Method for preparing mesoporous boron nitride with uniform pores by adopting molten mass bubble template process
A template method and boron nitride technology, applied in chemical instruments and methods, nitrogen compounds, inorganic chemistry, etc., can solve problems such as complex process, template residue, and difficulty in obtaining regular mesoporous boron nitride materials, and achieve a simple technical route , Reagents are cheap and easy to obtain, and the technical difficulty is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take 100 grams of boric acid, 400 grams of urea and 1 gram of ferric nitrate and mix them evenly, raise the temperature in an electric furnace to 300 degrees Celsius at a rate of 10 degrees Celsius / min and keep it for 20 minutes to obtain a precursor, and then cure it at 700 degrees Celsius for 1 minute. Put the precursor into the graphite boat nitrogen-hydrogen mixture (5wt% H 2 / N 2 ) under the protection of the atmosphere, the temperature was raised to 1000°C at a heating rate of 10°C / min, and the crude product was obtained by keeping the temperature for 2 hours. Then the crude product was placed in 500 ml of 0.5 mol / L hydrochloric acid and stirred at 60°C for 2 hours. The product was filtered, washed with deionized water and ethanol, and dried for 12 hours. 40 g of product were obtained.
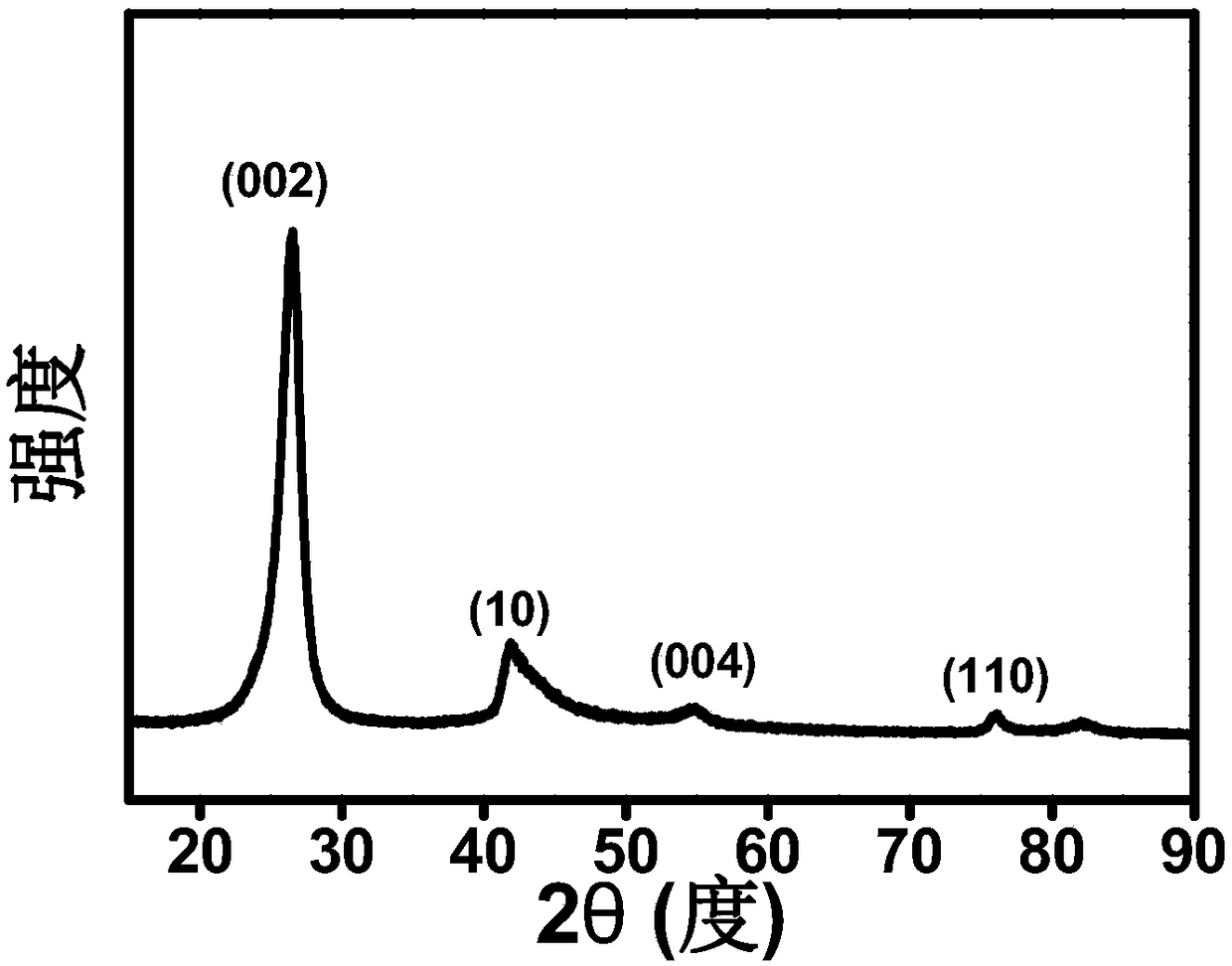
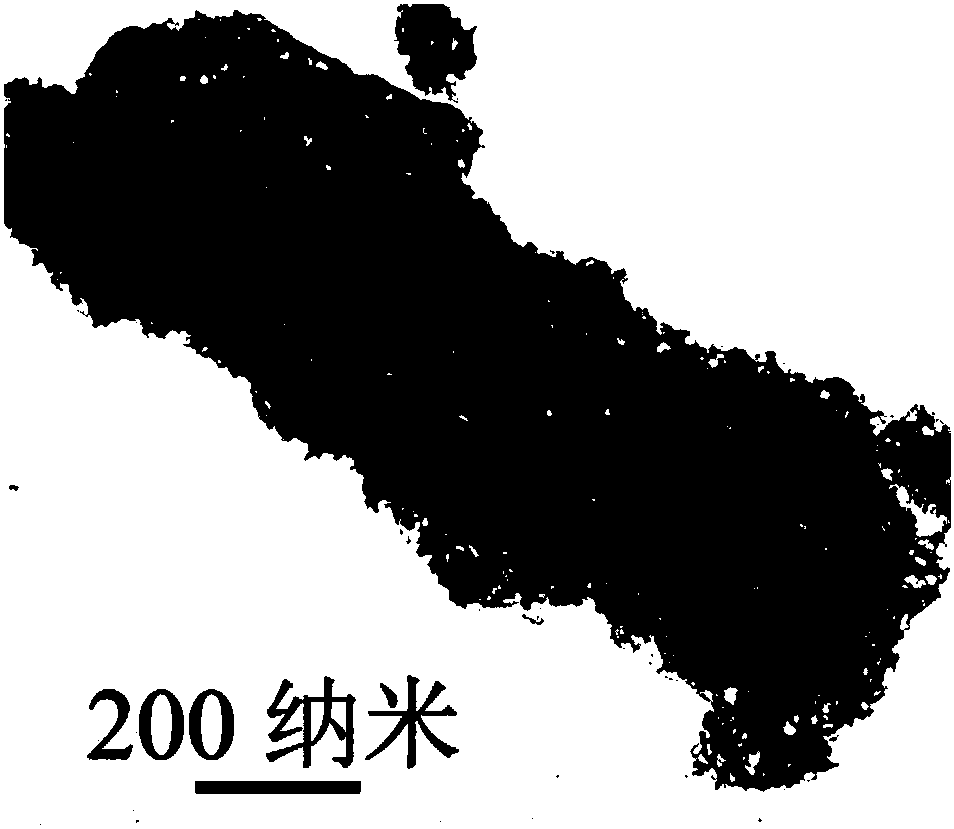
[0033] figure 1 Show that the obtained product is pure boron nitride, figure 2 It is a transmission electron microscope photo of the obtained product. It can be seen that t...
Embodiment 2
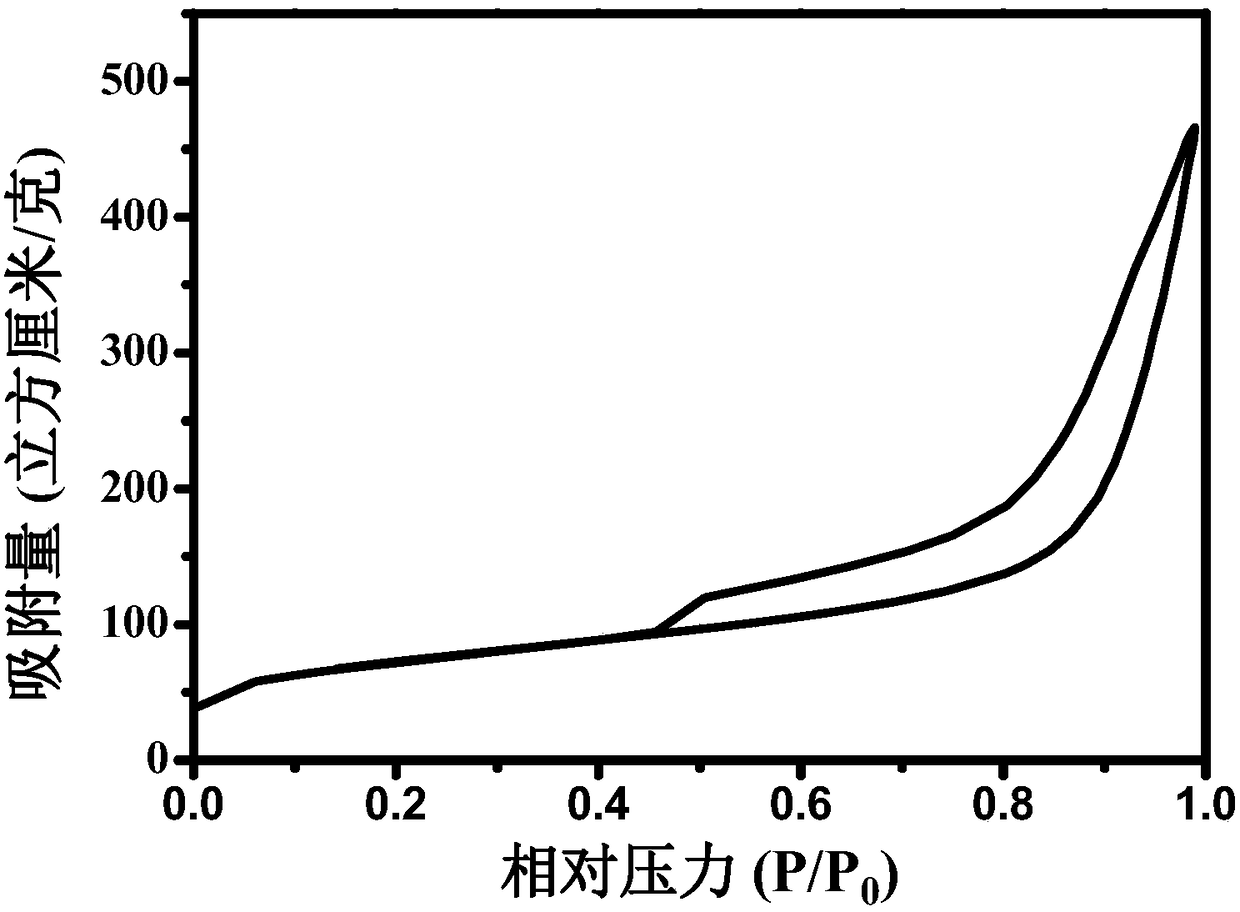
[0035] Take 100 grams of boric acid, 270 grams of urea, 30 grams of ammonium carbonate and 2 grams of cobalt nitrate and mix them evenly, heat them up to 220 degrees Celsius at a heating rate of 10 degrees Celsius / min and keep them warm for 2 hours in an electric furnace to obtain a precursor, which will be cured at 700 degrees Celsius for 10 minute. Put the precursor into the graphite boat nitrogen-hydrogen mixture (2 wt. %H 2 / N 2 ) under the protection of the atmosphere, the temperature was raised to 1000°C at a heating rate of 10°C / min, and the crude product was obtained by keeping the temperature for 2 hours. Then the crude product was placed in 500 ml of 3 mol / L hydrochloric acid and stirred at 40°C for 4 hours. The product was filtered, washed with deionized water and ethanol, and dried. The specific surface area of the sample was measured to be 220 m 2 / g, the most probable pore diameter is 25nm, and the pore size mainly ranges from 8 to 50 nm.
Embodiment 3
[0037] Take 10 grams of boric acid, 90 grams of boron oxide powder, 800 grams of urea, and 5 grams of ferric nitrate and mix them evenly. Heat the temperature in an electric furnace to 500 degrees Celsius for 6 minutes at a heating rate of 10 degrees Celsius / min, and then cure at 700 degrees Celsius for 20 minutes. Put the precursor into the graphite boat nitrogen-hydrogen mixture (20wt.% NH 3 / N 2 ) under the protection of an atmosphere, the temperature was raised to 900°C at a heating rate of 10°C / min, and the crude product was obtained by keeping the temperature for 3 hours. Then the crude product was placed in 500 ml of 1 mol / L hydrochloric acid and stirred at 50°C for 4 hours. The product was filtered, washed with deionized water and ethanol, and dried for 12 hours. The specific surface area of the obtained product was 150 m 2 / g, TEM picture see Figure 5 . The most probable pore diameter is 23 nm, and the pore size mainly ranges from 8 to 50 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com