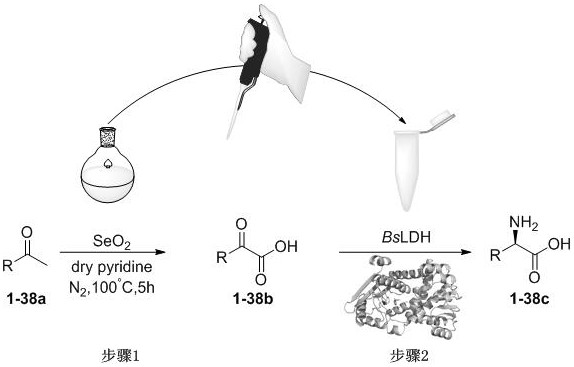
Amino acid dehydrogenase mutant and application thereof
A technology of amino acid dehydrogenase and leucine dehydrogenase, which is applied in the cross field of chemical catalysis and enzyme catalysis to achieve the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Extraction of Bacillus subtilis (B.subtilis) genomic DNA
[0049] Streak and activate B.subtilis frozen at -80°C on LB plate, pick a single colony and inoculate it in 4mL LB liquid medium, cultivate overnight at 37°C, centrifuge at 3000rpm for 5min with a 1.5mL centrifuge tube, discard the supernatant to collect the bacteria , can be repeated several times to obtain a sufficient number of cells; b) extract the Bacillus subtilis genome according to the operating instructions of the TIANGEN Bacteria Genomic DNA Extraction Kit (TIANamp Bacteria DNAKit).
Embodiment 2
[0050] Cloning of embodiment 2 wild-type BsLDH gene
[0051] With the Bacillus subtilis genomic DNA that embodiment 1 obtains as template, carry out PCR reaction, reaction system is as follows:
[0052]
[0053] Amplification program: 94°C, 10min; 34 cycles (94°C: 30s, 45°C: 30s, 72°C: 30s); 72°C, 10min.
[0054] Primer: BsLDH-EcoRI-F CCG GAATTC GATGGAACTTTTTAAATAT
[0055] BsLDH-XhoI-R CCG CTCGAG ACGTCTGCTTAATAC
[0056] Among them, restriction endonuclease cut sites are underlined.
[0057] The DNA fragments amplified by PCR were purified using a gel recovery kit. E.coliDH5α containing the pET-28b-MBP plasmid was cultured overnight in LB liquid medium at 37°C and 200 rpm, and the plasmid was extracted using the reference TIANprep MiniPlasmid Kit.
[0058] The target fragment and plasmid pET-28b-MBP were double-digested with restriction endonucleases EcoRI and XhoI, and the digestion system was as follows:
[0059]
[0060]
[0061] The digested products wer...
Embodiment 3E
[0062] Preparation and transformation of embodiment 3E.coli Rosetta (DE3) competent cells
[0063] a) by CaCl 2 Preparation method Prepare competent cells of E.coli Rosetta (DE3); b) Add 15 μL of T4 ligase reaction solution to 150 μL of competent cell suspension, swirl gently to mix, and ice-bath for 30 minutes; c) Incubate at 42°C Heat shock for 60s, ice bath for 2min, add 800μL LB liquid medium. After static culture at 37° C. for 1 h, shaking culture at 150 rpm for 1 h at the same temperature; d) 150 μL of transformation solution was respectively spread on LB resistance plates.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com