Monomer with anion recognizing function, oligomer and preparation method thereof
A technology for anion recognition and function, applied in the field of preparation of monomers and oligomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
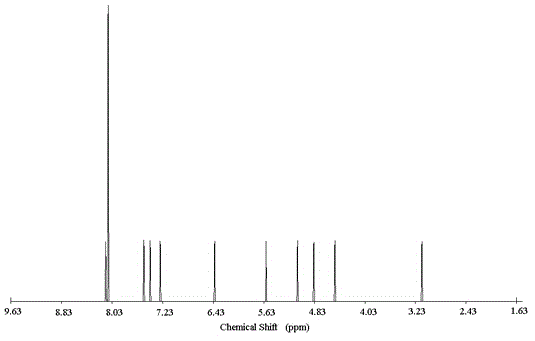
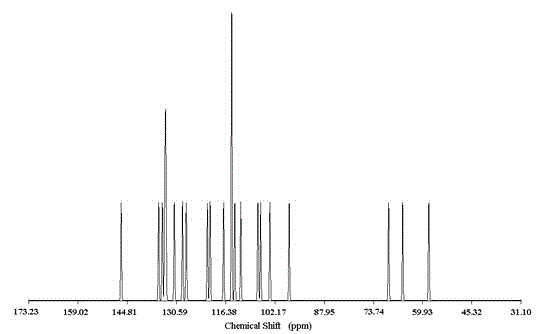
[0052] Degassed DMF / NET with 2-amino-5-iodobenzyl alcohol 2mmol, triphenylphosphine 33umol, bis-(triphenylphosphine)-dichloropalladium 16umol, 48umol CuI 3 (V / V, 2.8ml / 8ml) solution in N 2 Add it into a three-necked flask containing 3.4mmol trimethylsilylacetylene under atmosphere, stir and react at room temperature for 22h, distill the reaction system under reduced pressure, dissolve the residue with dichloromethane, wash with hydrochloric acid solution, and wash the organic layer with anhydrous MgSO 4 After drying, filtration, vacuum distillation and concentration, separation and purification on silica gel column chromatography with ethyl acetate / n-hexane (1 / 2, v / v) as eluent, the intermediate product 2-amino-5-trimethyl Silynyl benzyl alcohol (light yellow liquid, weight 0.35g, yield 90%).
[0053]Dissolve 1.8mmol of 2-amino-5-trimethylsilynyl benzyl alcohol in a mixed solvent of 30.6ml of MeOH / THF (V / V, 0.6ml / 30ml), and add 1M tetrabutylammonium fluoride THF solution 0.6...
Embodiment 2
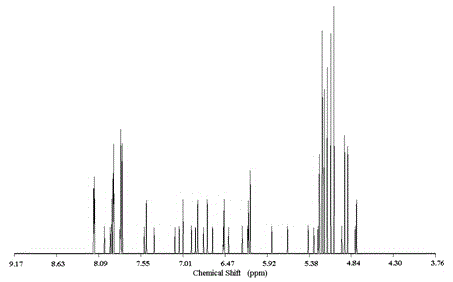
[0058] 2-amino-5-iodobenzyl alcohol 2mmol, triphenylphosphine 33umol, bis-(triphenylphosphine)-dichloropalladium 16umol, CuI (9.1mg, 48umol) degassed DMF / NET 3 (V / V, 2.8ml / 8ml) solution in N 2 Add it into a three-necked flask containing 3.4mmol trimethylsilylacetylene under atmosphere, stir and react at room temperature for 24h, distill the reaction system under reduced pressure, dissolve the residue with dichloromethane, wash with hydrochloric acid solution, and wash the organic layer with anhydrous MgSO 4 After drying, filtration, vacuum distillation and concentration, separation and purification on silica gel column chromatography with ethyl acetate / n-hexane (V / V, 1 / 2) as eluent, the intermediate product 2-amino-5-trimethyl Silynyl benzyl alcohol (light yellow liquid, weight 0.55g, yield 91%).
[0059] Dissolve 1.8mmol of 2-amino-5-trimethylsilynyl benzyl alcohol in 30.6ml of a mixed solvent of MeOH / THF (V / V, 0.6ml / 30ml), and add 1M tetrabutylammonium fluoride in THF Solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com