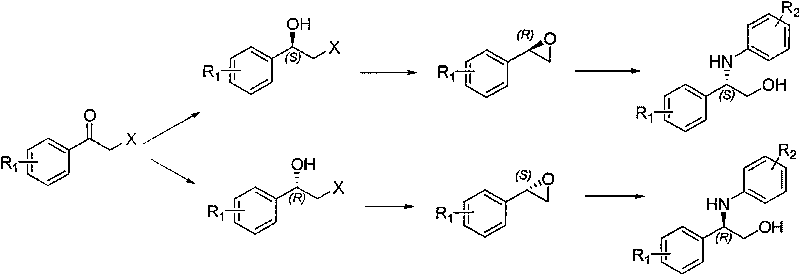
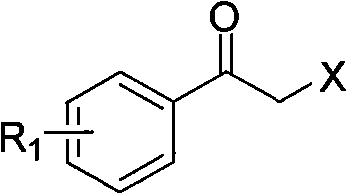
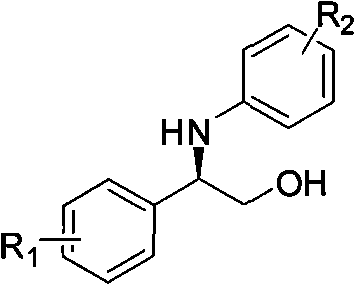
Synthesis method of derivative of chiral Beta-amino-alcohol and part of final products thereof
A synthesis method and technology for final products, which are applied in the field of chemical synthesis methods of chiral beta-amino alcohol derivatives and some final products thereof, can solve the problem of large enzyme requirements, limited application of pharmaceutical intermediates, and unsuitable for large-scale production. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of (R)-2-bromo-1-(4-bromophenyl)ethanol
[0028] Add 1.2L tetrahydrofuran (10mL / g), 32.8g borane dimethyl sulfide (1eq.) to the 2L reaction flask, add 5.9g R-methyloxazolidine (0.05eq.) to the system, after the addition is complete, place on ice Cool the bath to 0°C and stir for 30 min; start to add 120g of 2-bromo-4-bromoacetophenone (1eq) dropwise at a rate of 1 drop / 3s, after the addition is completed, follow the GC until the end of the reaction, add 240mL of methanol (2mL / g ) to terminate the reaction, the heat release is obvious, then add 240mL hydrochloric acid (18%) dropwise, shrink the system, extract with 240mL dichloromethane (2mL / g), wash the organic phase 3 times with saturated sodium bicarbonate solution (360mL*3), Saturated brine was washed 3 times (360mL*3), after drying, the organic phase was shrunk to obtain 112.7g of product, yield 93%, gas chromatography purity (GC): 97.8%, enantiomeric purity (ee) (using manual High performance liq...
Embodiment 2
[0037] (1) Preparation of (R)-2-chloro-1-phenylethanol
[0038] Add 1.5L 4-tetrahydrofuran (15mL / g), 58g borane dimethyl sulfide (1.5eq.) to the 2L reaction flask, add 11.4g R-methyloxazolidine (0.08eq.) to the system, and the addition is complete , cooled to -3°C in an ice bath and stirred for 40min; began to drop 80g of 2-chloro-acetophenone (1eq) at a rate of 1 drop / 2s. ) to terminate the reaction, the heat release is obvious, then add 160mL dilute sulfuric acid (18%) dropwise, shrink the system, extract with 160mL dichloromethane (2mL / g), and wash the organic phase 3 times with saturated potassium bicarbonate solution (150mL*3) , washed with saturated brine 3 times (150mL*3), after drying, the organic phase was concentrated to obtain 75g of product, yield 98.7%, gas chromatography purity (GC): 98.4%, enantiomeric purity (ee): 97.5%.
[0039] HNMR: (300MHz, CDCl3), δ7.333-7.399 (-C6H5, m), δ4.903 (-CH, d), δ3.707 (-CH2, dd), 2.681 (-OH, d).
[0040] (2) Preparation of (...
Embodiment 3
[0047] (1) Preparation of (R)-2-bromo-1-(4-chlorophenyl)ethanol
[0048] Add 1.2L tetrahydrofuran (12mL / g) and 27.9g borane dimethyl sulfide (0.9eq.) to the 2L reaction flask, and add 5.9g R-methyloxazolidine (0.05eq.) to the system. After the addition is complete, Cool down to -5°C in an ice bath and stir for 45 minutes; start to add 100g of 2-bromo-4-chloroacetophenone (1eq) dropwise at a rate of 1 drop / 2s. / g) to terminate the reaction, the heat release is obvious, then add 200mL hydrochloric acid (18%) dropwise, shrink the system, extract with 250mL dichloromethane (2.5mL / g), and wash the organic phase 3 times with saturated sodium carbonate solution (200mL*3 ), washed with saturated brine 3 times (200mL*3), after drying, the organic phase was shrunk to obtain 96.8g of product, yield 96%, gas chromatography purity (GC): 97.8%, enantiomeric purity (ee): 98.9%.
[0049] HNMR: (300MHz, CDCl3), δ7.465 (-C6H4, d), δ7.188 (-C6H4, d), δ4.915 (-CH, m), δ3.752 (-CH2, d), δ3. 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com