Leucine dehydrogenase mutant and application thereof
A technology for leucine dehydrogenase and mutants, which is applied to leucine dehydrogenase mutants and their application fields, can solve the problems of high downstream purification cost, difficult engineering amplification, low enzyme activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Construction of engineering strains
[0026] Bacillus thuringiensis (the strain Bacillus thuringiensis serovarkurstaki YBT-1520 preserved in our laboratory) was inoculated in LB medium, cultured at 37°C for 12 hours, collected by centrifugation, and the genomic DNA of Bacillus thuringiensis was extracted using a bacterial genome extraction kit. Using primers BtLeuDH-1 and BtLeuDH-2 to clone Bacillus thuringiensis genomic DNA as a template to obtain Bacillus thuringiensis leucine dehydrogenase gene BtLeuDH, its amino acid sequence is shown in SEQ ID NO.1, and its nucleotide sequence is shown in SEQ ID Shown in NO.2.
[0027] The BtLeuDH gene and expression vector pET28a were digested with restriction endonucleases BamHI and XhoI at 37°C for 4 hours, and then ligated with T4 ligase; the constructed recombinant plasmid pET28a-BtLDH was introduced into E.coli BL21(DE3), and contained Kanamycin (Kan) was cultured overnight on LB plates to obtain engineering ba...
Embodiment 2
[0030] Example 2: Leucine dehydrogenase protein engineering
[0031] (1) Acquisition of mutant library
[0032] Error-prone PCR amplification of BtLeuDH gene fragments, error-prone PCR reaction system: 50 μL system containing 1×Taq DNA polymerase buffer, 0.2mmol / L dATP and dGTP, 1mmol / L dCTP and dTTP, 2-5mmol / L Mg 2+ , 0.2~0.4mmol / L Mn 2+ , 2pmol / μL upstream and downstream primers (see primers BtLeuDH-1 and BtLeuDH-2 in Example 1), using the plasmid pET28a-BtLDH in Example 1 as a template. Error-prone PCR cycle conditions: 94°C for 1.5min, 60°C for 1min, 72°C for 1min, 29 cycles; 72°C for 10min. Among them, by adjusting Mg 2+ and Mn 2+ Libraries with different mutation frequencies can be obtained. The purified mutant BtLeuDH gene fragment was double-digested with BamHI and XhoI, and connected with the same double-digested plasmid pET28a to obtain a mutant recombinant plasmid. The recombinant plasmid containing the mutant gene was transformed into E.coli BL21(DE3), and th...
Embodiment 3
[0036] Embodiment 3: Enzyme activity determination of leucine dehydrogenase mutant BtLDH007
[0037] Seed medium formula: LB medium, yeast powder 5g / L, tryptone 10g / L, NaCl 10g / L.
[0038] Fermentation medium formula: glycerin 8g / L, yeast powder 8g / L, soybean peptone 10g / L, K 2 HPO 4 12H 2 O6.0g / L, KH 2 PO 4 10.0g / L.
[0039] Components of feed medium: glycerol 500g / L, MgSO 4 ·7H 2 O 10g / L.
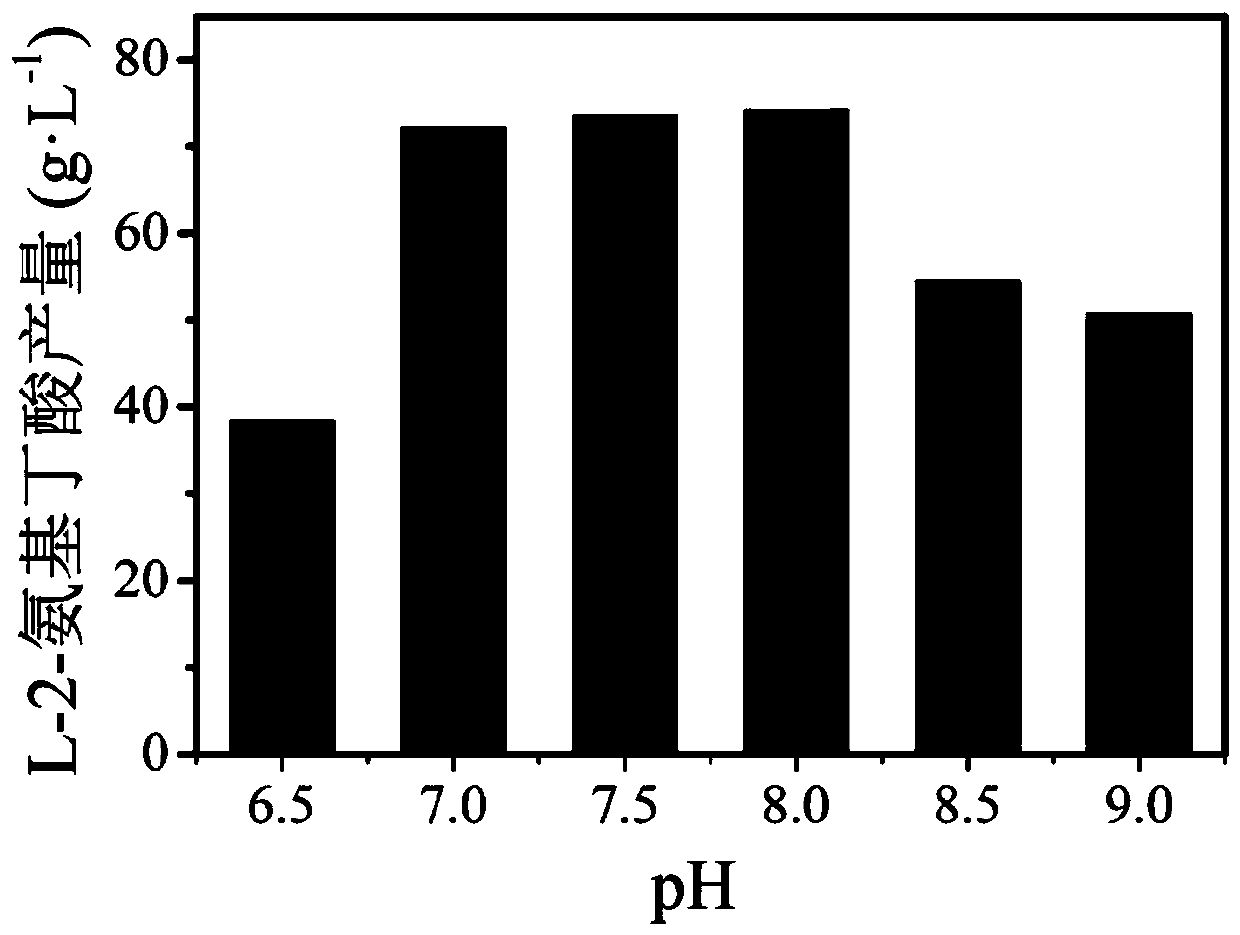
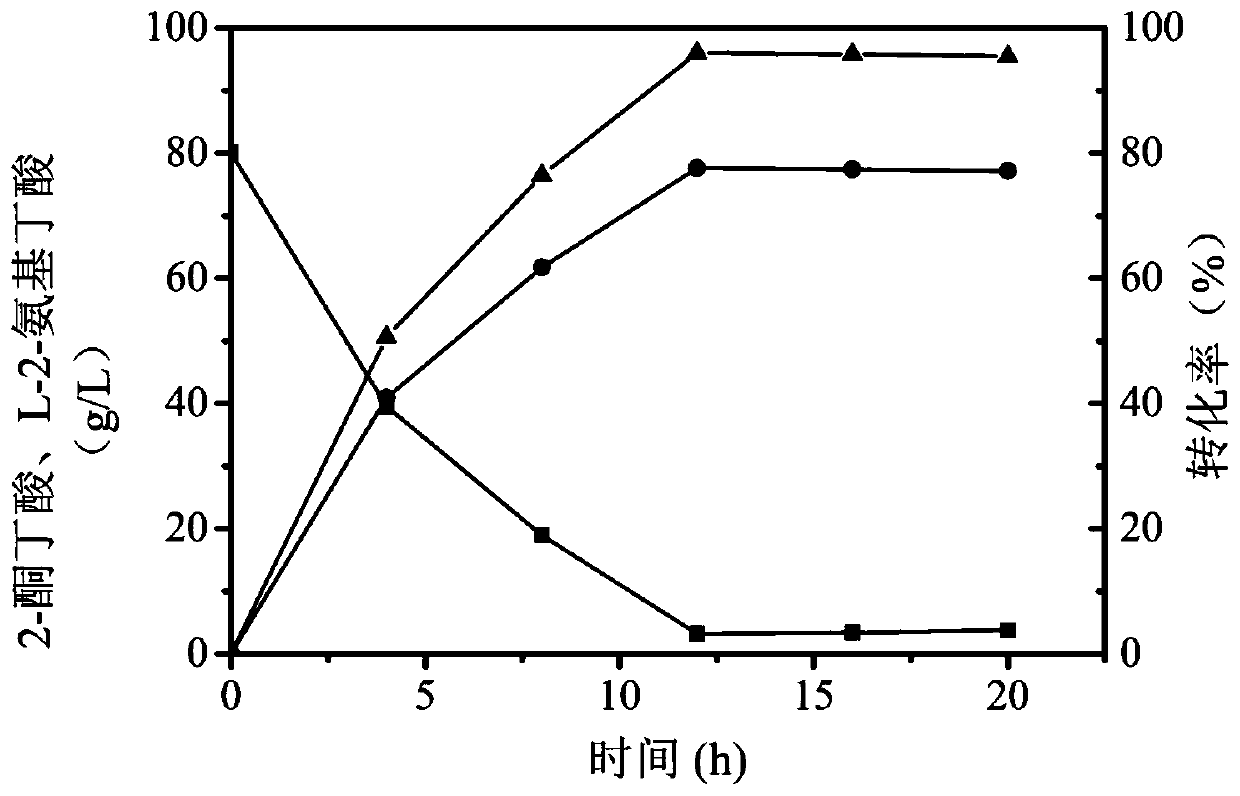
[0040] Coenzyme regeneration system: Since leucine dehydrogenase catalyzes the synthesis of L-2-aminobutyric acid from 2-ketobutyric acid, coenzyme NADH is required, and the coenzyme is expensive, so the coenzyme NADH regeneration system is coupled to increase the number of cycles of NADH , thereby saving costs and improving conversion efficiency. The system consists of: ammonium formate concentration is 20g / L, NAD + The concentration is 0.6g / L, the enzyme activity of formate dehydrogenase is 1000U / L, with ammonium formate as substrate, NAD + Convert to NADH to realize coenzym...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com