Synthesis method of chiral epoxy compound and intermediate products and final product
A technology for epoxy compounds and synthesis methods, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of long routes and low resolution yields, achieve good industrial value, process optimization, and improve The effect of raw material utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
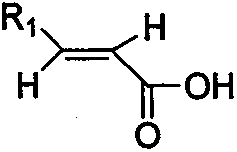
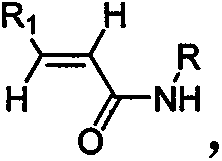
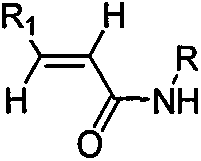
[0039] (1) Preparation of N-cyclopropyl-trans-2-hexenamide
[0040] Add 2.3kg methylene chloride (15ml / g) and 114g trans-2-hexenoic acid (1eq.) to the 5L reaction vessel, add 243gN'N-carbonyldiimidazole (1.5eq.) dropwise to the system, and the addition is complete , the system was refluxed for 4.5h; the temperature was lowered to 10°C, and 142g of cyclopropylamine (2.5eq.) was added dropwise. Incubate at 10±2°C for 12h. The organic phase was washed with an equal volume of 1.5 kg saturated brine, dried, filtered with suction, and the filtrate was spin-dried to obtain a crude product. The crude product was recrystallized with a mixed solvent of 1.7kg ethyl acetate and 216g cyclohexane, the ratio of the mixed solvent to the crude product was 15mL: 1g; wherein the volume ratio of ethyl acetate and cyclohexane was 8mL: 1mL to obtain a solid, the yield 76.8%.
[0041] 1HNMR (300MHz, CDCl3), δ0.501 (cyclopropyl CH2, m), δ0.772 (CH3, m), δ0.916 (CH2, m), δ1.460 (CH2, m), δ2.132 ( ...
Embodiment 2
[0053] (1) Preparation of N-cyclobutyl-trans-2-heptenamide
[0054] Add 3kg of dichloromethane (18ml / g) and 128g of trans-2-heptenoic acid (1eq.) to the 10L reaction vessel, add 356g of thionyl chloride (3eq.) dropwise to the system, after the addition is complete, the system is refluxed for 6h ; Cool to 10 ° C, dropwise added 213g cyclobutylamine (3eq.). Incubate at 10±4°C for 12h. The organic phase was washed with an equal volume of 2 kg saturated brine, dried, filtered with suction, and the filtrate was spin-dried to obtain a crude product. The crude product was recrystallized with a mixed solvent of ethyl acetate and cyclohexane (20 mL / g), wherein 2.5 kg of ethyl acetate and 277 g of cyclohexane were used to obtain a solid with a yield of 74.8%.
[0055] 1HNMR (300MHz, CDCl3), δ1.95 (cyclobutyl CH2, m), δ2.42 (cyclobutyl CH2, m), δ4.06 (cyclobutyl CH, m), δ0.94 (CH3, m ), δ1.33 (CH2, m), δ1.86 (CH2, m), δ6.08 (vinyl H, m), δ6.32 (vinyl H, m), δ8.34 (NH, m) .
[0056] ...
Embodiment 3
[0068] (1) Preparation of N-cyclohexyl-trans-2-octenamide
[0069] Add 2.2kg dichloroethane (12ml / g) in 5L reaction vessel, 142g trans-2-octenoic acid (1eq.), add dropwise 191g1-(3-dimethylaminopropyl)-3-ethane Carbodiimide hydrochloride (1eq.), the dropwise addition was completed, and the system was refluxed for 6h; the temperature was lowered to 10°C, and 149g of cyclohexylamine (1.5eq.) was added dropwise. Incubate at 10±2°C for 12h. The crude product was recrystallized from a mixed solvent of ethyl acetate and cyclohexane (12 mL / g), wherein 1.7 kg of ethyl acetate and 292 g of cyclohexane were used to obtain a solid with a yield of 72.1%.
[0070] 1HNMR (300MHz, CDCl3), δ1.52 (cyclohexyl CH2, m), δ1.39 (cyclohexyl CH2, m), δ1.68 (cyclohexyl CH2, m), δ3.28 (cyclohexyl CH, m) , δ0.94(CH3, m), δ1.33(CH2, m), δ1.86(CH2, m), δ6.52(vinyl H, m), δ6.26(vinyl H, m), δ8.18 (NH, m).
[0071] (2) Preparation of (2S, 3R)-N-cyclohexyl-2,3-epoxy-octylamide
[0072] a. Add 3.9kg tetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com