Synthetic method of glycol mono-hydrogenated nopyl ether and carboxylic ester thereof and application thereof
A technology of monohydrogenated nopyl ether carboxylate and monohydrogenated nopyl ether, which is applied in the application field of antibacterial, and achieves the effects of mild conditions, high product yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
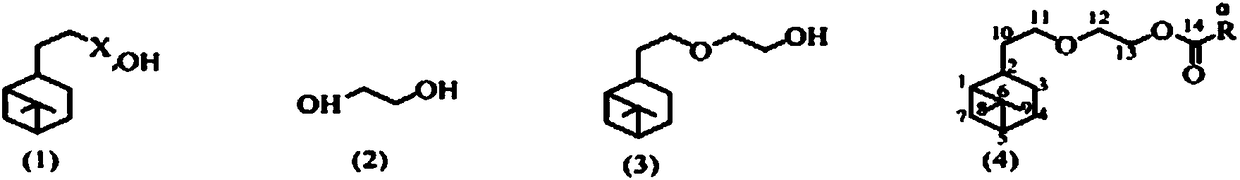
[0019] Synthesis of ethylene glycol monohydronopyl ether: add 0.1mol hydronopyl bromide, 20g ethylene glycol, 30g 1,4-dioxane and 5.0g powdered sodium hydroxide in a 150mL conical flask, Put in a magnetic stirring bar, place it on the magnetic stirrer and install a reflux condenser, stir and heat to reflux. After 3 hours of reaction, the reaction solution was sampled for gas chromatographic analysis. If it is found that there is no more hydrogenated nobyl bromide in the reaction solution, stop heating, cool to room temperature, transfer the reaction solution to a separatory funnel, wash twice with saturated brine, dry over anhydrous sodium sulfate, and then recover by distillation Solvent, ethylene glycol monohydrogenated nopyl ether was distilled off under vacuum, b.p. 133~135℃ / 200 Pa, colorless liquid, yield 91%, GC purity 97.3%.
[0020] IR,ν max (cm -1 ): 1121(C-O-C).
[0021] 1 H NMR, δ H / ppm: 3.693(2H,t,J 1 =J 2 =4.6Hz, 13- CH 2 ),3.495(2H,t,J 1 =J 2 =4.6Hz,...
Embodiment 2
[0024] General method for the synthesis of ethylene glycol monohydrogenated nopyl ether carboxylate:
[0025] In a 100mL conical flask, add 0.03mol ethylene glycol monohydronopolyl ether, 0.06mol carboxylic acid, 25g water-carrying agent, 0.3g p-toluenesulfonic acid, magnetic stirring bar, install water separator and condenser, stir, Heating until the steam in the condenser tube is condensed, the reaction continues, and the turbidity no longer appears in the water separator for another 10 minutes, stop heating, cool, transfer the reaction solution to a separatory funnel, and sequentially use saturated sodium bicarbonate solution and saturated brine. Wash, dry with anhydrous sodium sulfate, recover the solvent by distillation, and evaporate the product by vacuum distillation.
Embodiment 3
[0027] The carboxylic acid is formic acid, the water-carrying agent is cyclohexane, and other experimental methods and conditions are the same as in Example 2, and ethylene glycol monohydrogenated nobyl ether formate is synthesized to obtain a colorless liquid, b.p.129~131℃ / 200Pa, obtain The yield was 94%, and the GC purity was 97.1%.
[0028] IR,ν max (cm -1 ): 1727 (C=O), 1125 (C-O-C).
[0029] 1 H NMR, δ H(ppm): 8.061 (1H, s, HCO), 4.273 (2H, t, J 1 =J 2 =4.6Hz, 13- CH 2 ), 3.614(2H,t,J 1 =J 2 =4.6Hz, 12- CH 2 ),3.440(2H,m, 11- CH 2 ),2.282(1H,m, 2- CH), 2.052(1H,m, 7- CH),1.918~1.801(5H,m, 10- CH 2,3- CH, 5- CH, 1- CH),1.663(2H,m, 4- CH 2 ),1.438(1H,m, 3- CH),1.143(3H,s, 9- CH 3 ),0.972(3H,s, 8- CH 3 ),0.853(1H,d, J=9.2Hz, 7- CH);
[0030] 13 C NMR, δ C (ppm): 70.228 (C -12 ), 68.203 (C -11 ), 63.054 (C -13 ), 46.298 (C -2 ), 41.322(C -5 ), 38.628 (C -6 ), 37.671 (C -1 ), 37.285 (C -10 ), 33.520 (C -7 ), 28.105 (C -9 ), 26.367(C -4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com