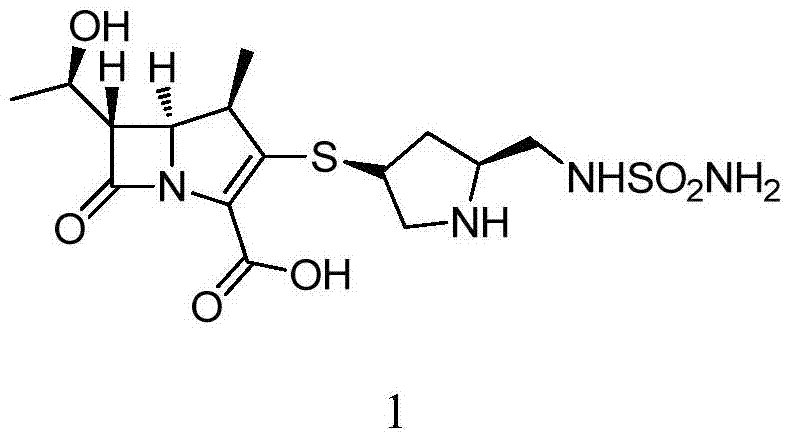
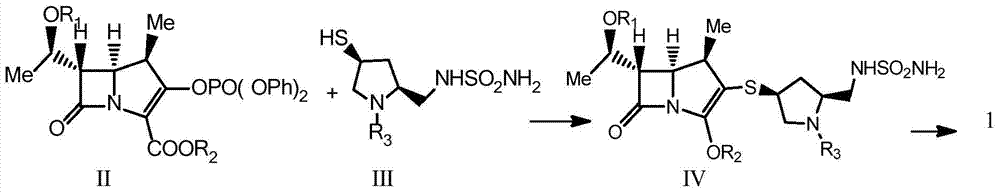
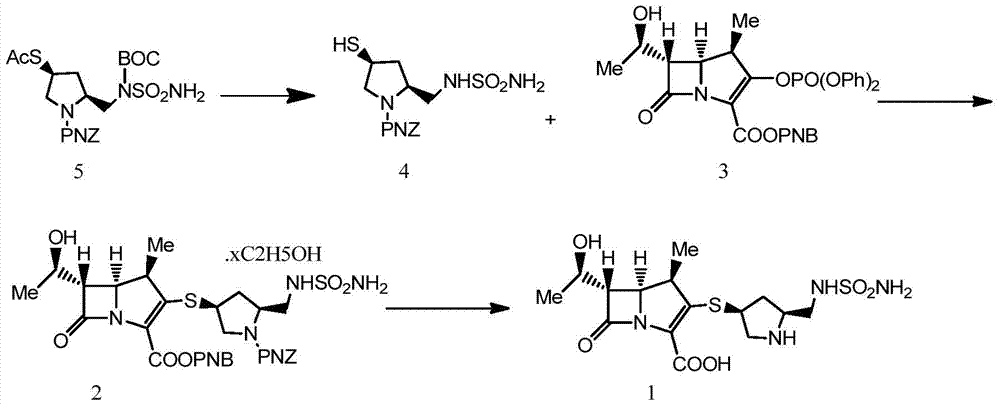
A kind of preparation method of doripenem
A doripenem and sulfur-based technology, which is applied in the field of preparation of doripenem, can solve problems such as difficult stratification, residual magnesium ions, and difficult crystallization, and achieve simplified post-processing, short crystallization time, and stable sex good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-thio-2-(N-sulfamoylamino)methylpyrrolidine
[0030] Dissolve 100.0g (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-acetylthio-2-(N-tert-butoxycarbonylsulfamoylamino)methylpyrrolidine (188mmol) in 500ml methanol , add 28ml of concentrated sulfuric acid dropwise, react at 60°C, after TLC monitors the reaction, cool the solution to below 10°C, adjust the pH to neutral with 10% NaOH solution, then concentrate, extract with ethyl acetate (250ml×3), Extracted and washed with 5% NaCl solution, dried, filtered, and concentrated to obtain an orange oil, which was directly used in the next reaction. MS-ESIm / z:346[M+H] + .
[0031] (1R,5S,6S)-2-[(3S,5S)-1-benzyl p-nitroformate-5-sulfamoylaminomethylpyrrolidine-3-sulfanyl]-6-[(1R) Preparation of -1-hydroxyethyl]-1-methyl-1-carbo-2-penem-3-carboxylic acid p-nitrobenzyl ester alcoholate
[0032] Dissolve (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-sulfanyl-2-(N-sulfamoylamino)methylpyrrolidine, ...
Embodiment 2
[0036] Preparation of (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-thio-2-(N-sulfamoylamino)methylpyrrolidine
[0037] Dissolve 100.0g (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-acetylthio-2-(N-tert-butoxycarbonylsulfamoylamino)methylpyrrolidine (188mmol) in 500ml methanol , add 28ml of concentrated sulfuric acid dropwise, react at 60°C, after TLC monitors the reaction, cool the solution to below 10°C, adjust the pH to neutral with 10% NaOH solution, then concentrate, extract with ethyl acetate (250ml×3), Extracted and washed with 5% NaCl solution, dried, filtered, and concentrated to obtain an orange oil, which was directly used in the next reaction.
[0038] (1R,5S,6S)-2-[(3S,5S)-1-benzyl p-nitroformate-5-sulfamoylaminomethylpyrrolidine-3-sulfanyl]-6-[(1R) Preparation of -1-hydroxyethyl]-1-methyl-1-carbo-2-penem-3-carboxylic acid p-nitrobenzyl ester alcoholate
[0039] Dissolve (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-sulfanyl-2-(N-sulfamoylamino)methylpyrrolidine, 107.2g (180mmol) carbapen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com