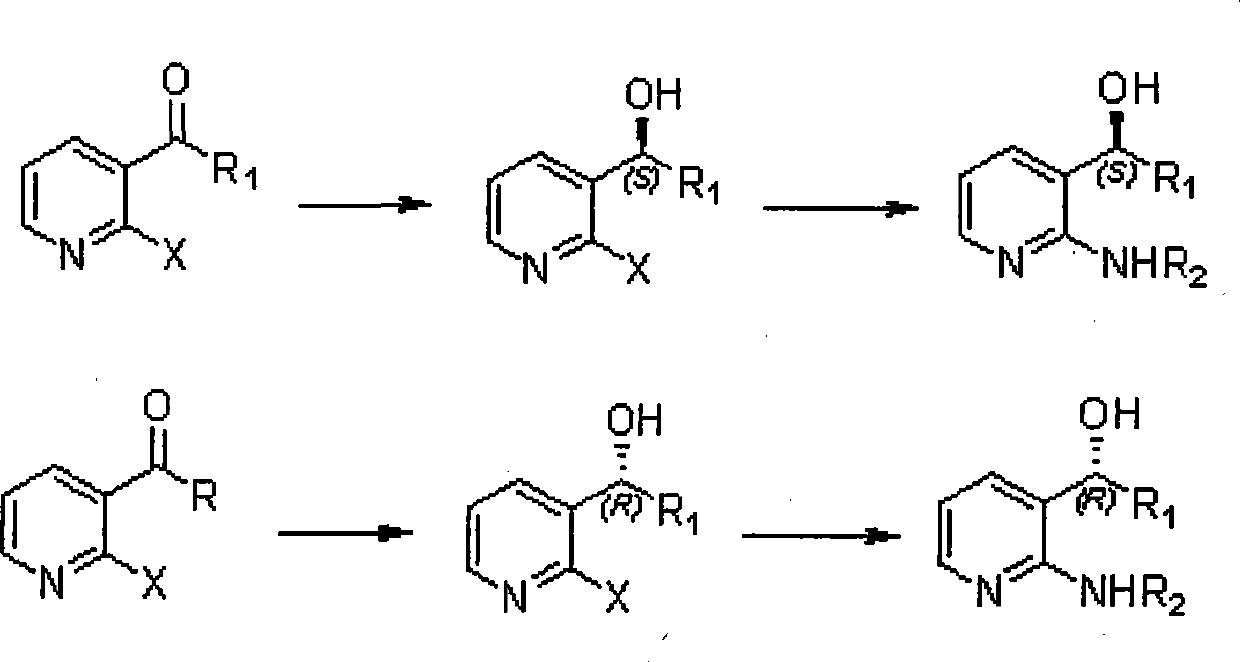
Method for synthesizing derivatives of chiral pyridyl aminoalcohols, and intermediate products and final products of same
The technology of an aromatic ring amino alcohol and a synthesis method, which is applied in the field of synthesis of derivatives, can solve the problems of low application value, difficult control of splitting conditions, and low purity (ee) value of easy racemic enantiomers of products, and achieves Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of 1-S-(2-fluoropyridin-3-yl)-1-propanol,
[0034] Add 1.7kg tetrahydrofuran (25mL / g), 34.2g borane dimethyl sulfide (0.9eq.) to the 5L reaction flask, add 8.3gS-methyloxazolidine (0.06eq.) to the system, after the addition is complete, 25 Stir at ~26°C for 30 minutes; start to add 76.6g of 2-fluoro-3-propionylpyridine (1eq) dropwise at a rate of 1 drop / s, after the addition is complete, follow the HPLC until the end of the reaction, add 1.2kg of methanol (20mL / g) Terminate the reaction, shrink the system, add the residue to water, extract with 2.3kg dichloromethane (23mL / g), shrink the organic phase to obtain 73.7g of product, yield 95%, liquid chromatography purity (HPLC): 98.8%, Enantiomeric purity (ee): 98.5%.
[0035] H-NMR: (300MHZ, CDCl3), δ 0.688 (-CH3, t), δ 1.352 (H on CH2 connected to CH3, m), δ 4.418 (H on CH connected to hydroxyl, m) , δ 5.099 (H on the -OH, d), δ 7.425 (H on the 5-position of the pyridine ring, m), δ 8.121 (H on the 4-p...
Embodiment 2
[0040] (1) Preparation of 1-R-(2-chloropyridin-3-yl)-1-cyclopropylmethanol;
[0041] Add 375.7g 2-methyltetrahydrofuran (30mL / g), 7.3g borane dimethyl sulfide (1.2eq.) to the 1L reaction flask, add 1.5g R-methyloxazolidine (0.07eq.) to the system , after the addition was completed, stir at 29-30°C for 40 minutes; start to add 14.6g of 2-chloro-3-cyclopropylformylpyridine (1eq) dropwise, 1 drop / s, after the addition was completed, follow the HPLC until the end of the reaction, add 172.6g Ethanol (15mL / g) was terminated, the system was shrunk to dryness, the residue was added to water, extracted with 484.2g (25mL / g) of dichloromethane, and the organic phase was dried to obtain 13.4g of the product, with a yield of 91%, liquid chromatography purity (HPLC ): 98%, enantiomeric purity (ee): 99%.
[0042] 1H-NMR: (300MHZ, CDCl3), δ 0.561 (cyclopropyl CH2, m), 4.398 (H on the -CH connected to the hydroxyl group, s), δ 4.499 (H on the -OH, s), δ 7.615 (H at the 5-position of the py...
Embodiment 3
[0047] (1) Preparation of 1-S-(2-chloropyridin-3-yl)-1-propanol;
[0048] Add 242.8g diethyl ether (40mL / g) and 1.0g diborane (0.7eq.) to the 1L reaction flask, and add 1.1g S-methyloxazoborane (0.08eq.) to the system. After the addition is complete, 25-26 Stir at ℃ for 35min; start to add 8.5g of 2-chloro-3-propionylpyridine (1eq) dropwise, 1 drop / s, after the dropwise addition is completed, follow the HPLC until the end of the reaction, add 147.7g of methanol (22mL / g) to terminate the reaction, and collect Dry the system, add the residue to water, extract with 229.5g ethyl acetate (30mL / g), shrink the organic phase to obtain 7.6g of product, yield 88%, liquid chromatography purity (HPLC): 98%, enantiomeric Purity (ee): 99%.
[0049] 1H-NMR: (300MHZ, CDCl3), δ 0.688 (-CH3, t), δ 1.252 (CH2, m), δ 4.418 (H linked to hydroxyl, m), δ 4.499 (-OH, d), δ 7.425 (H at the 5-position of the pyridine ring, m), δ 8.121 (H at the 4-position of the pyridine ring, d), 8.391 (H at the 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com