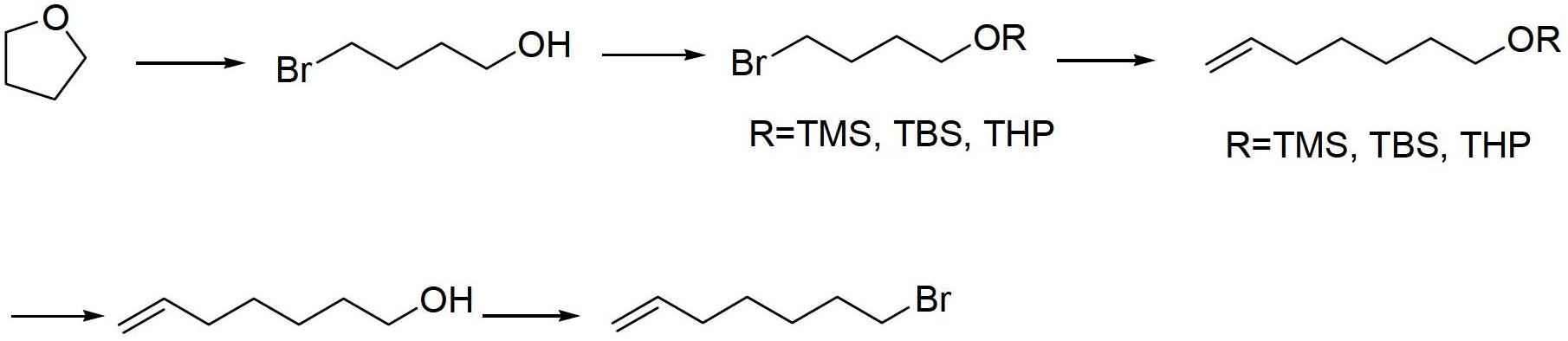
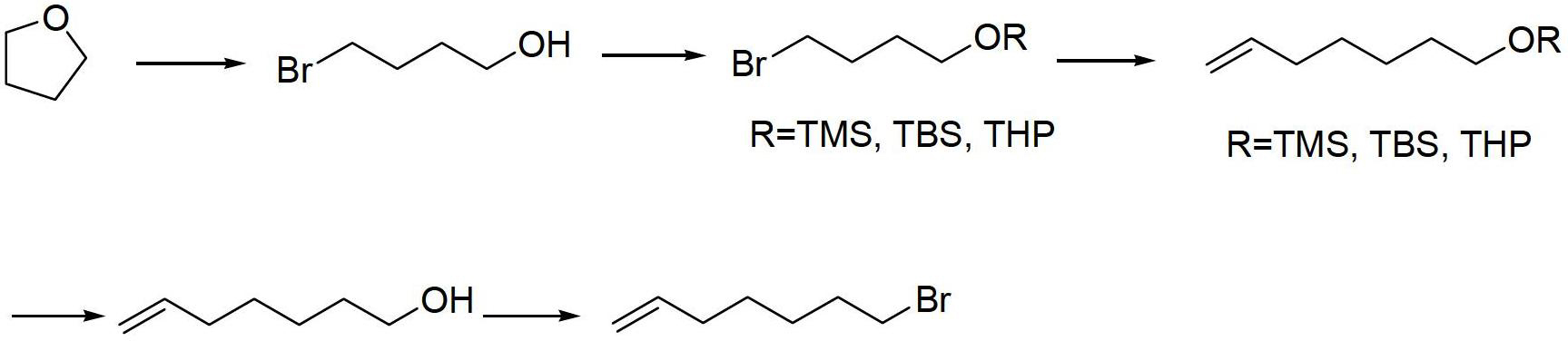
Method for preparing 7-bromine-1-heptylene
A technology of heptene, the main raw material, applied in the field of synthesis of heptene derivatives, which can solve the problems of producing a large amount of waste residue, not suitable for large-scale production, and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: A kind of preparation 7-bromo-1-heptene The method is characterized in that the specific preparation steps are as follows:
[0032] (1) Ring-opening reaction: Add 200kg of main raw materials tetrahydrofuran and 19kg (0.07eq) of concentrated sulfuric acid to a 1000L reactor in sequence, control the temperature of the system at 2±2°C, and slowly add 420kg (0.9eq) of 48% hydrobromic acid aqueous solution dropwise , after dropping, raise the temperature to 80±2°C, keep it warm for 3h, after the reaction is completed, cool down to 0±2°C, adjust the pH of the system to 7-8 with 23kg (0.1eq) of sodium bicarbonate, extract, wash the organic phase, and concentrate to obtain Oily product 4-bromo-1-butanol 286kg, yield 67.5%, gas chromatography purity (GC): 97.0%;
[0033] (2) Hydroxyl protection: Add the main raw material 4-bromo-1-butanol to the 1000L reactor in sequence 50kg, 333kg of dichloromethane (1g / 5mL), 13kg of p-toluenesulfonic acid (1g / 0.25g), tempe...
Embodiment 2
[0037] Embodiment 2: A kind of preparation 7-bromo-1-heptene The method is characterized in that the specific preparation steps are as follows:
[0038] (1) Ring-opening reaction: Add 150kg of main raw materials tetrahydrofuran and 24kg (0.1eq) of trifluoroacetic acid to a 1000L reactor in sequence, control the temperature of the system at 10±2°C, and slowly add 525kg (1.5eq) of 48% hydrobromic acid aqueous solution dropwise ), after dropping, raise the temperature to 90±2°C, keep it warm for 4 hours, after the reaction is completed, cool down to 5±2°C, use 44kg (0.2eq) of sodium carbonate to adjust the pH to 7~8, extract, wash the organic phase, concentrate, The oily product 4-bromo-1-butanol was obtained 181kg, yield 56.8%, gas chromatography purity (GC): 96.5%;
[0039] (2) Hydroxyl protection: add the main raw material 4-bromo-1-butanol to the 500L reactor in sequence 35kg, N,N-dimethylformamide 263kg (1g / 8mL), imidazole 35kg (1g / 1g), temperature control 25±2°C, drop...
Embodiment 3
[0043] Embodiment 3: A kind of preparation 7-bromo-1-heptene The method is characterized in that the specific preparation steps are as follows:
[0044] (1) Ring-opening reaction: Add 100kg of main raw materials tetrahydrofuran and 4.8kg (0.02eq) of p-toluenesulfonic acid to a 500L reactor in sequence, control the temperature of the system at -5±2°C, and slowly add 117kg of 48% hydrobromic acid aqueous solution dropwise (0.5eq), after dripping, raise the temperature to 70±2°C, keep it warm for 1h, after the reaction is completed, lower the temperature to -5±2°C, use 7kg (0.05eq) of potassium bicarbonate to adjust the pH to 7~8, extract, organic phase Washing, concentration, the oily product 4-bromo-1-butanol is obtained 134kg, yield 63.3%, gas chromatography purity (GC): 96.0%;
[0045] (2) Hydroxyl protection: add the main raw material 4-bromo-1-butanol to the 500L reactor in sequence 70kg, methyl tert-butyl ether 104kg (1g / 2mL), triethylamine 7kg (1g / 0.1g), temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com