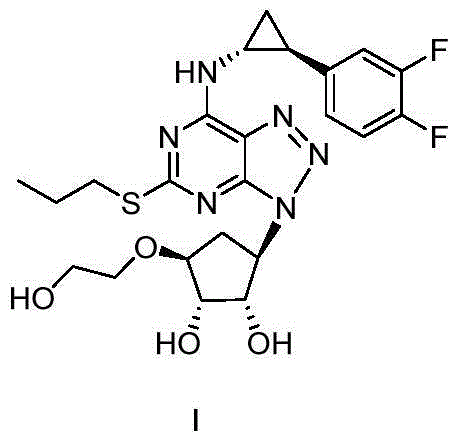
Method for preparing ticagrelor solution
A technology of ticagrelor and solution, applied in chemical instruments and methods, blood diseases, pharmaceutical formulations, etc., can solve the problems of reduced yield, increased complexity of preparation process, increased annual production cost and raw material cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 P = Trityl, P 1 = Hydrogen, P 2 = hydrogen, the preparation method of ticagrelor solution
[0057] The reaction scheme is as follows:
[0058]
[0059] The crude product of ticagrelor is prepared according to the method of Chinese patent CN1200940C, with a purity of 90% (HPLC analysis purity) and a content of 88% (mass fraction).
[0060] In the three-necked round-bottomed flask of 100ml, add content and be 88% ticagrelor crude product (5.94g, 10.0mmol), triphenylchloromethane (3.62g, 13.0mmol) and dichloromethane (60ml), stir well mixed. The temperature was raised to 25-30° C., triethylamine (1.32 g, 13.0 mmol) was added dropwise, and the reaction was stirred at this temperature. After reacting for 3-4 hours, HPLC traced the completion of the reaction, and added saturated ammonium chloride solution (50 ml) to quench the reaction. The organic phase was separated and the aqueous phase was extracted once with dichloromethane (50ml). The combined organic...
Embodiment 2
[0062] Example 2 P = tert-butyldiphenylsilyl, P 1 = Hydrogen, P 2 = hydrogen, the preparation method of ticagrelor solution
[0063] The reaction scheme is as follows:
[0064]
[0065]The crude product of ticagrelor is prepared according to the method of Chinese patent CN1200940C, with a purity of 90% (HPLC analysis purity) and a content of 88% (mass fraction).
[0066] In a 100ml three-necked round-bottomed flask, the content of 88% crude ticagrelor (5.94g, 10.0mmol), tert-butyldiphenylchlorosilane (3.30g, 12.0mmol) and dichloromethane (60ml) was added , and mix well under stirring. The temperature was raised to 25-30° C., triethylamine (1.21 g, 12.0 mmol) was added dropwise, and the reaction was stirred at this temperature. After reacting for 2 to 3 hours, HPLC traced the completion of the reaction, and added saturated ammonium chloride solution (50 ml) to quench the reaction. The organic phase was separated and the aqueous phase was extracted once with dichlorometh...
Embodiment 3
[0068] Example 3 P=4-phenylbenzoyl, P 1 = Hydrogen, P 2 = hydrogen, the preparation method of ticagrelor solution
[0069] The reaction scheme is as follows:
[0070]
[0071] The crude product of ticagrelor is prepared according to the method of Chinese patent CN1200940C, with a purity of 90% (HPLC analysis purity) and a content of 88% (mass fraction).
[0072] In a 100ml three-necked round-bottomed flask, add ticagrelor crude product (5.94g, 10.0mmol), triethylamine (1.21g, 12.0mmol), 4-(N,N-dimethylamino) with a content of 88% Pyridine (0.05g) and dichloromethane (50ml) were mixed evenly under stirring. The temperature was raised to 25-30° C., a dichloromethane solution of 4-phenylbenzoyl chloride (2.60 g, 12.0 mmol) was added dropwise, and the reaction was stirred at this temperature. After reacting for 4 hours, HPLC traced the completion of the reaction, and added saturated ammonium chloride solution (50 ml) to quench the reaction. The organic phase was separated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com