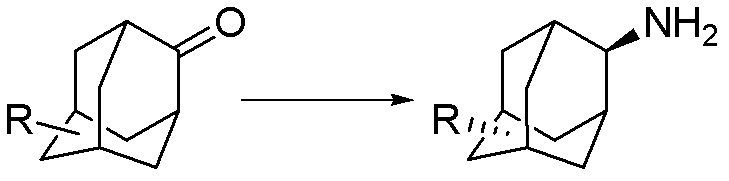
Synthesis method for trans-form amantadine compound
A technology of amantadine and a synthesis method, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., can solve the problems of environmental pressure, complicated operation, lengthy steps, etc., and achieves mild chemical reaction conditions and technology. The technology is mature and the effect of saving a lot of costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of preparation trans-5-carboxylate-2-adamantanamine The method is characterized in that the specific preparation steps are as follows:
[0030] (1) Add methanol 1169kg (1g / 20mL), 5% Pd / C 5.25kg (1g / 0.07g) and main raw material 2-adamantanone-5-formic acid to the 3000L reactor 75kg;
[0031] (2) The reactor was replaced with nitrogen for 3 times, and after stirring evenly, 164kg (25eq) of liquid ammonia was introduced into the system;
[0032] (3) Control the temperature at 45±2°C, and pass hydrogen at 1.0±0.05MPa until the reaction is complete;
[0033] (4) After the reaction is completed, add 30% sodium methylate methanol solution to the system to make the product completely salify, press filter, wash the filter cake with methanol, combine the filtrates, adjust the acidity with hydrochloric acid to pH=8.3, and centrifuge to obtain the product trans 5 -Carboxylic acid-2-amantadine 66.7kg, yield 88.5%, liquid chromatography purity (HPLC) 99.8%.
Embodiment 2
[0035] A preparation of trans 5-hydroxyl-2-adamantanamine The method is characterized in that the specific preparation steps are as follows:
[0036] (1) Add 711kg of ethanol (1g / 15mL), 10% Pd / C3kg (1g / 0.05g) and 5-hydroxy-2-adamantanone as the main raw material to the 1500L reactor in sequence 60kg;
[0037] (2) The reaction kettle was replaced with nitrogen for 3 times, and after stirring evenly, 123kg (20eq) of liquid ammonia was introduced into the system;
[0038] (3) Control the temperature at 40±2°C, and pass hydrogen at 0.8±0.05MPa until the reaction is complete;
[0039] (4) After the reaction is completed, add 30% sodium ethylate ethanol solution to the system to make the product completely salify, press filter, wash the filter cake with ethanol, combine the filtrates, adjust the acidity to pH=8 with sulfuric acid, and centrifuge to obtain the product trans 5 -Hydroxy-2-adamantanamine 54kg, yield 89.4%, liquid chromatography purity (HPLC): 99.2%.
Embodiment 3
[0041] A kind of preparation trans 2-adamantanamine-5-ammonium sulfonate The method is characterized in that the specific preparation steps are as follows:
[0042] (1) Add 1570kg (1g / 25mL) of isopropanol and 10% Pd(OH) to the 3000L reactor in sequence 2 / C 8kg(1g / 0.1g), 2-adamantanone-5-ammonium sulfonate 80kg;
[0043] (2) The reaction kettle was replaced with nitrogen for 3 times, and after stirring evenly, 178kg (30eq) of liquid ammonia was introduced into the system;
[0044] (3) Control the temperature at 60±2°C, and pass hydrogen at 1.2±0.05MPa until the reaction is complete;
[0045](4) After the reaction is completed, add 30% sodium hydroxide solution to the system to make the product completely salify, press filter, wash the filter cake with isopropanol, combine the filtrates, adjust the acidity with acetic acid to pH=8.5, and centrifuge to obtain the product trans 2-Adamantadine-5-ammonium sulfonate 70kg, yield 87.1%, liquid chromatography purity (HPLC): 99....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com