Monomer for identifying halogen anions, polymer and preparation method of monomer and polymer
An anion and polymer technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylic acid amides, etc., can solve problems such as lack of synergistic identification of anions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
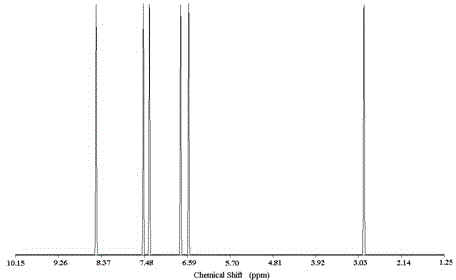
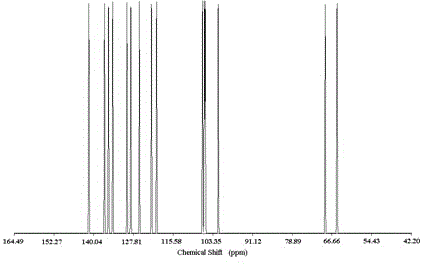
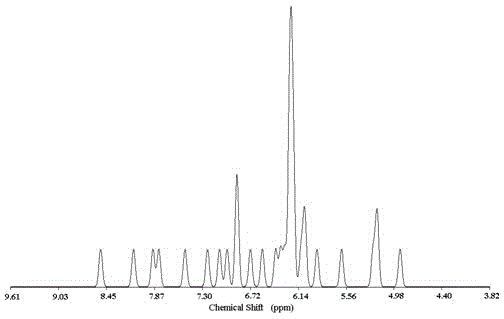
Image
Examples
Embodiment 1
[0055] Dissolve 1.0 mmol of p-aminophenylacetylene, 1.3 mmol of pentafluorobenzoic acid and 0.2 mmol of N,N-dimethyl-4-aminopyridine in 5 ml of anhydrous CH 2 Cl 2 In, then add 1.5mmol of dimethylaminopropyl ethyl carbonamide hydrochloride (EDC-HCl), 23 DEG C of stirring reaction 24h. react with CH 2 Cl 2 as a solvent, the reaction system was washed with water, the organic layer was separated, and the aqueous layer was washed with CH 2 Cl 2 Extracted 3 times, the obtained organic layers were combined and washed with concentrated brine, the organic layer was separated and washed with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated by distillation under reduced pressure. Using ethyl acetate as the eluent, the crude product concentrate was separated and purified on silica gel column chromatography to obtain the target product N -(p-ethynyl)-phenyl-2-X-tetrafluorobenzamide (pale yellow crystals, yield 92%).
[0056] Under the protection of Ar ...
Embodiment 2
[0058] Dissolve 1.0 mmol of p-aminophenylacetylene, 1.3 mmol of 2-chloro-tetrafluorobenzoic acid and 0.2 mmol of N,N-dimethyl-4-aminopyridine in 5 ml of anhydrous CH 2 Cl 2 In, then add 1.5mmol of dimethylaminopropyl ethyl carbonamide hydrochloride (EDC-HCl), 25 DEG C of stirring reaction 26h. react with CH 2 Cl 2 as a solvent, the reaction system was washed with water, the organic layer was separated, and the aqueous layer was washed with CH 2 Cl 2 Extracted 3 times, the obtained organic layers were combined and washed with concentrated brine, the organic layer was separated and washed with anhydrous Na 2 SO 4After drying and filtering, the filtrate was concentrated by distillation under reduced pressure. Using ethyl acetate as the eluent, the crude product concentrate was separated and purified on silica gel column chromatography to obtain the target product N -(p-ethynyl)-phenyl-2-chloro-tetrafluorobenzamide (pale yellow crystals, yield 92%).
[0059] Under the prot...
Embodiment 3
[0061] Dissolve 1.0 mmol of p-aminophenylacetylene, 1.3 mmol of 2-I-tetrafluorobenzoic acid and 0.2 mmol of N,N-dimethyl-4-aminopyridine in 5 ml of anhydrous THF, and then add 1.5 mmol of di Methylaminopropyl ethyl carbonamide hydrochloride (EDC-HCl), stirred at 23°C for 30h. The reaction used THF as a solvent, distilled the reaction system under reduced pressure, diluted the distillation residue with 50 ml of ethyl acetate, washed with 20 ml of water and 20 ml of hydrochloric acid (1 M aq. Water Na 2 SO 4 After drying and filtering, the filtrate was concentrated by distillation under reduced pressure. Using ethyl acetate as the eluent, the crude product concentrate was separated and purified on silica gel column chromatography to obtain the target product N -(p-ethynyl)-phenyl-2-X-tetrafluorobenzamide (pale yellow crystals, about 92.5% yield).
[0062] Under the protection of Ar gas, the 2.69mmol N -(p-ethynyl)-phenyl-2-I-tetrafluorobenzamide monomer was dissolved in a S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com