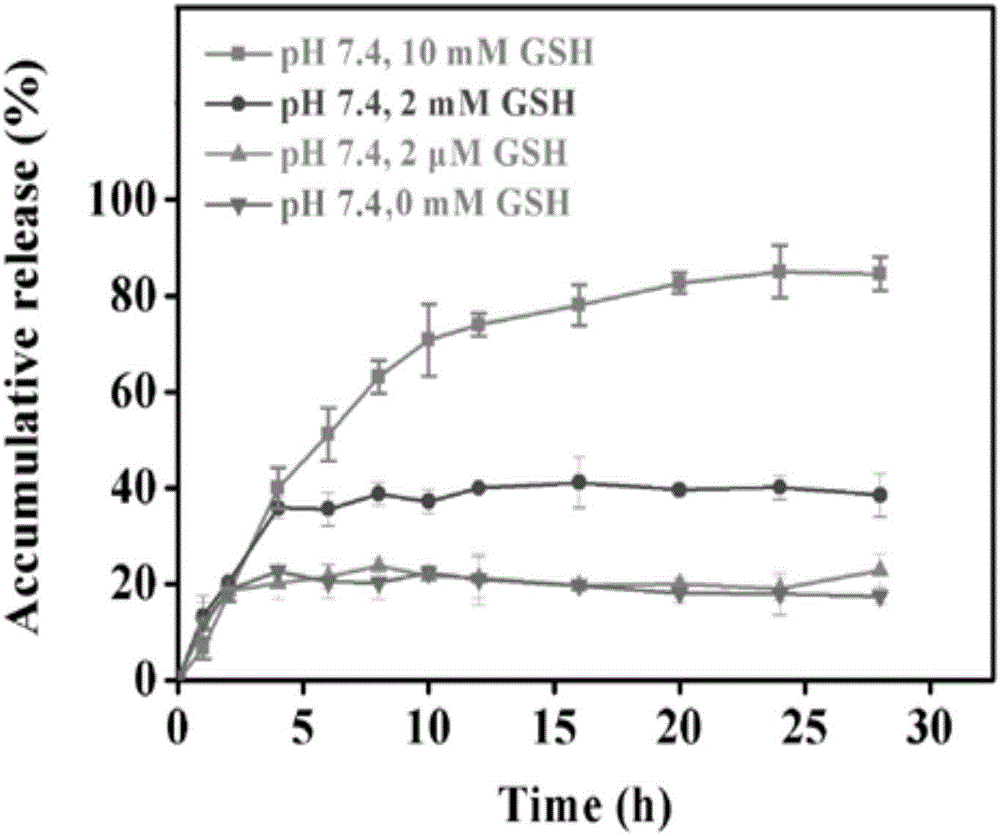
The invention relates to reducing response magnetic drug-loaded nanoparticles with synergetic anti-cancer interaction and a preparation method of the reducing response magnetic drug-loaded nanoparticles. The drug-loaded nanoparticles are prepared from a polymer prodrug and inorganic nanoparticles, which are connected through a disulfide bond; and the inorganic nanoparticles include surface-aminated superparamagnetism ferroferric oxide nanoparticles (SPION-NH2) and aminated selenium nanoparticles (NH2-R-SeNPs) employing an amino-containing high polymer as a template. The preparation method specifically comprises the following steps: (1) mixing an SPION-NH2 solution with an NH2-R-SeNPs dispersing liquid and adjusting the pH value to be 4.5-5.5; (2) dissolving the polymer prodrug connected through the disulfide bond by using ultrapure water and adjusting the pH value to be 5; and (3) dropwise adding the solution obtained in step (1) to the solution obtained in step (2), adjusting the pH value of a system to be 7.4, stirring the solution at room temperature for 10-14h to obtain the reducing response magnetic drug-loaded nanoparticles with synergetic anti-cancer interaction. The drug-loaded nanoparticles have the advantages of specific responsiveness in a targeted area, synergetic interaction and a low toxic or side effect.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
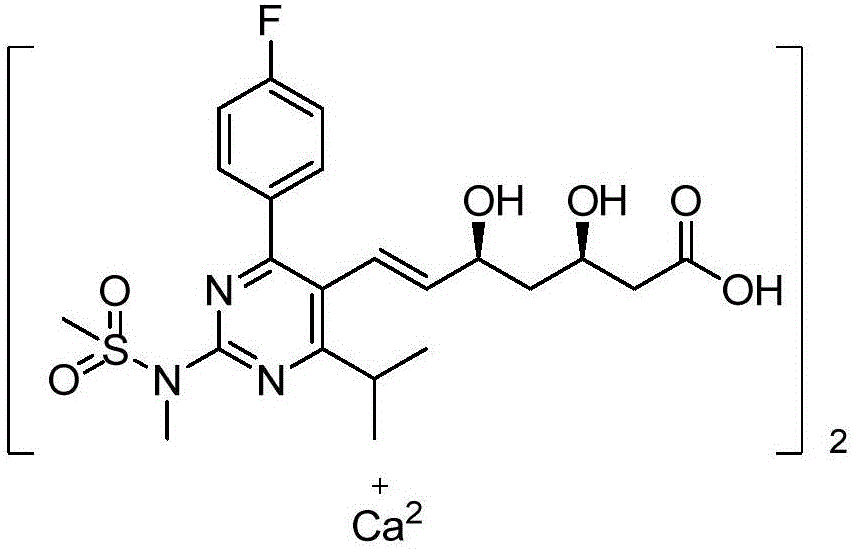
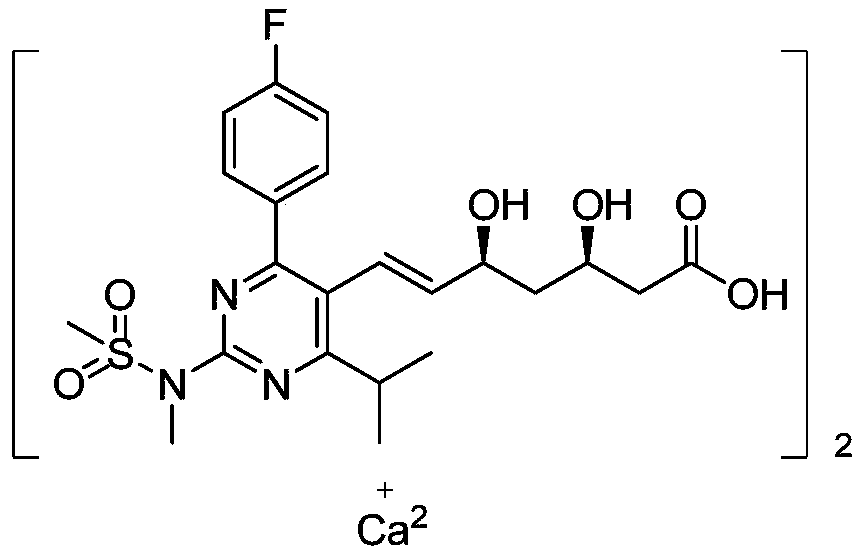
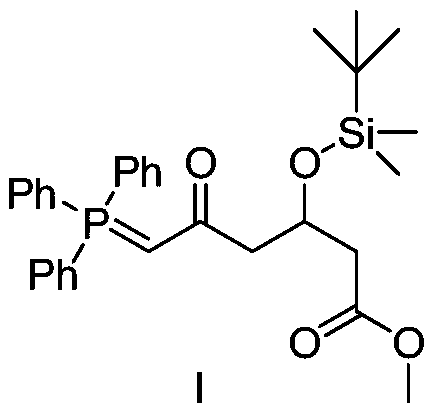
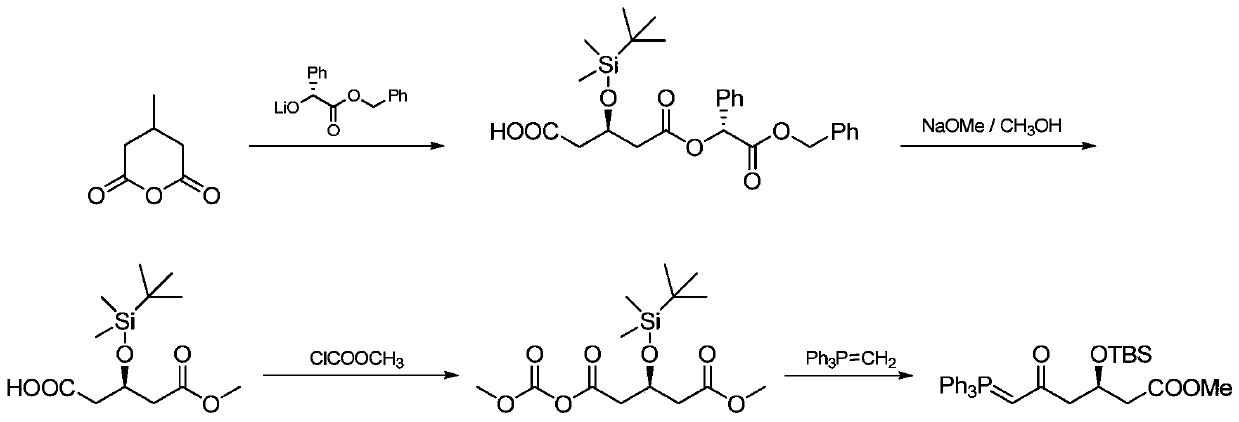
Preparation method of novel rosuvastatin calcium intermediate
ActiveCN106749032AHigh purityEasy to operateGroup 5/15 element organic compoundsBulk chemical productionOperabilityRosuvastatin Calcium
The invention provides a preparation method of a novel rosuavastatin calcium intermediate I which is suitable for industrial large-scale production. The preparation method comprises the step of enabling triphenyl methyl phosphorus bromide to react with a compound II, thus obtaining an intermediate I. The preparation method provided by the invention is safe and simple and is strong in operability, and a final finished product which is high in efficiency and purity can be obtained.
Owner:ZHEJIANG YONGTAI PHARMA
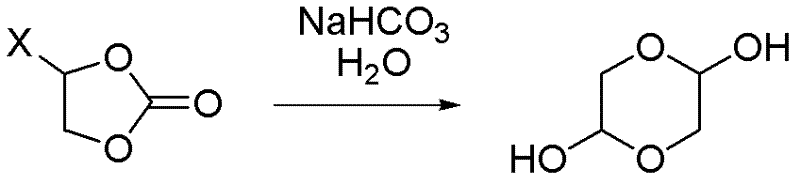
Synthetic method of 1,4-dioxane-2,5-diol
ActiveCN102351838ALow priceMeet the needs of large-scale productionOrganic chemistryChemical reactionCompound (substance)
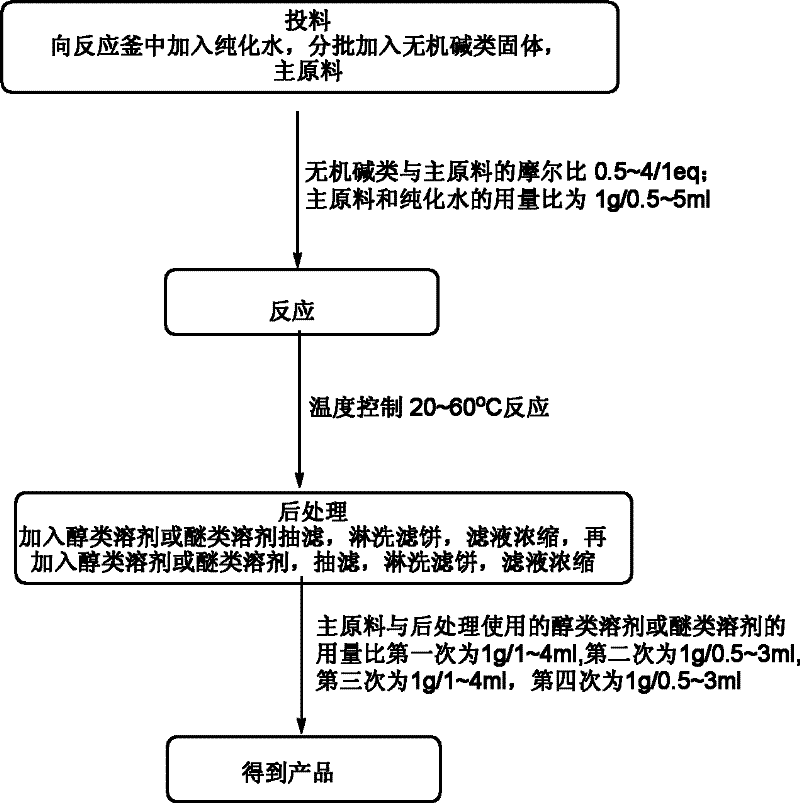
A synthetic method of 1,4-dioxane-2,5-diol is characterized in that concrete preparation comprises the following steps of: (1) feeding; (2) reacting; (3) post-processing; (4) preparation of the product. The invention has following advantages: 1, raw materials adopted in the invention are easily available and are cheap in price; the raw materials are all commercial raw materials or raw materials which are easy to prepare, and can satisfy the requirement of large scale production; 2, by the adoption of a simple hydrolysis reaction and simultaneously a batch charging method, the solvent amount is less, the energy and post-processing time are saved, the operation is simple, the synthetic method is suitable for large scale industrial production; 3, the condition of chemical reactions adopted in the invention is mild; there is no high temperature or low temperature reaction during the whole reaction process; during the production process, the technology is reasonable, the production is safe and reliable, and the cost is low; there is no environmental pollution; the synthetic method is suitable for large scale industrial production.
Owner:ASYMCHEM LAB TIANJIN +1
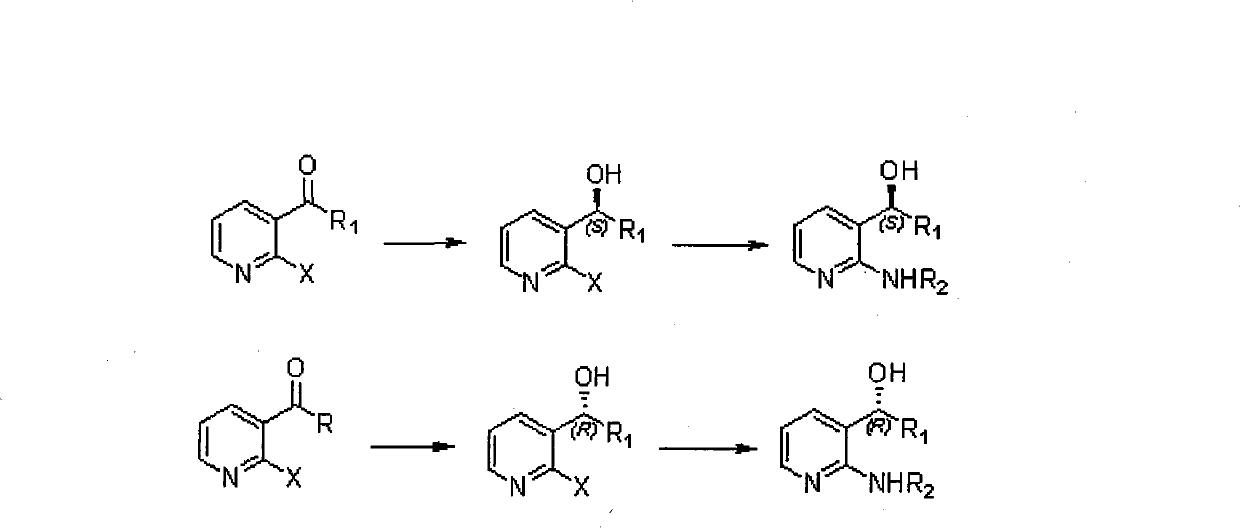
Method for synthesizing derivatives of chiral pyridyl aminoalcohols, and intermediate products and final products of same
ActiveCN101519374ALow priceMeet the needs of large-scale productionOrganic chemistryAlcoholChemical reaction
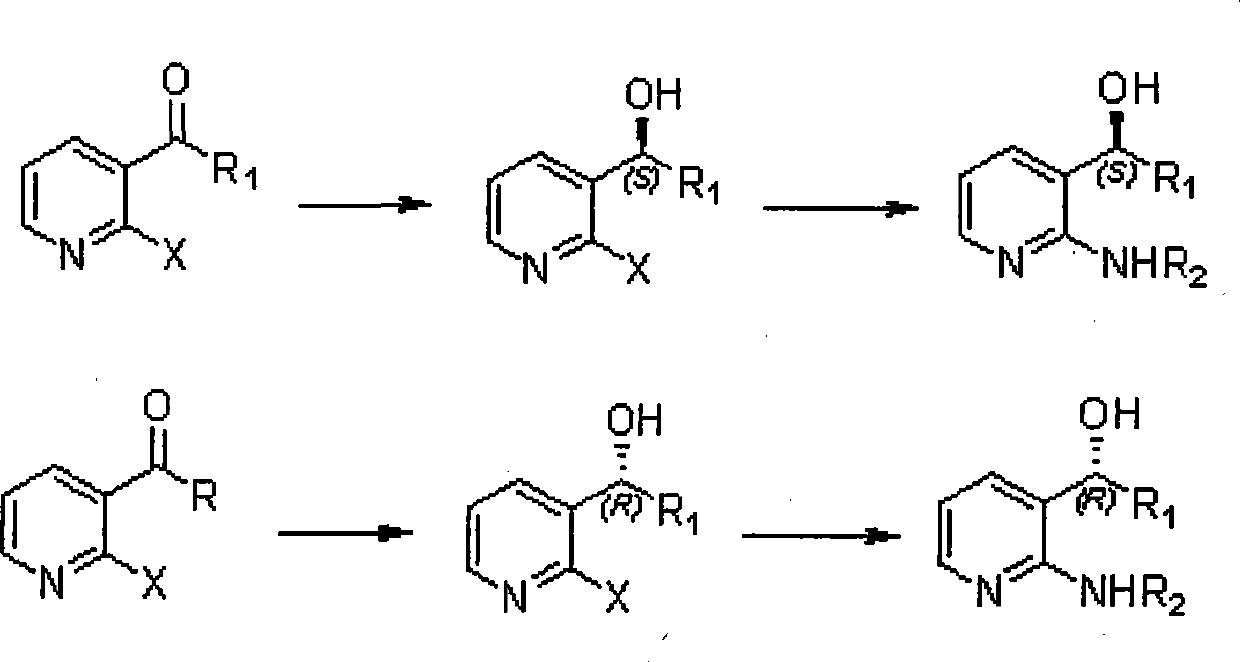
The invention relates to a method for synthesizing the derivatives of chiral pyridyl aminoalcohols, and intermediate products and final products of the same. Initial materials are selected from commercial raw materials on the market or easy-to-prepare raw materials of halogenated pyridine alkyl ketone or aromatic ring ketones, wherein X is F or Cl, R1 is C1 to C8 alkyls or C3 to C8 cycloalkyls; and final products are obtained through a process of chemical reactions under mild conditions, wherein R1 is C1 to C8 alkyls or C3 to C8 naphthene base, R2 is H or C1 to C8 alkyls, or C3 to C8 cycloalkyls or C7 to C9 benzyls, and chiral centers of alcohols have an S or R structure. The method provides a new thought and means for preparing the derivatives of chiral pyridyl aminoalcohols; the intermediate products are that the chiral centers of alcohols have an S or R structure; and the final products are that the chiral centers of alcohols have an S or R structure.
Owner:ASYMCHEM LAB TIANJIN +3
Monomer for identifying halogen anions, polymer and preparation method of monomer and polymer
InactiveCN104557601ASimple technical routeReduce consumptionMaterial analysis by observing effect on chemical indicatorOrganic compound preparationBenzoic acidPolymer science
The invention discloses a monomer N-(p-acetenyl)-phenyl-2-X-tetrafluorobenzamide (X=F, Cl, Br and I) for identifying halogen anions, a polymer, and a method for preparing the monomer and the polymer. The method comprises the following steps: by taking 4-ethynylaniline and hexafluorobenzoic acid as main initial raw materials, taking N,N-dimethyl-4-aminopyridine as a catalyst, finally synthesizing a target small molecule compound N-(p-acetenyl)-phenyl-2-X-tetrafluorobenzamide; and by taking a precious metal complex Rh+(2,5-nbd)[(eta6-C6H5)B-(C6H5)3] (triphenyl-eta6-phenylboron-2,5-norborneol diene rhodium, Rh(nbd)BPh4) as a catalyst, thereby obtaining the poly-N-(p-acetenyl)-phenyl-2-X-tetrafluorobenzamide by virtue of a coordination polymer orientation method. The obtained small molecule monomer and polymer molecular structures simultaneously contain hydrogen bond donors and halogen bond donors, and the monomer and polymer can identify the halogen anions by virtue of coordination of hydrogen bonds and halogen bonds. Meanwhile, the preparation method has the advantages of the reaction conditions are mild, the reaction process is simple, the method is easy to control, the yield of the obtained product is high and the like.
Owner:TIANSHUI NORMAL UNIV
Monomer with anion recognizing function, oligomer and preparation method thereof
InactiveCN104672108AReduce energy consumptionReaction Process SafetyUrea derivatives preparationOrganic compound preparationPhenyl groupPara-nitrophenyl
The invention discloses a N-p-nitrophenyl-N-(p-acetenyl-2-pentafluorobenzoic acid benzoyloxycarbonyl)-phenyl-urea monomer, an oligomer and a preparation method thereof. As a target product of the technology, the N-p-nitrophenyl-N-(p-acetenyl-2-pentafluorobenzoic acid benzoyloxycarbonyl)-phenyl-urea and the oligomer structure have urea structure units which can be used as hydrogen-bond donors, also have aromatic-ring structure units which can be used as anion-n donors, and when the urea structure units and the aromatic-ring structure units are combined with the anions, the synergy on the space geometry is achieved, i.e., the N-p-nitrophenyl-N-(p-acetenyl-2-pentafluorobenzoic acid benzoyloxycarbonyl)-phenyl-urea can recognize anions by the synergistic effect of two weak bonds such as hydrogen bonds and anion-n simultaneously, the target-product oligomer of the N-p-nitrophenyl-N-(p-acetenyl-2-pentafluorobenzoic acid benzoyloxycarbonyl)-phenyl-urea has the same space geometrical structure with the monomer thereof, i.e., a plurality of anions also can be recognized and combined by the synergistic effect of the two weak bonds such as the hydrogen bonds and the anion-n.
Owner:TIANSHUI NORMAL UNIV
Method for preparing ticagrelor solution
InactiveCN105859720AGood physical propertiesPromote crystallizationOrganic active ingredientsPharmaceutical delivery mechanismSolventBy-product
The invention discloses a method for preparing a ticagrelor solution. According to the invention, hydroxy of a ticagrelor I crude product is protected by a protective group, then a crude product of a compound II is separated and purified to obtain the high purity compound II; the hydroxy protective group of the high purity compound II is removed under proper reaction conditions, a reaction mixture is post-processed and then the by-product is removed to obtain the high purity ticagrelor I, the ticagrelor I, a solvent or / and pharmaceutic adjuvant form a solution, after the solvent is removed, ticagrelor or a mixture containing ticagrelor and accessory with medicinal requirement can be obtained. The method has the advantages of mild reaction condition, simple operation, low cost, and environmental protection, and is suitable for industrial production.
Owner:SHANGHAI FANGNAN PHARMA
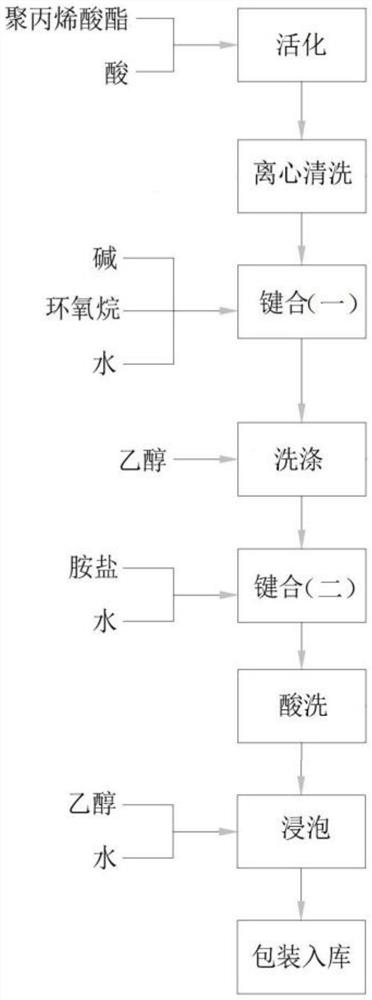
Amine salt type polyacrylate emulsion and preparation method thereof
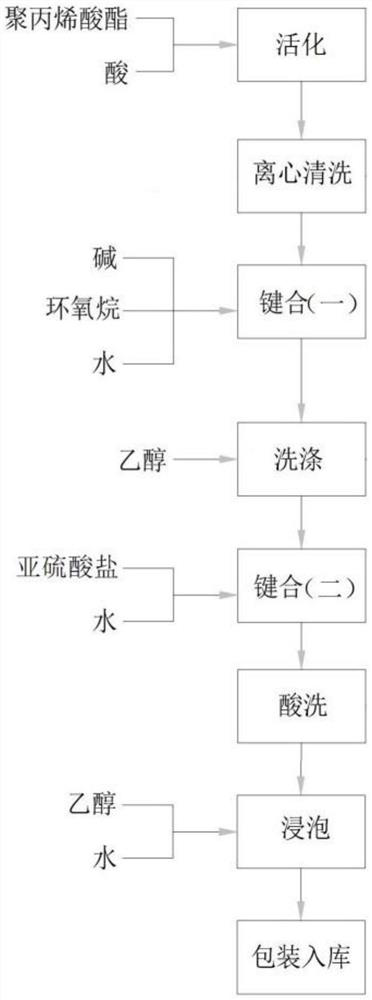
The invention provides a preparation method of an amine salt type polyacrylate emulsion, which comprises the following steps: activating polyacrylate-based spheres, carrying out two-time bonding reaction on the activated microspheres, and carrying out repeated cleaning and solution washing treatment to finally obtain the amine salt type polyacrylate emulsion. According to the preparation method, reaction is carried out in a water phase system, the reaction temperature is mild, and the pressure is close to normal pressure, so that reaction condition control is easily realized; the amine salt type polyacrylate emulsion obtained by the preparation method is uniform in particle size, the particle size is 15-90 [mu] m, the pore size is that the amine salt type polyacrylate emulsion has better hydrophilicity, non-specific adsorption with biological samples is avoided to the greatest extent, compared with other hydrophobic fillers, the pore size is larger, and the hydrophobic filler has better hydrophobic property. The method is more suitable for separation and purification of biological samples with larger molecular weight; meanwhile, the preparation method is simple in process, and the used raw materials are easy to obtain, so that the production cost is low.
Owner:赛分科技扬州有限公司
Synthesis method of chiral epoxy compound and intermediate products and final product
ActiveCN101691338BLow priceReduce generationOrganic compound preparationCarboxylic acid amides preparationEpoxyPtru catalyst
The invention discloses a synthesis method of a chiral epoxy compound and intermediate products and a final product beta-amino acid derivative. The chiral epoxy compound is obtained by utilizing commercialized raw materials in markets as initial raw materials and then obtaining a chiral intermediate in the process of chemical reaction under mild conditions. In the method, the novel chiral catalyst method is utilized, a great amount of novel chiral catalyst ligand is synthesized by adopting the method in documents, epoxidation is carried out on olefin and then olefin is applied to the synthesis of the beta-amino acid derivative, thereby improving the use ratio of raw materials and optimizing the process from the origin. Epoxidation is carried out on unsaturated amide by the novel chiral ligand, and then open-loop deprotection is carried out to obtain the product. The value of product obtained by oxidation reaction is 80-99%, the yield coefficient thereof is 80-95%, and the chemical purity thereof is 90-99%, therefore, the chiral epoxy compound can be used for mass production and has very good industrial value.
Owner:ASYMCHEM LIFE SCI TIANJIN
Sulfated polyacrylate emulsion and preparation method thereof
The invention provides a preparation method of sulfated polyacrylate emulsion, which comprises the following steps: activating polyacrylate-based spheres, carrying out two-time bonding reaction on the activated microspheres, and carrying out repeated cleaning and solution washing treatment to finally obtain the sulfated polyacrylate emulsion. According to the preparation method, reaction is carried out in a water phase system, the reaction temperature is mild, and the pressure is close to normal pressure, so that reaction condition control is easily realized; the sulfated polyacrylate emulsion obtained through the preparation method is uniform in particle size, the particle size ranges from 15 micrometers to 90 micrometers, the pore diameter is as follows: the sulfated polyacrylate emulsion has better hydrophilicity, non-specific adsorption with biological samples is avoided to the maximum extent, compared with other hydrophobic filler, the pore diameter is larger, and the hydrophobicity is better than that of other hydrophobic filler. The method is more suitable for separation and purification of biological samples with larger molecular weight; meanwhile, the preparation method is simple in process, and the used raw materials are easy to obtain, so that the production cost is low.
Owner:赛分科技扬州有限公司
Synthetic method of glycol mono-hydrogenated nopyl ether and carboxylic ester thereof and application thereof
ActiveCN108084022AReduce pollutionHigh yieldBiocideOrganic compound preparationVinyl etherChemical reaction
The invention discloses a synthetic method of glycol mono-hydrogenated nopyl ether and carboxylic ester thereof and application thereof. The method comprises the following steps of adding hydrogenatednopyl halide and glycol into organic solvents; performing backflow reaction under the effect of hydroxide; after the reaction is completed, performing washing, drying, solvent recovery and vacuum distillation to obtain glycol mono-hydrogenated nopyl ether; then, performing backflow reaction on glycol mono-hydrogenated nopyl ether and carboxylic acid under the participation of catalysts and watercarrying agents; performing synthesis to obtain five kinds of glycol mono-hydrogenated nopyl ether carboxylic ester. The synthesized glycol mono-hydrogenated nopyl ether and the five kinds of glycol mono-hydrogenated nopyl ether carboxylic ester can be used for inhibiting plant pathogenic fungi. The compound has the advantages of safety, nontoxicity, low environment pollution and the like. The chemical reaction conditions are mild; the equipment is simple; the operation is simple and convenient; the product yield is high; the purity is high.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
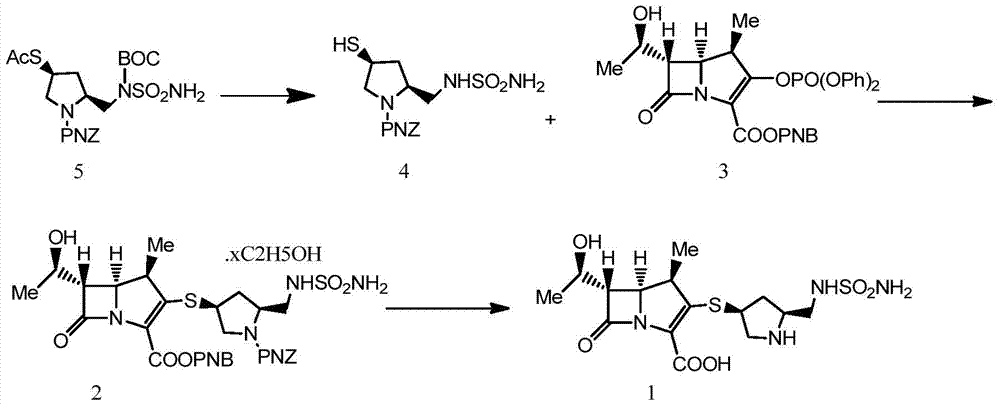
A kind of preparation method of doripenem
The invention belongs to the field of medicine synthesis and particularly relates to a doripenem preparation method. The method includes the steps that a compound 5 reacts with concentrated sulfuric acid in methanol to obtain a compound 4; the compound 4 and p-Nitrobenzyl-6-(1-hydroxyethyl)-1-azabicyclo(3.2.0)heptane-3,7-dione-2-carboxylate (a compound 3) are subjected to a condensation reaction under the condition that N,N-diisopropylethylamine exists, water and ethyl acetate are added and stirred after the reaction, an ethyl acetate layer is collected, alcohol is added in ethyl acetate collection liquid, crystallization is carried out, and a compound 2 is obtained; the product is dissolved through ethyl acetate; after a monopotassium phosphate solution and a phase transfer reagent of triethylbenzylammonium chloride are added, zinc powder is added into the ethyl acetate / monopotassium phosphate solution in batches to react and obtain doripenem. According to the method, reaction conditions are moderate, the technology is simple, and the conversion rate and the yield are high. Two-phase reaction is used in deprotection reaction, and the after-treatment process is simplified. The zinc powder which is cheap is used, so that the method is economical, and a new concept and a new method are provided for doripenem preparation.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Synthetic method of 1,4-dioxane-2,5-diol
ActiveCN102351838BLow priceMeet the needs of large-scale productionOrganic chemistryChemical reactionCompound (substance)
A synthetic method of 1,4-dioxane-2,5-diol is characterized in that concrete preparation comprises the following steps of: (1) feeding; (2) reacting; (3) post-processing; (4) preparation of the product. The invention has following advantages: 1, raw materials adopted in the invention are easily available and are cheap in price; the raw materials are all commercial raw materials or raw materials which are easy to prepare, and can satisfy the requirement of large scale production; 2, by the adoption of a simple hydrolysis reaction and simultaneously a batch charging method, the solvent amount is less, the energy and post-processing time are saved, the operation is simple, the synthetic method is suitable for large scale industrial production; 3, the condition of chemical reactions adopted in the invention is mild; there is no high temperature or low temperature reaction during the whole reaction process; during the production process, the technology is reasonable, the production is safe and reliable, and the cost is low; there is no environmental pollution; the synthetic method is suitable for large scale industrial production.
Owner:ASYMCHEM LAB TIANJIN +1
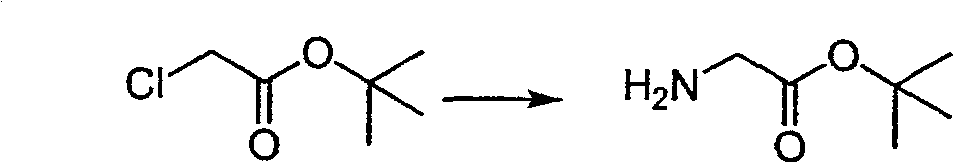
Production technique of tert-butyl glycinate adapted for industrial production
ActiveCN100560564CEasy to getLow priceOrganic compound preparationAmino-carboxyl compound preparationGlycineAcetic acid
Owner:ASYMCHEM LAB FUXIN
A kind of preparation method of novel rosuvastatin calcium intermediate
ActiveCN106749032BHigh purityEasy to operateGroup 5/15 element organic compoundsBulk chemical productionOperabilityRosuvastatin Calcium
The invention provides a preparation method of a novel rosuavastatin calcium intermediate I which is suitable for industrial large-scale production. The preparation method comprises the step of enabling triphenyl methyl phosphorus bromide to react with a compound II, thus obtaining an intermediate I. The preparation method provided by the invention is safe and simple and is strong in operability, and a final finished product which is high in efficiency and purity can be obtained.
Owner:ZHEJIANG YONGTAI PHARMA
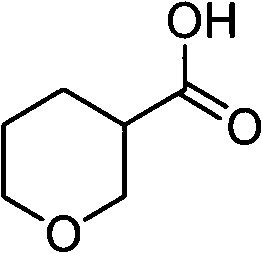
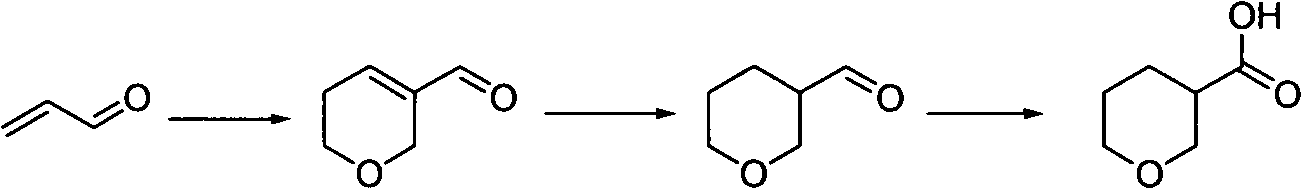
Tetrahydropyrane-3-formic acid preparation method
InactiveCN103420964ARaw materials are cheap and easy to getReduce manufacturing costOrganic chemistryHydrogenMetal catalyst
The present invention discloses a tetrahydropyrane-3-formic acid preparation method, wherein acrolein is adopted as a raw material, and the preparation method comprises the following three steps: (1) acrolein is subjected to a ring closure reaction under an acid effect to obtain 5,6-dihydro-2-H pyran-3-formaldehyde, (2) the 5,6-dihydro-2-H pyran-3-formaldehyde and hydrogen are subjected to a reduction reaction under a metal catalyst effect to obtain tetrahydropyrane-3 formaldehyde, and (3) the tetrahydropyrane-3 formaldehyde and a Jones reagent are subjected to an oxidation reaction to obtain tetrahydropyrane-3-formic acid.
Owner:ASCEPION PHARMA
A kind of preparation method and application of thermosetting epoxy resin shape memory polymer
ActiveCN110305297BThe catalytic effect of the reaction is goodImprove catalytic performanceEpoxyPolymer science
The invention discloses a preparation method and application of a thermosetting epoxy resin shape memory polymer. The method comprises the steps of reacting epoxy resin, thiocyanic acid and a basic catalyst under vacuum conditions at 80 DEG C to 150 DEG C; followed by natural cooling to obtain a thermosetting epoxy resin shape memory polymer. The invention also provides a preparation method of a thermosetting epoxy resin shape-memory polymer material and a shape-memory restoration method. The thermosetting epoxy resin shape-memory polymer prepared by the method of the invention has good chemical stability and thermal stability, exhibits good shape-memory effect when its glass transition temperature is above its glass transition temperature, and has good mechanical properties.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
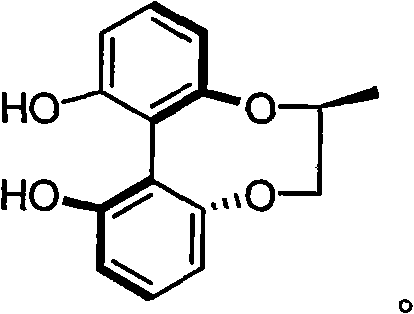
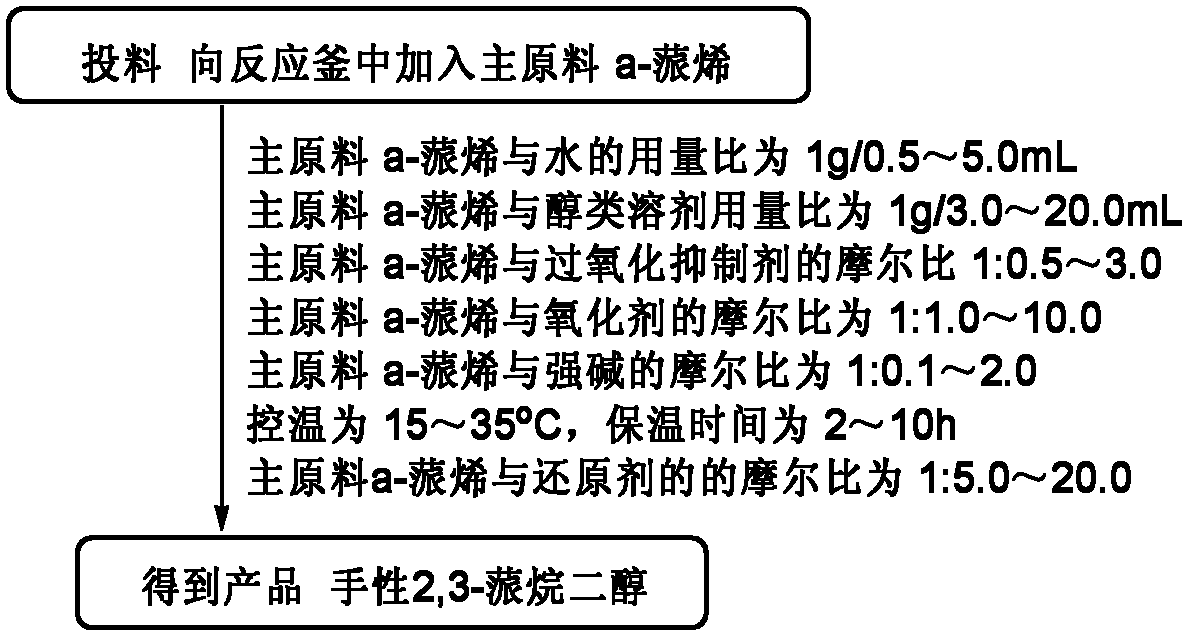
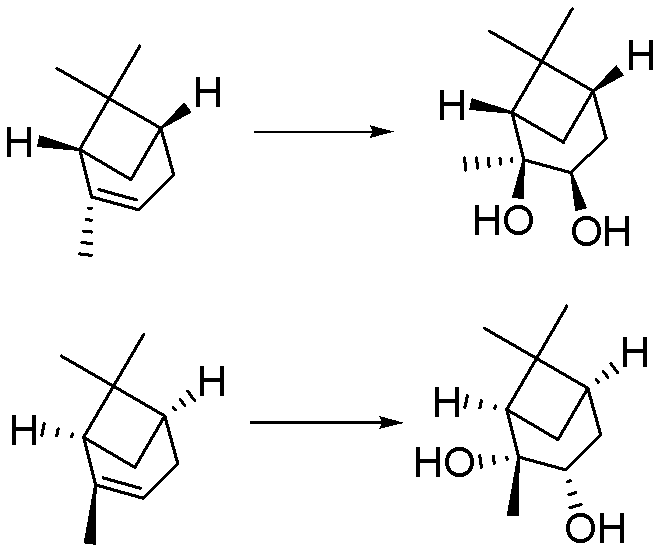
Synthesis method of chiral 2,3-pinanediol
ActiveCN102584538BReduce generationImprove protectionPreparation by oxidation reactionsChemical reactionEnantiomer
The invention relates to a synthesis method of chiral 2,3-pinanediol. The structural formula of chiral 2,3-pinanediol is showed in the specification. The synthesis method comprises the step of adding an oxidation inhibitor and alkali liquor containing an oxidant in alpha-pinene which is commercialized in the market and selected as a starting material for reaction so that a final product, namely chiral 2,3-pinanediol, can be obtained. According to the method, a target product with high liquid phase purity and high ee (enantiomeric excess) value of a product enantiomer can be obtained, wherein the liquid phase purity is stabilized above 98%, the ee value of the enantiomer is stabilized above 99% and the yield is 60.7-76.6%. According to the synthesis method, the materials are easy to obtain, the price is cheap, the chemical reaction condition is mild during the production process, the technical condition is stable, the operation is simple, pollution is less; the synthesis method is suitable for on-scale production, and a new idea and method can be provided for preparing chiral 2,3-pinanediol.
Owner:ASYMCHEM LAB TIANJIN +4
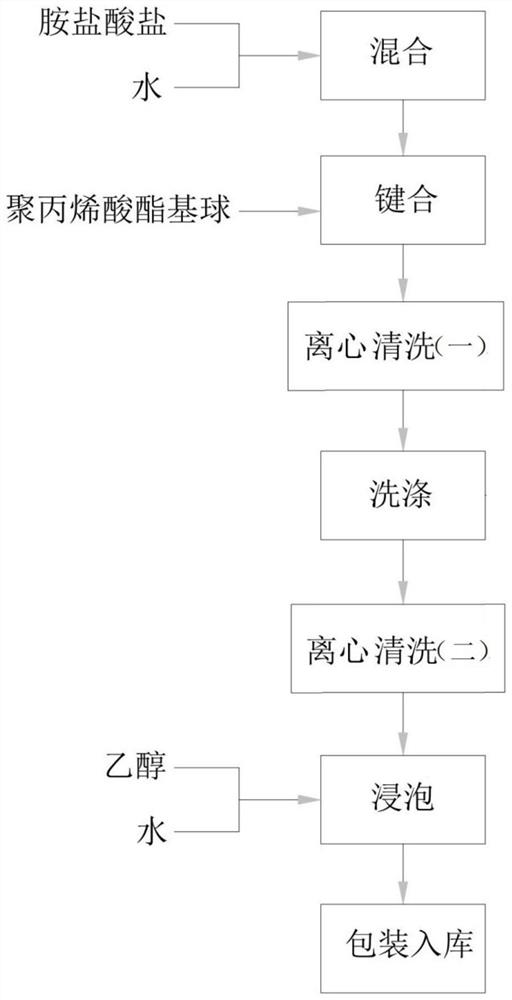
Polyacrylate-based ball and amine salt modification method thereof
PendingCN114262465ASimple processSimple ingredientsOther chemical processesAlkali metal oxides/hydroxidesChemistryNon specific adsorption
The invention provides an amine salt modification method for polyacrylate-based balls, which comprises the following steps: carrying out bonding reaction on activated polyacrylate-based balls, and carrying out repeated cleaning and solution washing treatment to finally obtain a polyacrylate emulsion. According to the amine salt modification method, the reaction is carried out in a water phase system, the reaction temperature is mild, the pressure is close to normal pressure, so that the reaction condition control is easily realized, the particle size of the obtained emulsion is 15-90 microns, the pore diameter is that the polyacrylate emulsion has better hydrophilicity, non-specific adsorption with biological samples is avoided to the greatest extent, and the application prospect is wide. Compared with other hydrophobic fillers, the hydrophobic filler has larger pore diameter and is more suitable for separation and purification of biological samples with larger molecular weight; meanwhile, the amine salt modification method is simple in process, and the used raw materials are easy to obtain, so that the production cost is relatively low.
Owner:赛分科技扬州有限公司
Monomers and polymers for recognition of halide anions and methods of preparation
InactiveCN104557601BMild chemical reaction conditionsSimple technical routeMaterial analysis by observing effect on chemical indicatorOrganic compound preparationPolymer science4-Aminopyridine
The invention discloses a monomer N-(p-acetenyl)-phenyl-2-X-tetrafluorobenzamide (X=F, Cl, Br and I) for identifying halogen anions, a polymer, and a method for preparing the monomer and the polymer. The method comprises the following steps: by taking 4-ethynylaniline and hexafluorobenzoic acid as main initial raw materials, taking N,N-dimethyl-4-aminopyridine as a catalyst, finally synthesizing a target small molecule compound N-(p-acetenyl)-phenyl-2-X-tetrafluorobenzamide; and by taking a precious metal complex Rh+(2,5-nbd)[(eta6-C6H5)B-(C6H5)3] (triphenyl-eta6-phenylboron-2,5-norborneol diene rhodium, Rh(nbd)BPh4) as a catalyst, thereby obtaining the poly-N-(p-acetenyl)-phenyl-2-X-tetrafluorobenzamide by virtue of a coordination polymer orientation method. The obtained small molecule monomer and polymer molecular structures simultaneously contain hydrogen bond donors and halogen bond donors, and the monomer and polymer can identify the halogen anions by virtue of coordination of hydrogen bonds and halogen bonds. Meanwhile, the preparation method has the advantages of the reaction conditions are mild, the reaction process is simple, the method is easy to control, the yield of the obtained product is high and the like.
Owner:TIANSHUI NORMAL UNIV
Method for synthesizing derivatives of chiral pyridyl aminoalcohols, and intermediate products and final products of same
ActiveCN101519374BLow priceMeet the needs of large-scale productionOrganic chemistryChemical reactionAlcohol
The invention relates to a method for synthesizing the derivatives of chiral pyridyl aminoalcohols, and intermediate products and final products of the same. Initial materials are selected from commercial raw materials on the market or easy-to-prepare raw materials of halogenated pyridine alkyl ketone or aromatic ring ketones, wherein X is F or Cl, R1 is C1 to C8 alkyls or C3 to C8 cycloalkyls; and final products are obtained through a process of chemical reactions under mild conditions, wherein R1 is C1 to C8 alkyls or C3 to C8 naphthene base, R2 is H or C1 to C8 alkyls, or C3 to C8 cycloalkyls or C7 to C9 benzyls, and chiral centers of alcohols have an S or R structure. The method provides a new thought and means for preparing the derivatives of chiral pyridyl aminoalcohols; the intermediate products are that the chiral centers of alcohols have an S or R structure; and the final products are that the chiral centers of alcohols have an S or R structure.
Owner:ASYMCHEM LAB TIANJIN +3
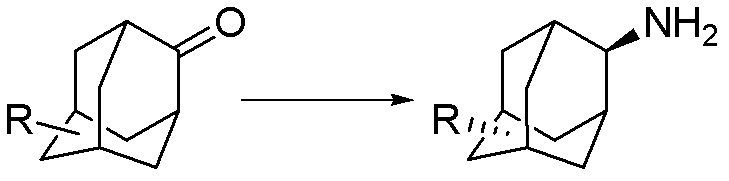
Synthesis method for trans-form amantadine compound
ActiveCN102503754BReduce dosageHigh purityOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsAmantadine
The invention relates to a synthesis method for a trans-form amantadine compound, which includes the steps of utilizing commercialize raw materials on the market or an easily prepared amantadine compound as initial materials, and carrying out reductive amination reaction between the initial raw materials and liquid ammonia under the action of a reductive agent and a catalyst, so that 100% of the trans-form amantadine compound, (wherein R refers to -H, -COOH, -OH, -SO2NH2, Cl and groups shown in the description) is obtained. The method has the advantages that raw materials are easy to obtain, both reaction purity and yield are high, loss of the trans-form amantadine compound is decreased by salifying for final treatment, production cost is reduced, and the method is applicable to large-scale production and provides a novel thinking for preparing the trans-form amantadine compound.
Owner:ASYMCHEM LAB TIANJIN +4
Vildagliptin preparation method
InactiveCN104844494ALow priceMeet the needs of large-scale productionOrganic chemistryAcetonitrileVildagliptin
The invention discloses a vildagliptin preparation method, and relates to the technical field of preparation of pyrrolidine heterocyclic compounds. The method comprises the following steps: 1, preparing an acetonitrile solution of S-1-chloroacetyl-2-cyanpyrrolidine; 2, adding acetonitrile into a reaction kettle, stirring, adding 3-amino-1-adamantanol, potassium carbonate and potassium iodide, heating, adding the acetonitrile solution of S-1-chloroacetyl-2-cyanpyrrolidine in a dropwise manner, and carrying out a heat insulation reaction after the dropwise addition until the reaction is completely carried out; and 3, post-processing: cooling, stirring, centrifuging, collecting the obtained filter cake, adding dichloromethane into the kettle, stirring, adding the filter cake, stirring, centrifuging, collecting the obtained filtrate, concentrating the filtrate to obtain a concentrate, re-crystallizing the concentrate by using isopropanol to obtain a crude product, carrying out hot washing on the crude product by using isopropanol, cooling, and centrifuging to obtain a product. The product is obtained through the one-step reaction by using the method, the yield is high, the chemical purity and the chiral purity are respectively greater than 99%, and the method has the advantages of short reaction time, low energy consumption and simple operation.
Owner:CANGZHOU SENARY CHEM SCI TEC
Method for synthesizing derivative of beta-amino acid and intermediate product thereof
ActiveCN101186587BLow priceMeet the needs of large-scale productionOrganic chemistryChemical reactionSynthesis methods
The invention provides a synthesis method of beta-amino acid derivative and a relative intermediate product, in particular to a synthesis method of the formula (I) and a relative intermediate product.The invention is characterized in that the invention uses commercialized material as formula (II) (trans-, E-type) as initial material, to obtain final product which formula is (III) via chemical reaction with mild condition. The invention has easily accessible starting material and stable technological condition, and is suitable for scaled industrial production.
Owner:ASYMCHEM LAB FUXIN
Synthesizing method, partial intermediate products and final products of chiral beta-alkamine derivative
ActiveCN101717341BLow priceMeet the needs of large-scale productionOrganic compound preparationAmino-hyroxy compound preparationArylChemical reaction
The invention relates to a synthesizing method, partial intermediate products and final products of a chiral beta-alkamine derivative. The synthesizing method of the chiral beta-alkamine derivative is characterized by comprising the steps of: selecting commercialized materials in the market and NH2R2 as initial materials, wherein R2 is a Cl-C6 alkyl group, a C3-C6 naphthenic base and an aryl group or an aryloxy; obtaining the intermediate products and the final products through a chemical reaction process with moderate conditions, wherein R1 and R2 are the Cl-C6 alkyl group, the C3-C6 naphthenic base and the aryl group or the aryloxy, and a chiral center is in an S or R shape. The invention has the advantages that the adopted materials are easy to obtain and at low price and can meet the requirements of large-scale production, chiral compounds are used as the initial materials, optical purity is retained in consequent reaction without finding racemization, and committed steps accord with the requirements of the current green chemistry; in addition, the invention has the advantages of simple synthesizing method, good selectivity, high yield coefficient, easy operation and good market benefits.
Owner:ASYMCHEM LIFE SCI TIANJIN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com