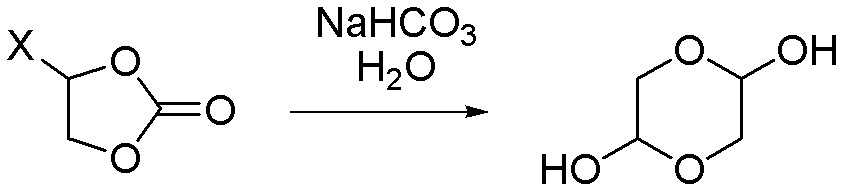Synthetic method of 1,4-dioxane-2,5-diol
A technology of dioxane and a synthesis method, applied in 1 field, can solve the problems of non-industrial application value, harsh reaction conditions, amplifying production costs, etc., and achieves energy saving and post-processing time, mild chemical reaction conditions, and production. Safe and reliable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
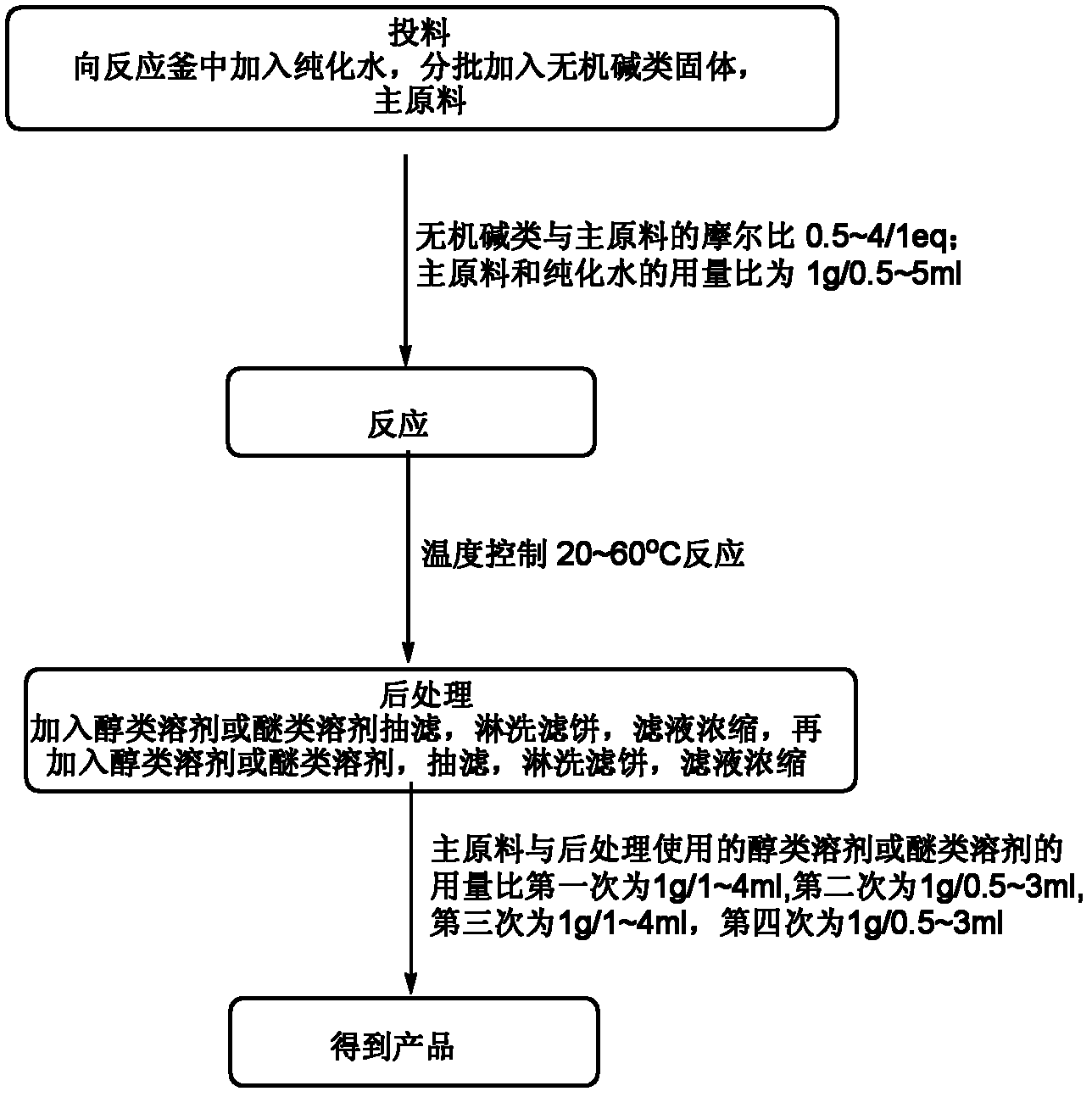
[0034] (1) Feeding: Add 85.0kg of purified water (0.5ml / 1g) to a 1000L reactor, heat up to 35-45°C, add 116.6kg of sodium bicarbonate solid (1.0eq) and 170.0kg (1.0eq) in 10 batches main raw material
[0035] (2) Reaction: heat preservation reaction at a temperature of 35 to 45°C;
[0036] (3) post-processing: after the reaction is completed, add 340L dioxane (2ml / 1g) to the reaction system, filter with suction, wash the filter cake with 85L dioxane (0.5ml / 1g), and concentrate the filtrate to no fraction. Then dissolve the crude product with 340L dioxane (2ml / 1g), filter with suction, and wash the filter cake with 85L dioxane (0.5ml / 1g), and concentrate the filtrate to no fraction;
[0037] (4) The obtained product: 72.0 kg of 1,4-dioxane-2,5-diol was obtained, with a yield of 43.2%, and a nuclear magnetic purity (NMR): 98.9%.
[0038] The test data of the final product are as follows: mp: 96~97℃, 1 H-NMR (CDCl3, 500HZ): δ2.0 (H on -OH), 5.44 (H on CH), 3.98, 3.73 (H on C...
Embodiment 2
[0040] (1) Feeding: Add 1158.5kg of purified water (5ml / 1g) to a 3000L reactor, heat up to 50-60°C, add 466kg of sodium carbonate solid (4.0eq) and 231.7kg (1.0eq) of main raw materials in 10 batches
[0041] (2) Reaction: at a temperature of 50-60°C, heat preservation reaction;
[0042] (3) Aftertreatment: after the reaction, add 686L methyl tert-butyl ether (4ml / 1g) to the reaction system, filter with suction, wash the filter cake with 514L methyl tert-butyl ether (3ml / 1g), and concentrate the filtrate to No fraction, then dissolve the crude product with 686L methyl tert-butyl ether (4ml / 1g), filter with suction, wash the filter cake with 514L methyl tert-butyl ether (3ml / 1g), and concentrate the filtrate until there is no fraction;
[0043](4) The obtained product: 65.0 kg of 1,4-dioxane-2,5-diol was obtained, with a yield of 39.0%, and a nuclear magnetic purity (NMR): 98.5%.
[0044] The test data of the final product are as follows: mp: 96~97℃, 1 H-NMR (CDCl3, 500HZ):...
Embodiment 3
[0046] (1) Feeding: Add 74kg of purified water (0.5ml / 1g) into a 500L reactor, heat up to 20-30°C, add 34.7kg of potassium bicarbonate solid (0.5eq) and 148.4kg (1.0eq) of main raw material
[0047] (2) Reaction: heat preservation reaction at a temperature of 20 to 30°C;
[0048] (3) Post-treatment: After the reaction is complete, add 117L methanol (1ml / 1g) to the reaction system, filter with suction, wash the filter cake with 59L methanol (0.5ml / 1g), concentrate the filtrate until there is no fraction, and then wash it with 117L methanol (1ml / 1g). / 1g) dissolve the crude product, filter with suction, and wash the filter cake with 59L methanol (0.5ml / 1g), and the filtrate is concentrated to no fraction;
[0049] (4) The obtained product: 33.0 kg of 1,4-dioxane-2,5-diol was obtained, with a yield of 39.6%, and a nuclear magnetic purity (NMR): 98.6%.
[0050] The test data of the final product are as follows: mp: 96~97℃, 1 H-NMR (CDCl3, 500HZ): δ2.0 (H on -OH), 5.44 (H on C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com