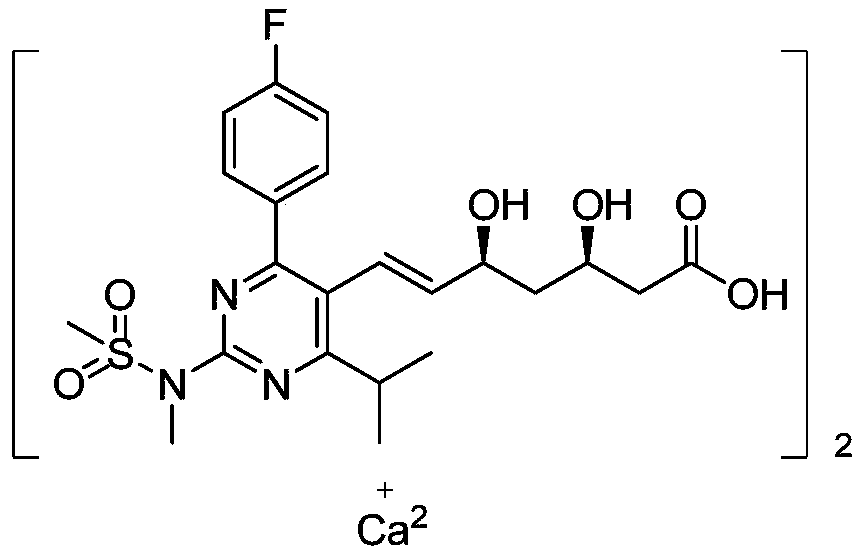
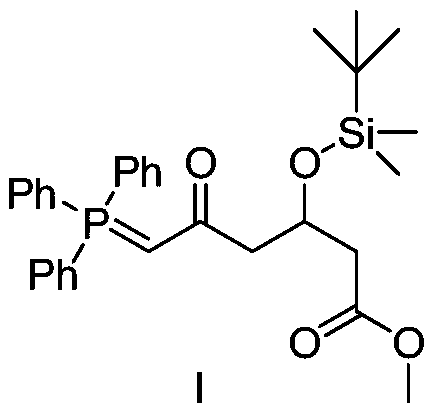
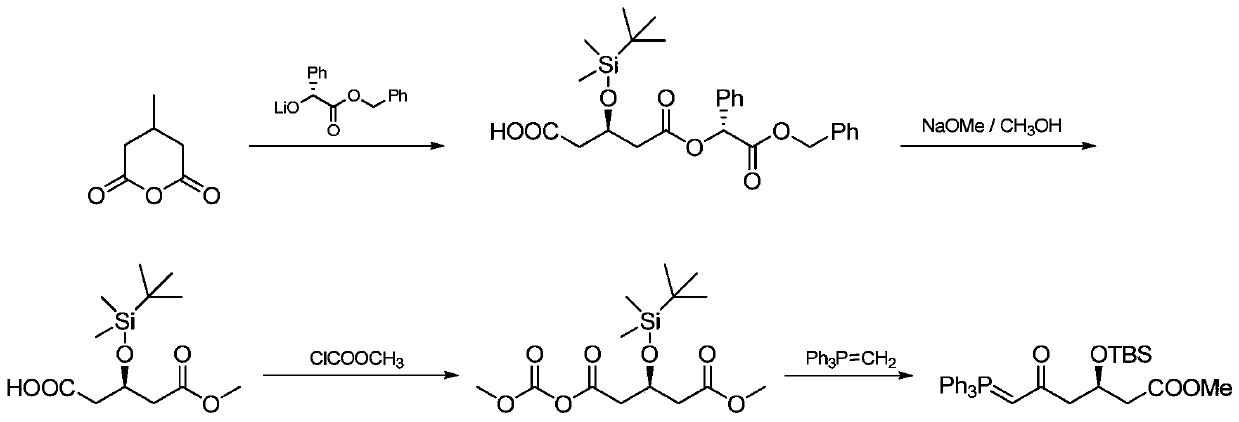
A kind of preparation method of novel rosuvastatin calcium intermediate
A technology of rosuvastatin calcium and intermediates, applied in the field of medicinal chemistry, can solve the problems of post-processing of toxic and harmful chemical reagents, long and cumbersome reaction steps, etc., and achieve the effects of three wastes treatment, mild chemical reaction conditions, and easy three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of (R)-methyl 3-(tert-butyldimethylsiloxane)-5-(1H-imidazol-1-yl)-5-oxopentanoic acid methyl ester (compound II)
[0047]
[0048] 30.0 g of compound III, 108.5 mmol, and 150 ml of dichloromethane were put into a three-necked reaction flask, and the mixture was stirred to dissolve. 18.5 g 114.1 mmol of carbonyldiimidazole was added in batches at a temperature of 20-30 °C. The reaction is stirred for 2 to 3 hours, and the sampling is controlled until the residual amount of compound III in the reaction solution is less than or equal to 0.5%, and the reaction is completed.
[0049] The above reaction solution was added to a flash silica gel column for column purification, and the eluate was collected. The solvent was removed by concentration under reduced pressure to obtain 31.9 g of a yellow oil. The yield was 90.0%, and the GC purity was 95.0%.
Embodiment 2
[0050] Example 2: Preparation of (R)-methyl 3-(tert-butyldimethylsiloxane)-5-(1H-imidazol-1-yl)-5-oxopentanoic acid methyl ester (compound II)
[0051]
[0052] 30.0 g of compound III, 108.5 mmol, and 150 ml of dichloromethane were put into a three-necked reaction flask, and the mixture was stirred to dissolve. 35.2 g 217.1 mmol of carbonyldiimidazole was added in batches at a temperature of 20-30 °C. The reaction is stirred for 2 to 3 hours, and the sampling is controlled until the residual amount of compound III in the reaction solution is less than or equal to 0.5%, and the reaction is completed.
[0053] The above reaction solution was added to a flash silica gel column for column purification, and the eluate was collected. The solvent was removed by concentration under reduced pressure to obtain 34.0 g of a yellow oil. The pure yield was 84.0%, and the GC purity was 88.0%.
Embodiment 3
[0054] Example 3: Preparation of (R)-methyl 3-(tert-butyldimethylsiloxane)-5-(1H-imidazol-1-yl)-5-oxopentanoic acid methyl ester (compound II)
[0055]30.0 g of compound III, 108.5 mmol, and 150 ml of dichloromethane were put into a three-necked reaction flask, and the mixture was stirred to dissolve. 40.5 g 249.6 mmol of carbonyldiimidazole was added in batches at a temperature of 20-30 °C. The reaction is stirred for 2 to 3 hours, and the sampling is controlled until the residual amount of compound III in the reaction solution is less than or equal to 0.5%, and the reaction is completed.
[0056] The above reaction solution was added to a flash silica gel column for column purification, and the eluate was collected. The solvent was removed by concentration under reduced pressure to obtain 34.5 g of a yellow oil. The pure yield was 80.0%, and the GC purity was 83.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com