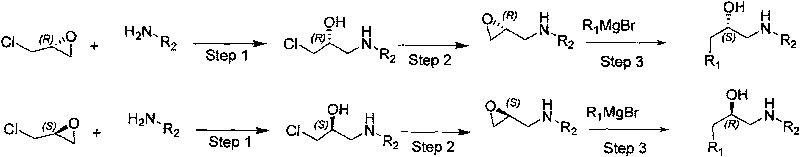
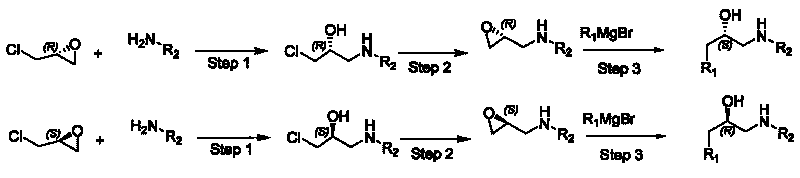
Synthesizing method, partial intermediate products and final products of chiral beta-alkamine derivative
A technology of amino alcohols and synthesis methods, which is applied in the field of synthesis of chiral β-amino alcohol derivatives, and can solve problems such as difficult control of reaction conditions, difficulty in obtaining raw materials, and unsuitability for large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Preparation of R-1-(3-bromophenylamino)-3-chloro-propane-2-ol,
[0033] Add 100g (R) epichlorohydrin (1eq) and 204.6g meta-bromoaniline (1.1eq) into a 1L four-neck flask, and add 12.1g of stannous chloride dihydrate (SnCl) to the system in two batches 2 .2H 2 O) (0.05eq), the addition is complete, stirring at 25±1℃, GC tracking until the reaction is over, 400mL water is added to terminate the reaction, the system is extracted with 600mL methyl tert-butyl ether, the organic phase is then washed with 150mL saturated brine, and reduced Dry organic phase, obtain product 185.9g, yield 65%, enantiomeric purity (ee): 99.8%, gas phase purity (GC): 94%.
[0034] H-NMR: (300MHz, DMSO-d 6 ), δ 2.991-3.058 (dd, CH2NH, 1H), δ 3.138-3.198 (dd, CH2NH, 1H), δ 3.553-3.608 (dd, CH2Cl, 1H), δ 3.648-3.699 (dd, CH2Cl , 1H), δ 3.800-3.836 (m, CH, 1H), δ 5.390 (b, NH, 1H), δ 5.971 (b, OH, 1H), δ 6.587 (d, Ph-H, 1H) ), δ 6.615 (d, Ph-H, 1H), δ 6.772 (s, Ph-H, 1H), δ 6.995 (t, Ph-H, 1H).
[003...
Embodiment 2
[0042] (1) Preparation of R-1-(4-fluorophenylamino)-3-chloro-propane-2-ol,
[0043] Add 150g (R) epichlorohydrin (1eq) and 189g p-fluoroaniline (1.05eq) into a 1L four-neck flask, and add 21.9g stannous chloride dihydrate (SnCl) to the system in four batches 2 .2H 2 O) (0.06eq), the addition is complete, stirring at 26±1℃, GC tracking to the end of the reaction, 400mL of water is added to terminate the reaction, the system is extracted with 800mL of methyl tert-butyl ether, the organic phase is washed with 200mL of saturated brine, and reduced Dry organic phase to obtain crude product 358g, methyl tert-butyl ether: petroleum ether = 1:3 recrystallized to obtain product 210g, yield 63.6%, enantiomeric purity (ee): 99.6%, gas phase purity (GC): 96%.
[0044] H-NMR: (300MHz, DMSO-d 6 ), δ3.032-3.075(dd, CH2NH, 1H), δ3.158-3.218(dd, CH2NH, 1H), δ3.576-3.630(dd, CH2Cl, 1H), δ3.670-3.719(dd, CH2Cl , 1H), δ 3.810-3.846 (m, CH, 1H), δ 5.503 (b, NH, 1H), δ 5.998 (b, OH, 1H), δ 6.501 (m, P...
Embodiment 3
[0053] (1) Preparation of R-1-(4-bromophenylamino)-3-chloro-propane-2-ol,
[0054] Add 50g (R) epichlorohydrin and 106.9g 4-bromoaniline (1.15eq) into a 500mL four-neck flask, add 7.05g lithium bromide (0.15eq) into the system in two batches, after the addition is complete, stir at 27±1℃, GC tracked to the end of the reaction, added 300 mL of water to terminate the reaction, the system was extracted with 600 mL of methyl tert-butyl ether, the organic phase was washed with 150 mL of saturated brine, and the organic phase was shrunk to obtain 71 g of product, with a yield of 50%, enantiomer Purity (ee): 99.7%, gas phase purity (GC): 95%.
[0055] H-NMR: (300MHz, DMSO-d 6 ), δ 2.701-3.078 (dd, CH2NH, 1H), δ 3.158-3.218 (dd, CH2NH, 1H), δ 3.532-3.587 (dd, CH2Cl, 1H), δ 3.627-3.678 (dd, CH2Cl , 1H), δ 3.806-3.847 (m, CH, 1H), δ 5.270 (b, NH, 1H), δ 5.983 (b, OH, 1H), δ 6.560 (d, Ph-H, 1H) ), δ 6.651 (d, Ph-H, 1H), δ 6.790 (d, Ph-H, 1H), δ 6.974 (d, Ph-H, 1H).
[0056] (2) Preparation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com