Method for synthesizing derivative of beta-amino acid and intermediate product thereof
A synthesis method and derivative technology, applied in the direction of organic chemistry, can solve the problems of high reaction conditions, unsuitable for large-scale production, high raw material prices, etc., and achieve the effects of low pollution, simple waste treatment methods, and cheap prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
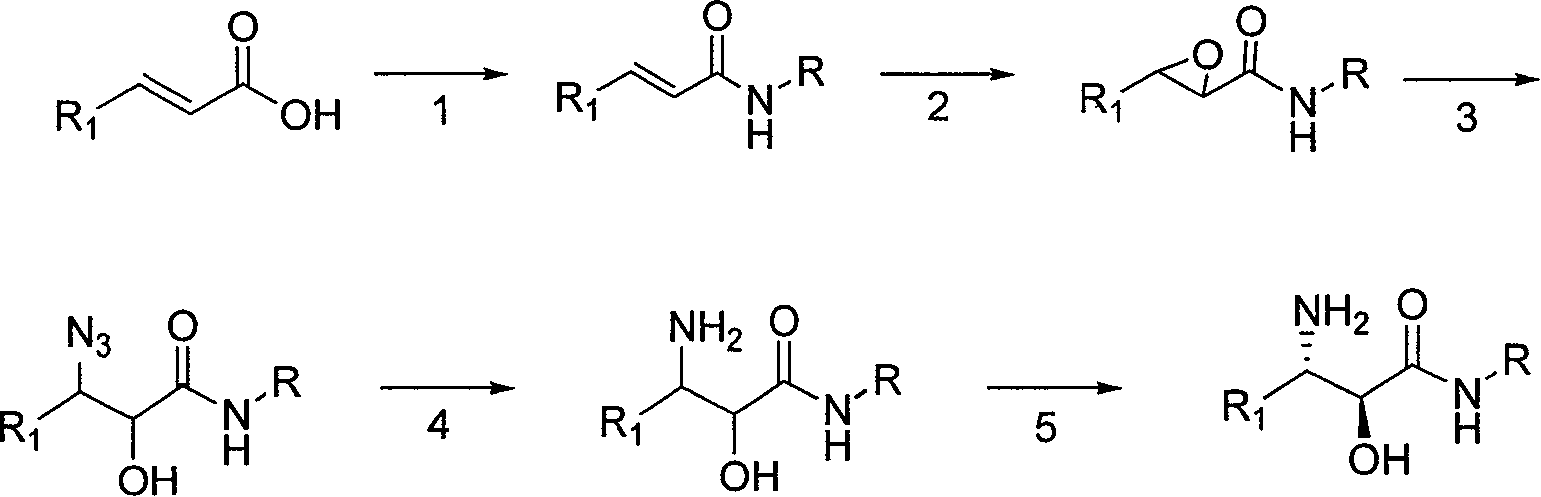
[0030] (1) Preparation of (E)-N-cyclopropyl-2-hexanamide
[0031]Add dichloromethane (10ml / g) and trans-2-hexenoic acid (1eq.) to the reaction kettle, add N'N-dicarbonyldiimidazole (1.1eq.) to the system, after the addition is complete, the system is refluxed for 4.5h; Cool down to 35°C and add cyclopropylamine (2.4eq.). Incubate at -15°C for 12h. The system was suction filtered and rinsed with dichloromethane. The organic phase was washed with brine, dried, filtered with suction, and the filtrate was spin-dried to obtain a crude product. The crude product was recrystallized from a mixed solvent of ethyl acetate and cyclohexane to obtain a solid with a yield of 80%.
[0032] 1HNMR (300MHz, CDCl3), δ0.501 (cyclopropyl CH2, m), δ0.772 (CH3, m), δ0.916 (CH2, m), δ1.460 (CH2, m), δ2.132 ( Cyclopropyl CH, m), δ6.160 (vinyl H, m), δ6.819 (vinyl H, m), δ7.294 (NH, m).
[0033] (2) Preparation of 3-propyloxirane-2-hexanoic acid cyclopropylamide
[0034] Add propionitrile (5L / mol...
Embodiment 2
[0046] (1) Preparation of (E)-N-n-octyl-2-butanamide
[0047] Add dichloroethane (20ml / g) and trans-2-butenoic acid (1eq.) to the reaction kettle, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt to the system acid salt (2eq.), after the addition was complete, the system was refluxed for 4.5h; the temperature was lowered to 10°C, and n-octylamine (5eq.) was added dropwise. Incubate at 20°C for 12h. The system was suction filtered and rinsed with dichloromethane. The organic phase was washed with brine, dried, filtered with suction, and the filtrate was spin-dried to obtain a crude product. The crude product was recrystallized from a mixed solvent of ethyl acetate to obtain a solid with a yield of 54%.
[0048] 1HNMR (300MHz, CDCl3), δ0.912 (n-octyl CH3, m), δ1.279 (4×n-octyl CH2, m), δ1.268 (n-octyl CH2 linked to CH3, m), δ1 .543 (CH2 of β linked to N, m), δ1.667 (CH3, m), δ2.934 (CH2 of α linked to N, m), δ6.198 (vinyl H, cis, m ), δ6.615 (vinyl H, m), δ7.548 (NH, ...
Embodiment 3
[0062] (1) Preparation of (E)-N-tert-butyl-2-hexanamide
[0063] Add dichloromethane (40ml / g) and trans-2-hexenoic acid (1eq.) to the reaction kettle, add thionyl chloride (1.1eq.) to the system, after the addition is complete, the system is refluxed for 4.5h; tert-butyl amine (3.5 eq.). Incubate at 16°C for 12h. The system was suction filtered and rinsed with dichloromethane. The organic phase was washed with brine, dried, filtered with suction, and the filtrate was spin-dried to obtain a crude product. The crude product was recrystallized from a mixed solvent of ethyl acetate and cyclohexane to obtain a solid with a yield of 58%.
[0064] 1HNMR (300MHz, CDCl3), δ0.871 (CH3, m), δ1.223 (tert-butyl CH3, s), δ1.296 (CH2, m), δ1.798 (CH2, m), δ6.103 ( Vinyl H, m), δ6.457 (vinyl H, m), δ7.357 (NH, m).
[0065] (2) Preparation of 3-propyloxirane-2-hexenoic acid tert-butylamide
[0066] Add acetonitrile (5L / mol) and water (1L / mol) to the reaction kettle at one time, start sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com