Patents
Literature
47 results about "Norleucine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
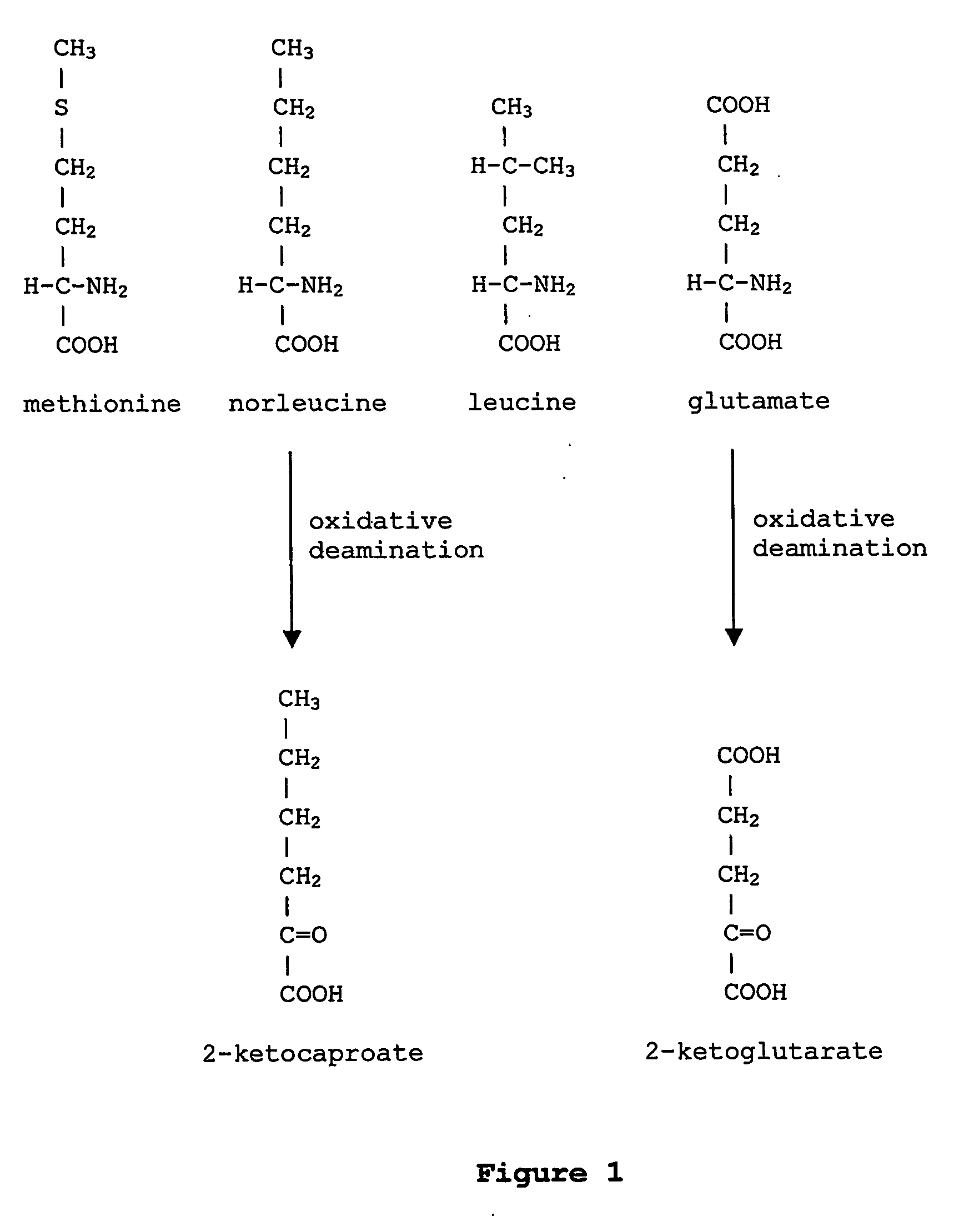
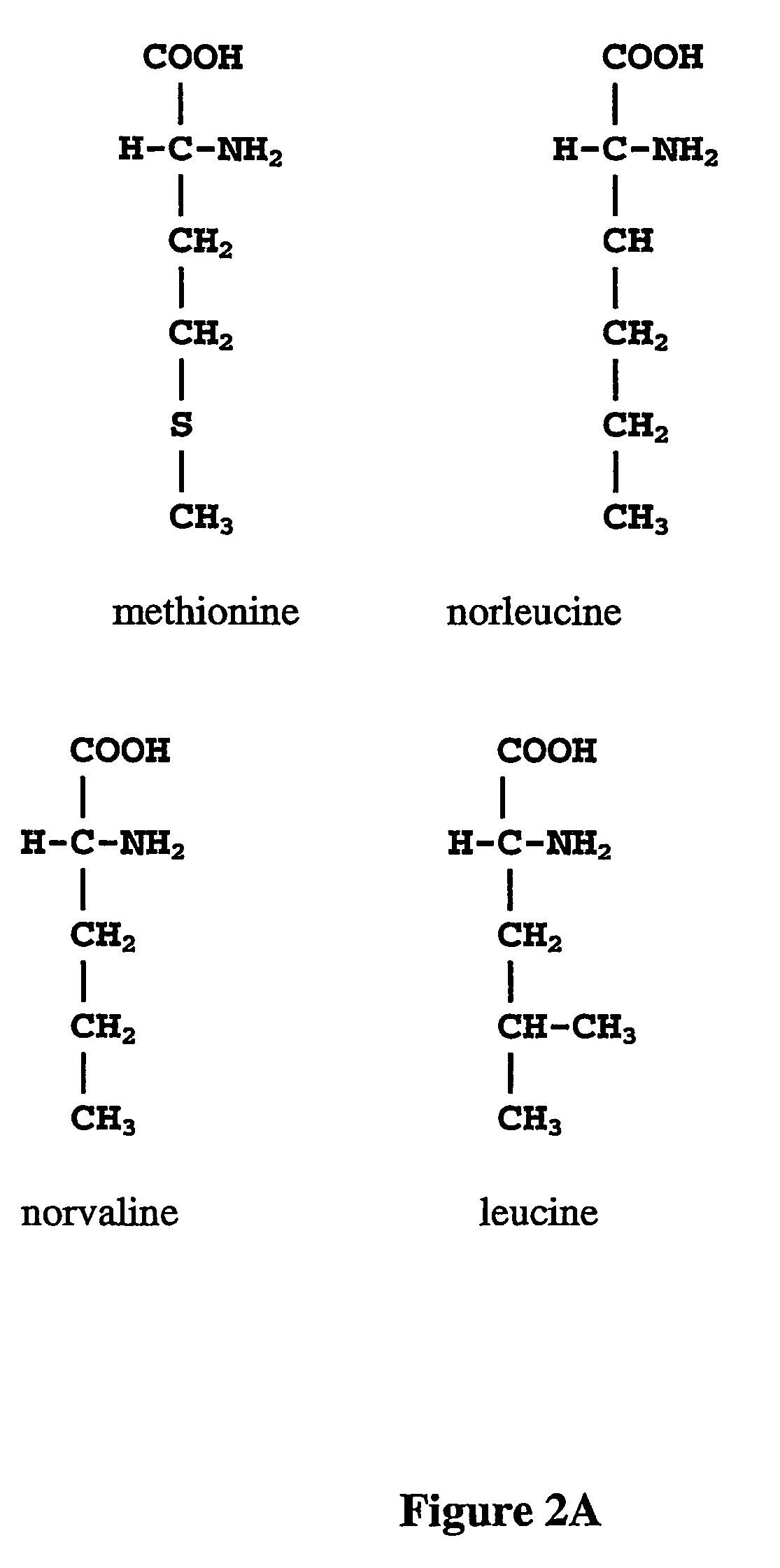
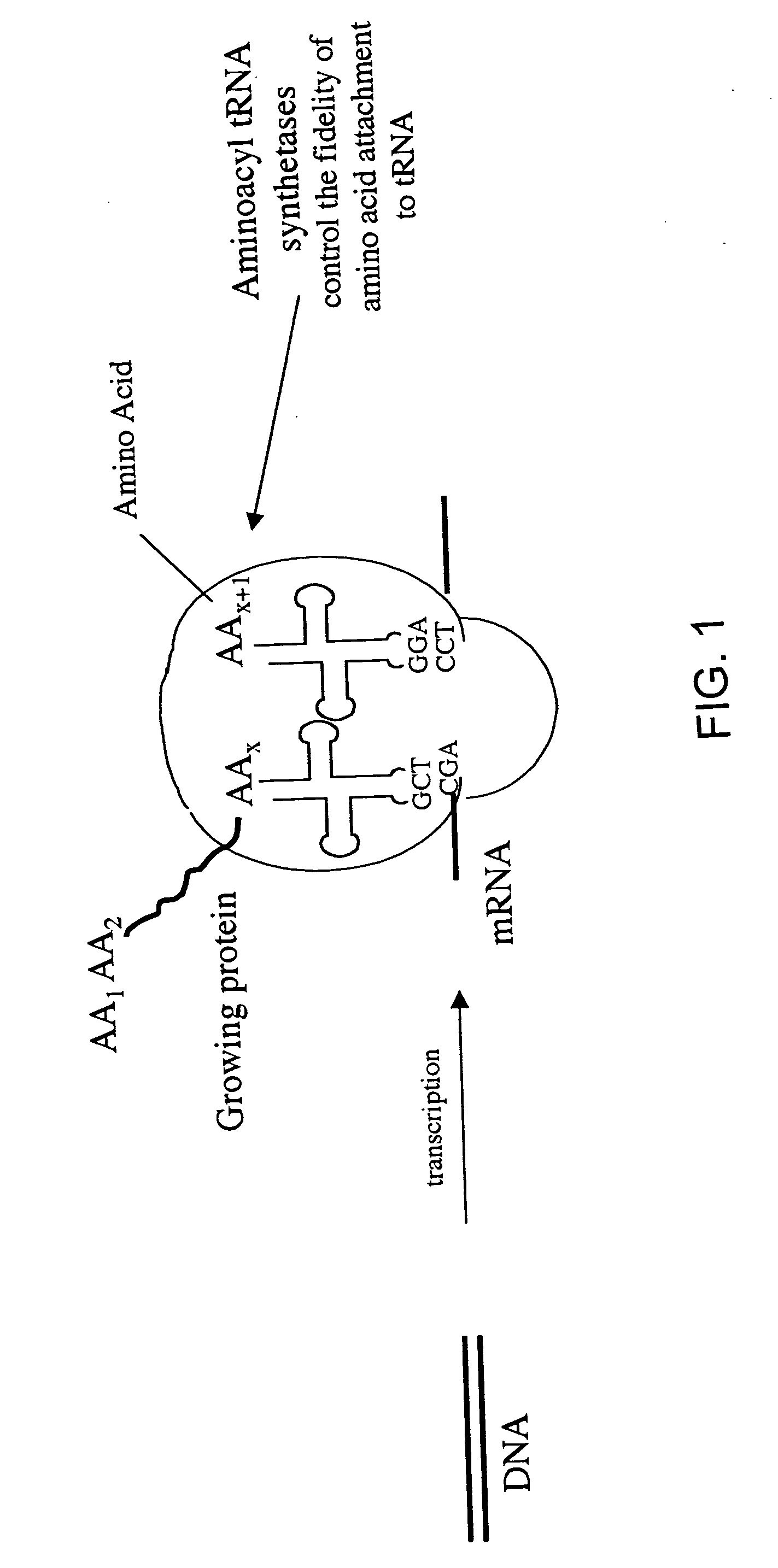
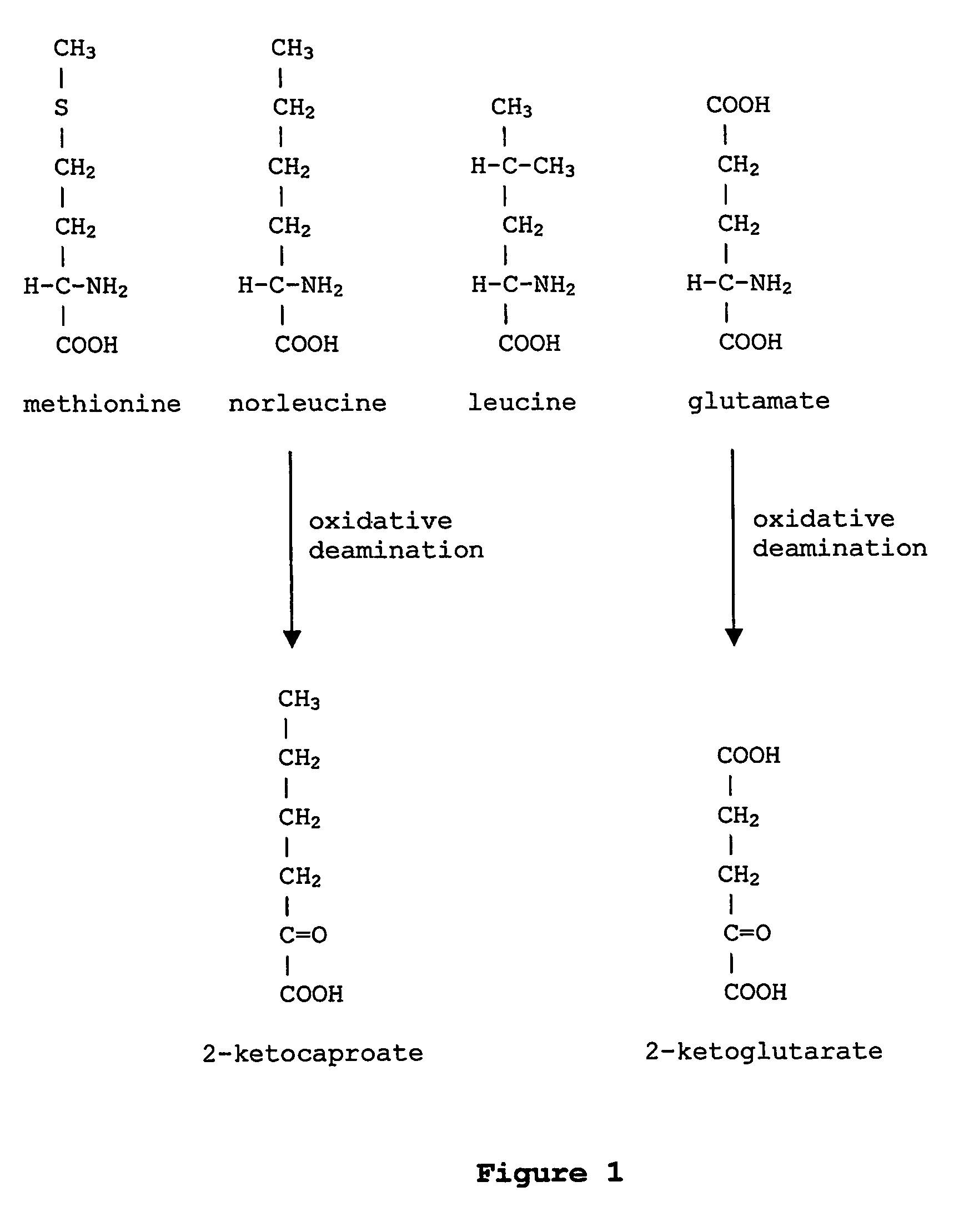
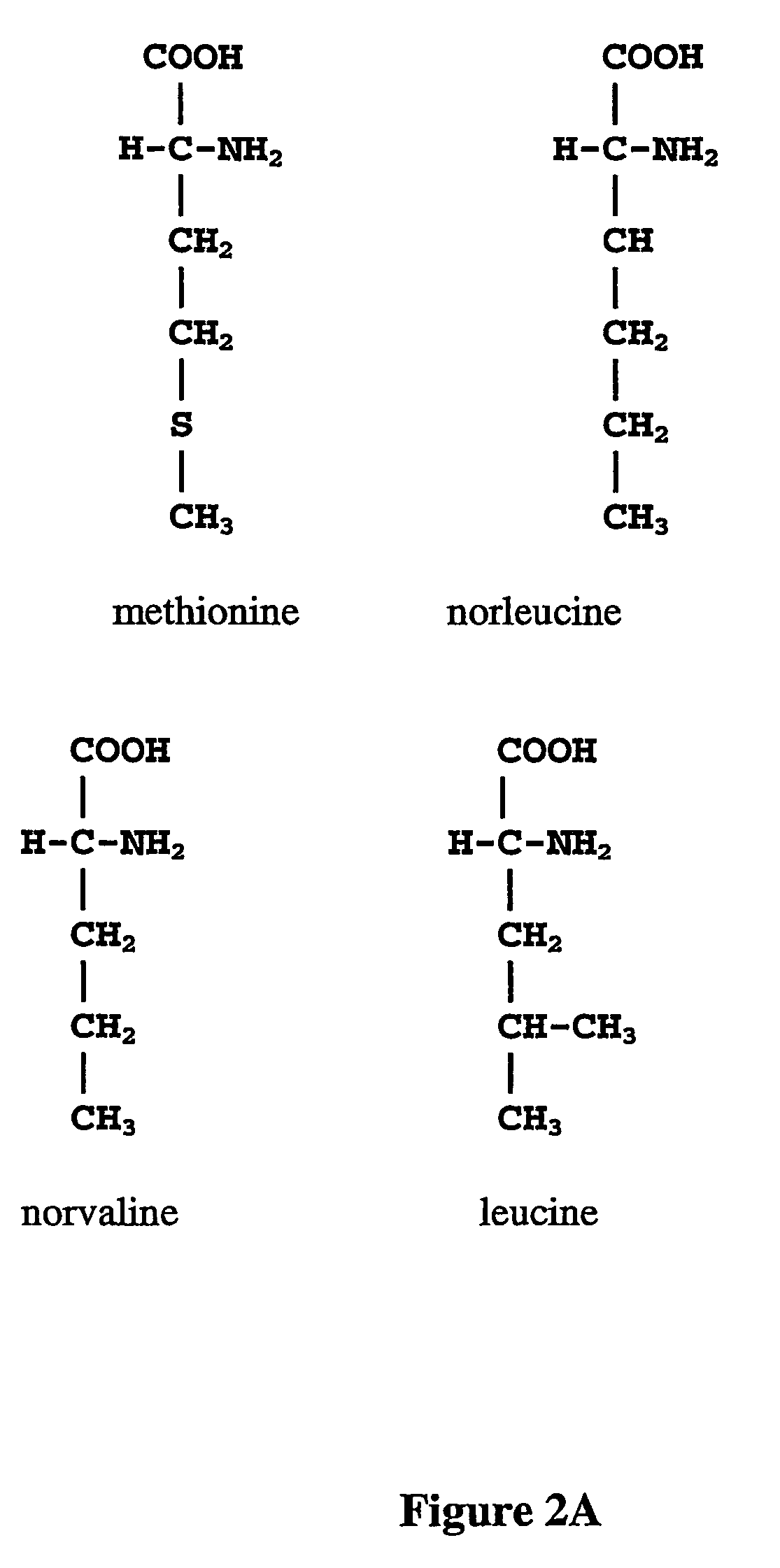
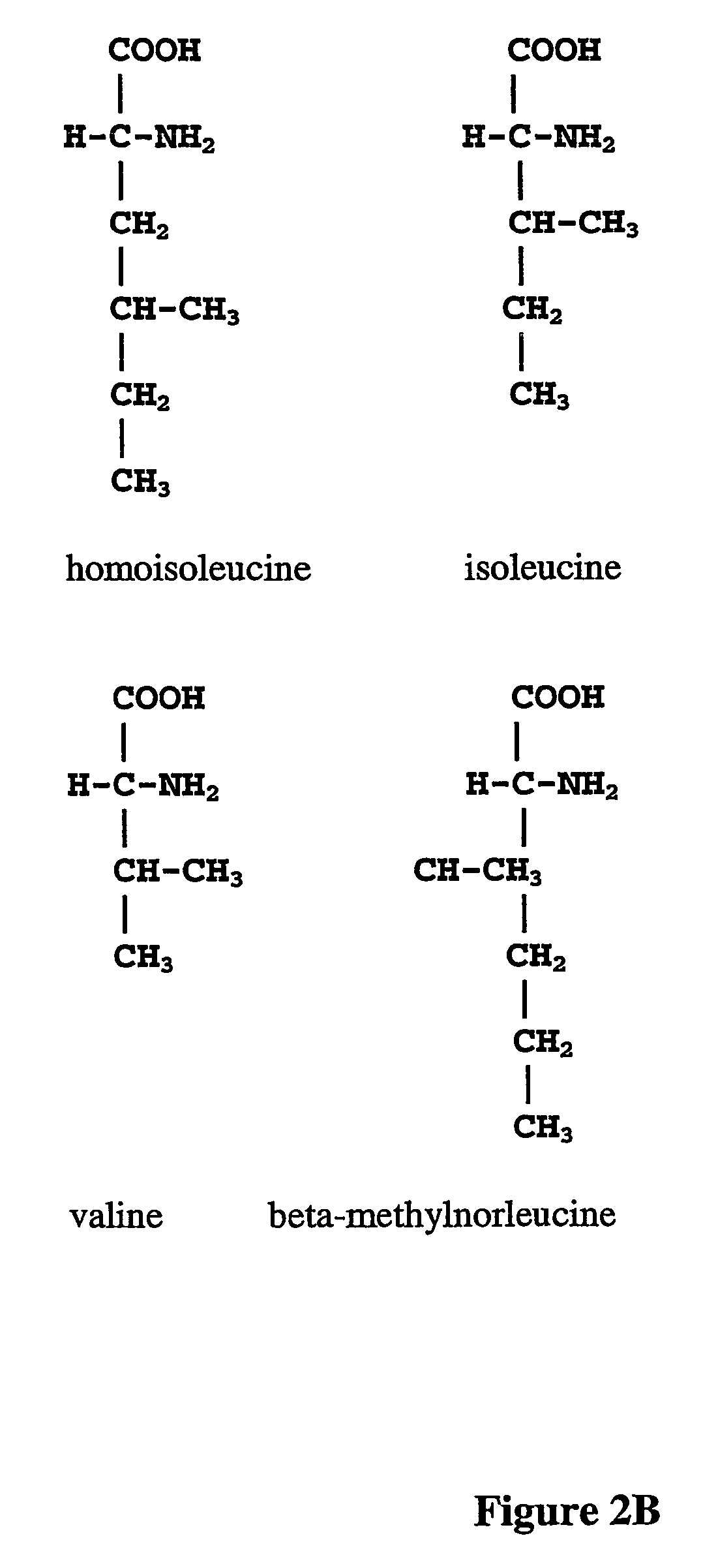
Norleucine (abbreviated as Nle) is an amino acid with the formula CH₃(CH₂)₃CH(NH₂)CO₂H. A systematic name for this compound is 2-aminohexanoic acid. The compound is an isomer of the more common amino acid leucine. Like most other α-amino acids, norleucine is chiral. It is a white, water-soluble solid.
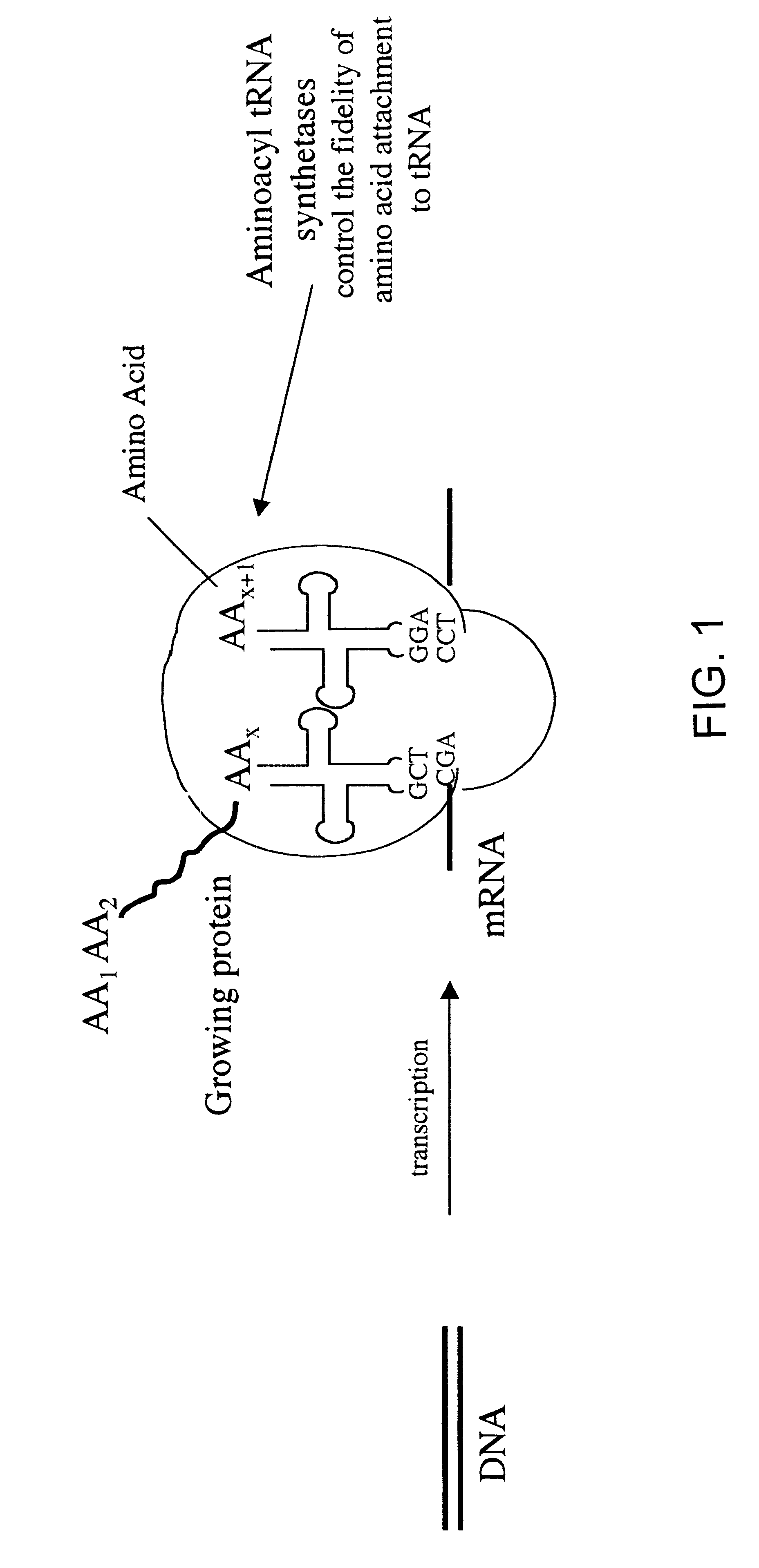
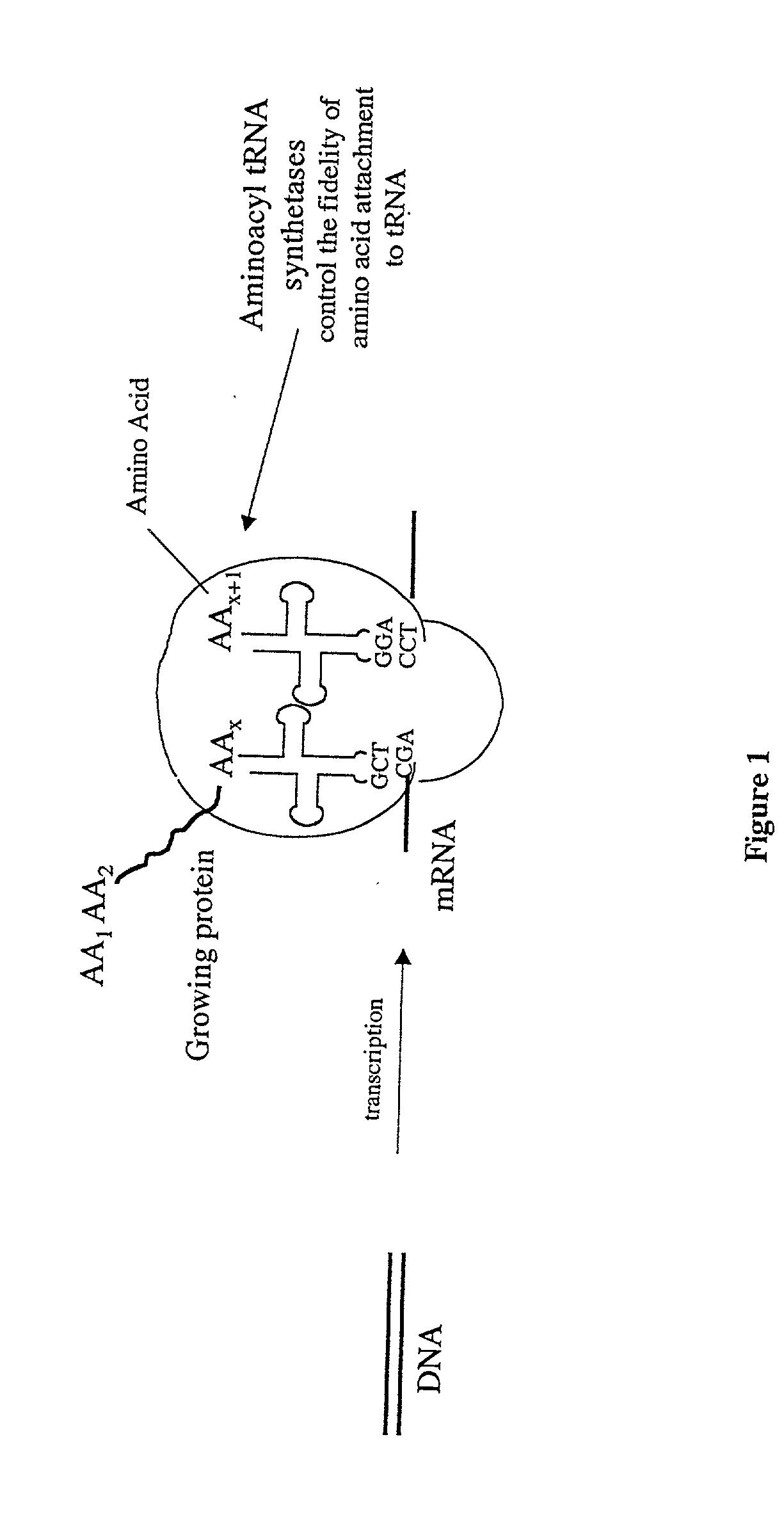
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS6586207B2High yieldRapid and predictable approachBacteriaOxidoreductasesMethionine biosynthesisDihydrofolate reductase
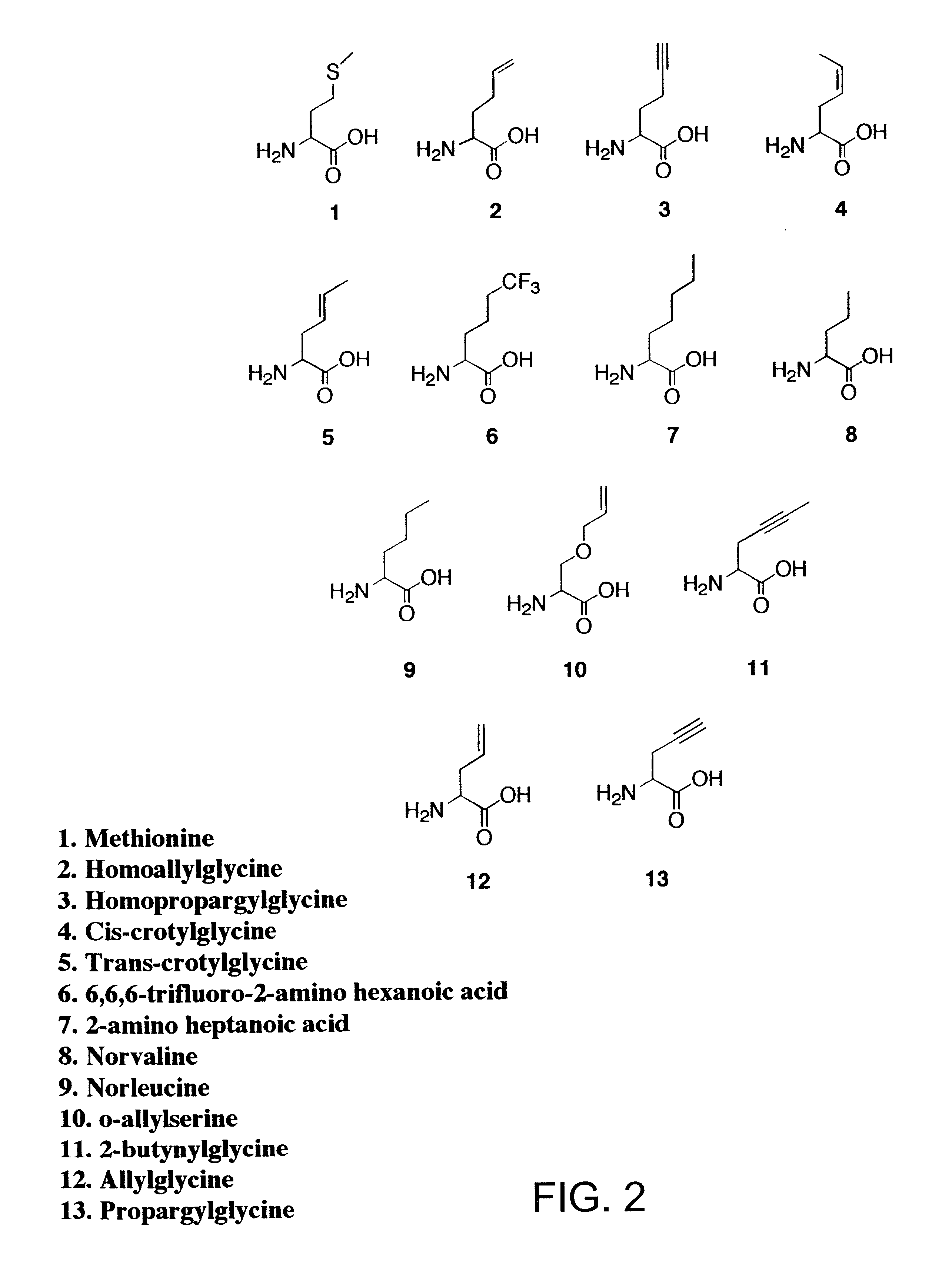
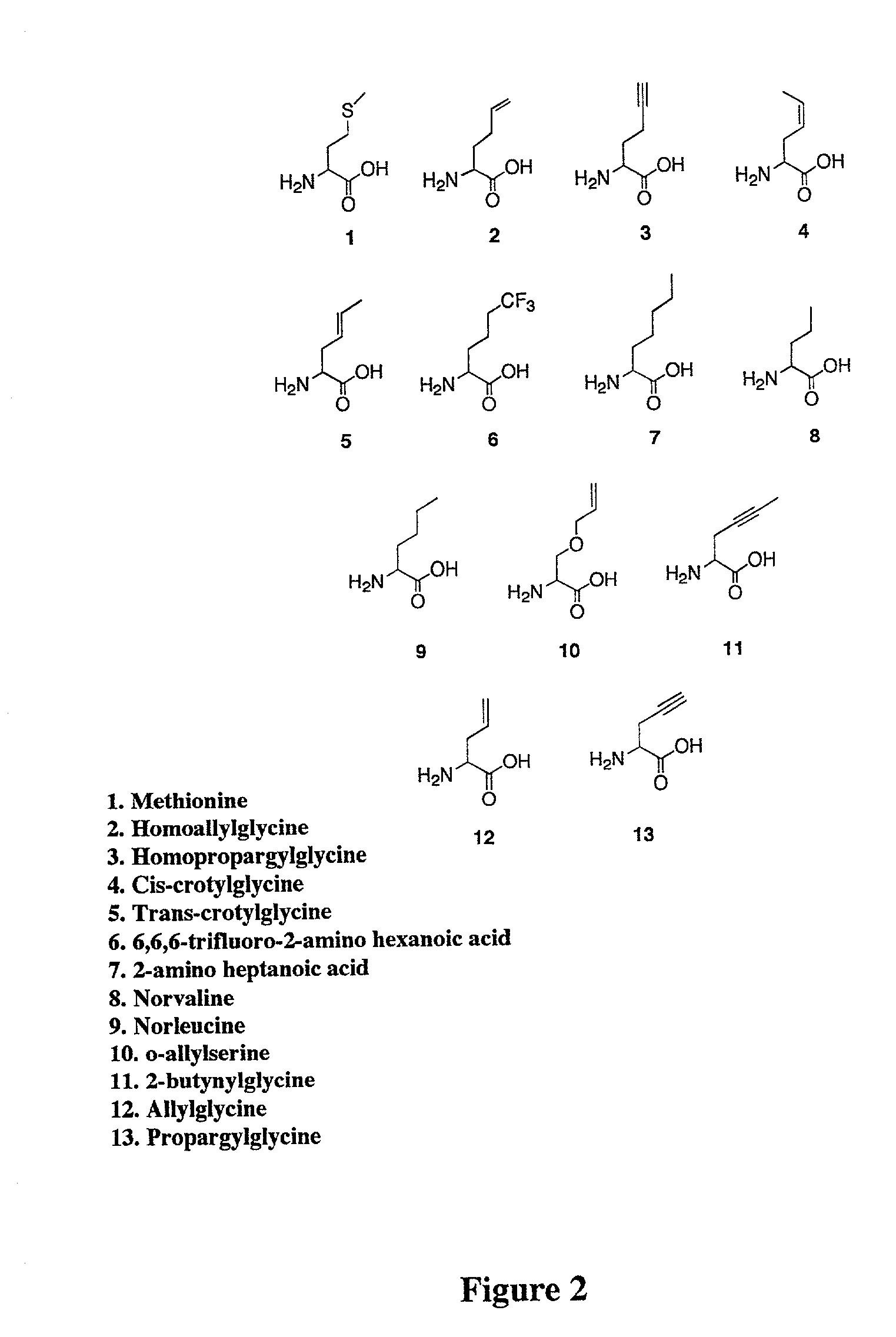
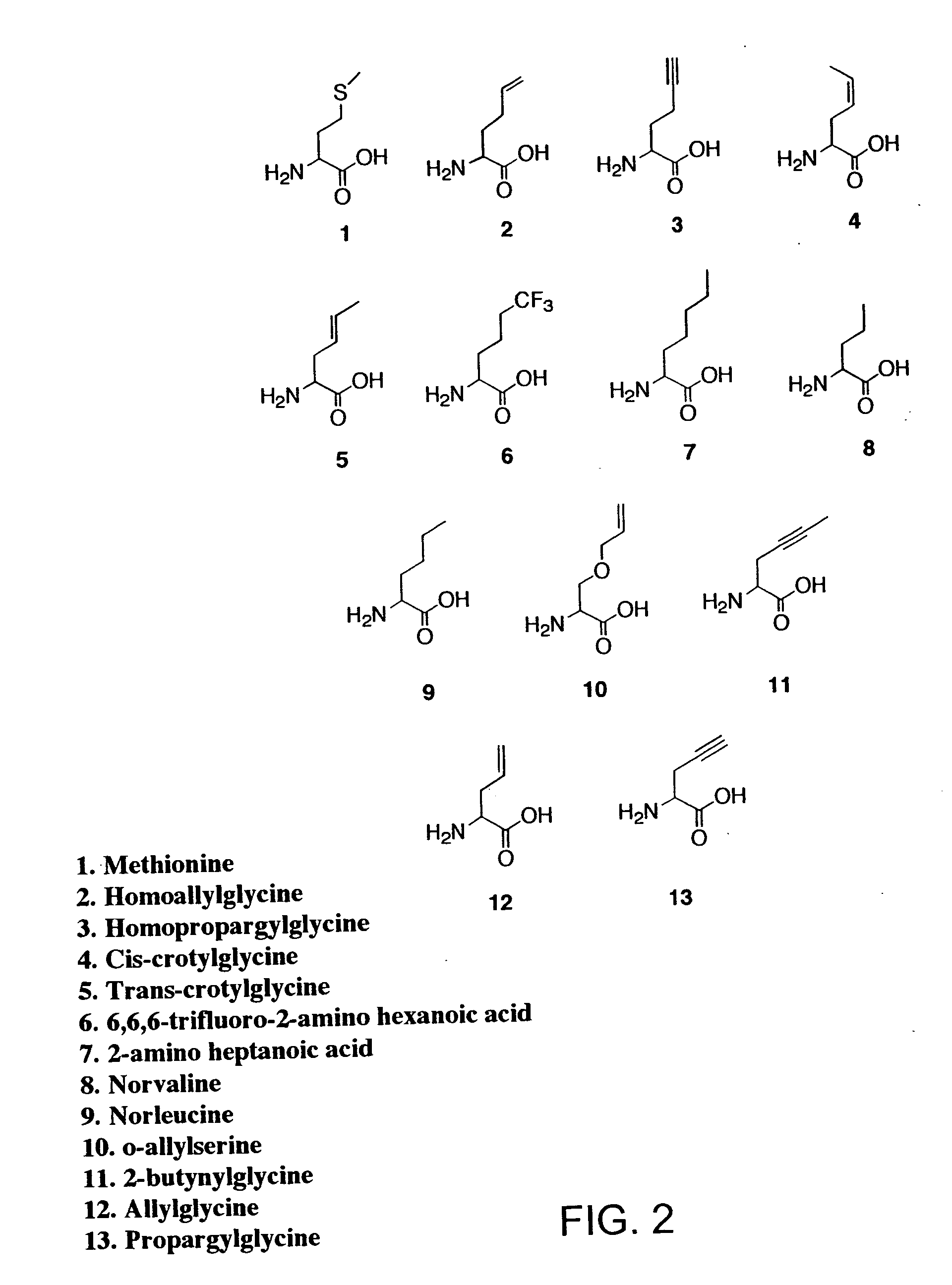
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS20020042097A1High yieldRapid and predictable approachFungiBacteriaMethionine biosynthesisDihydrofolate reductase
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
Epitope analogs
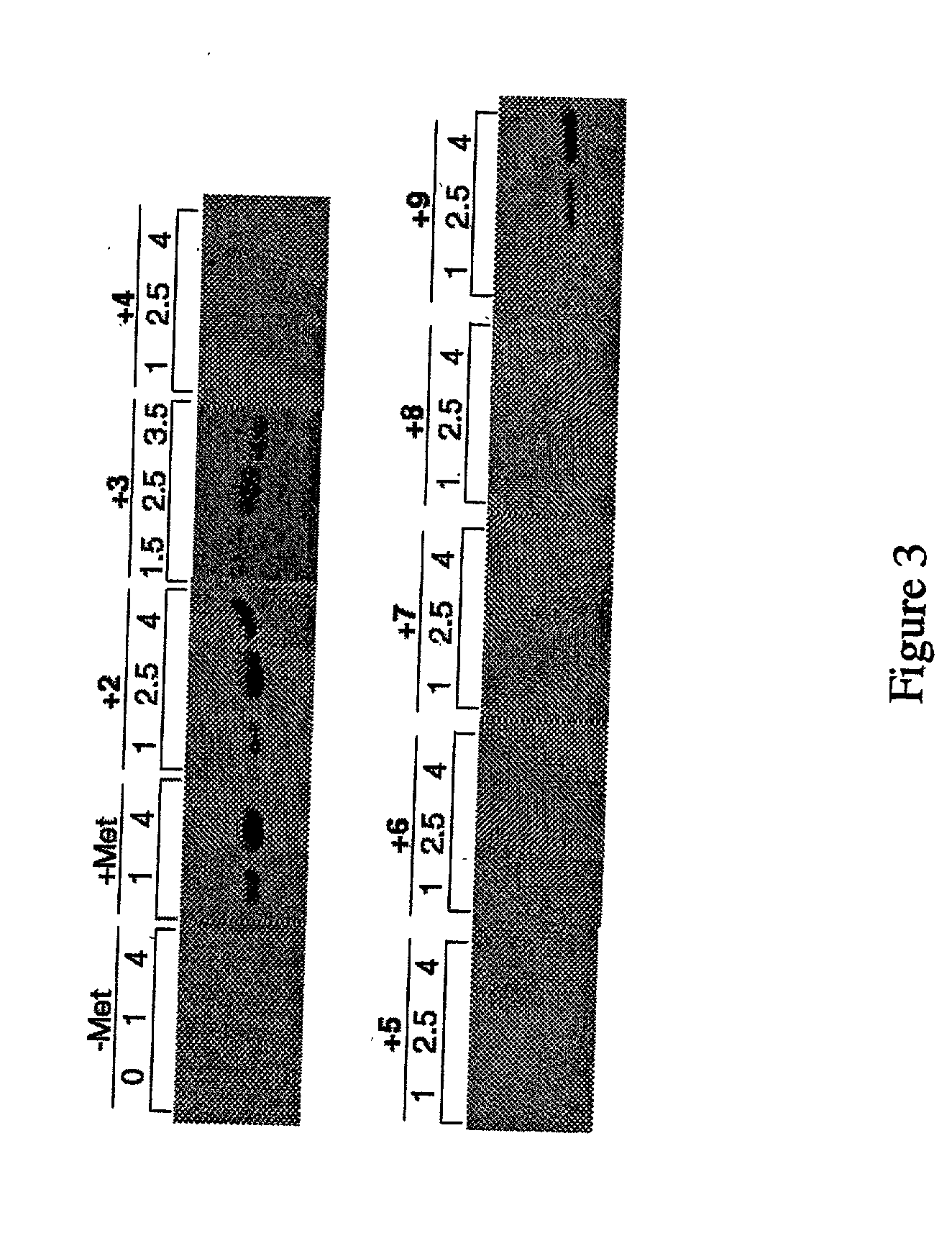
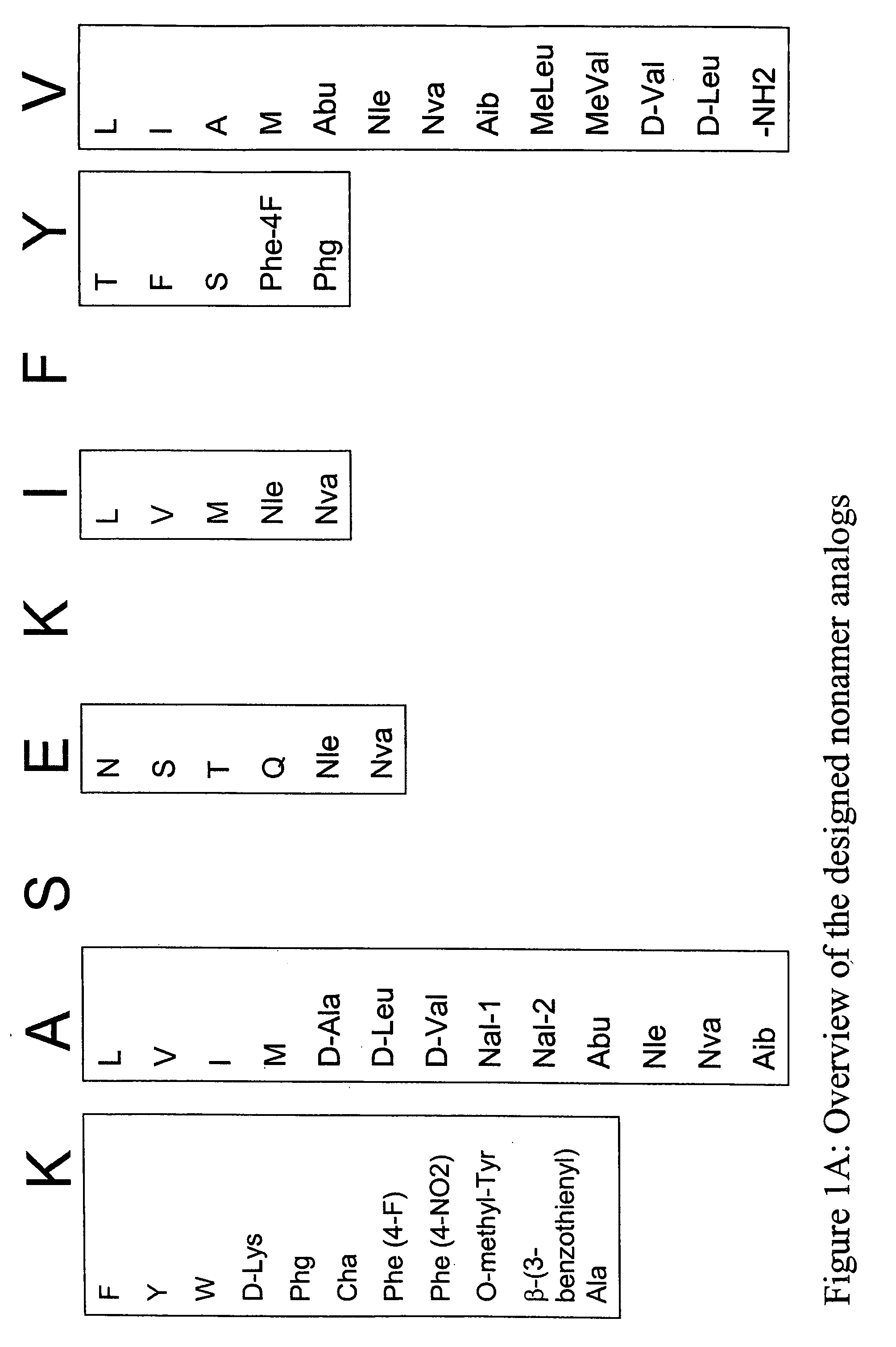
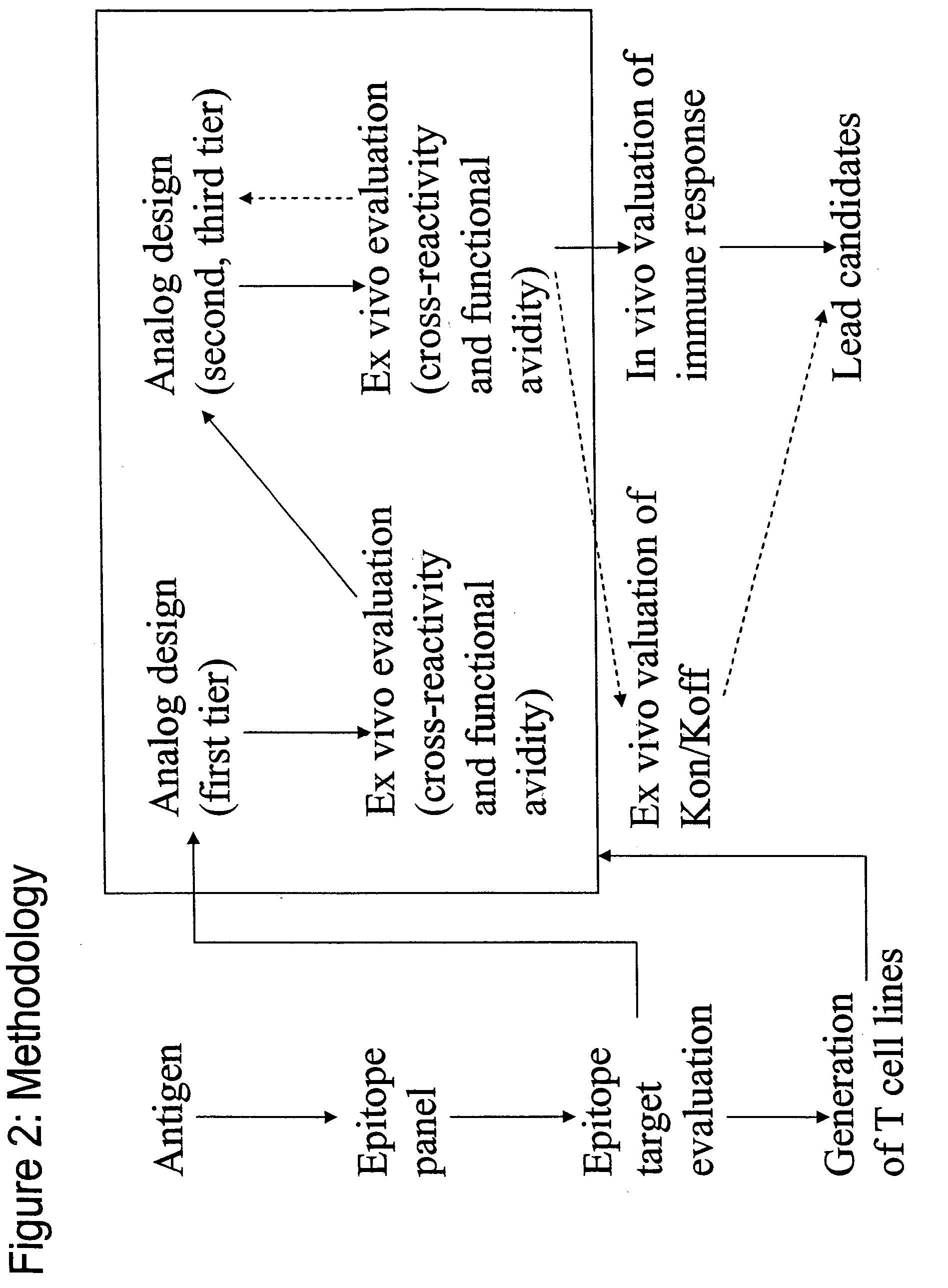
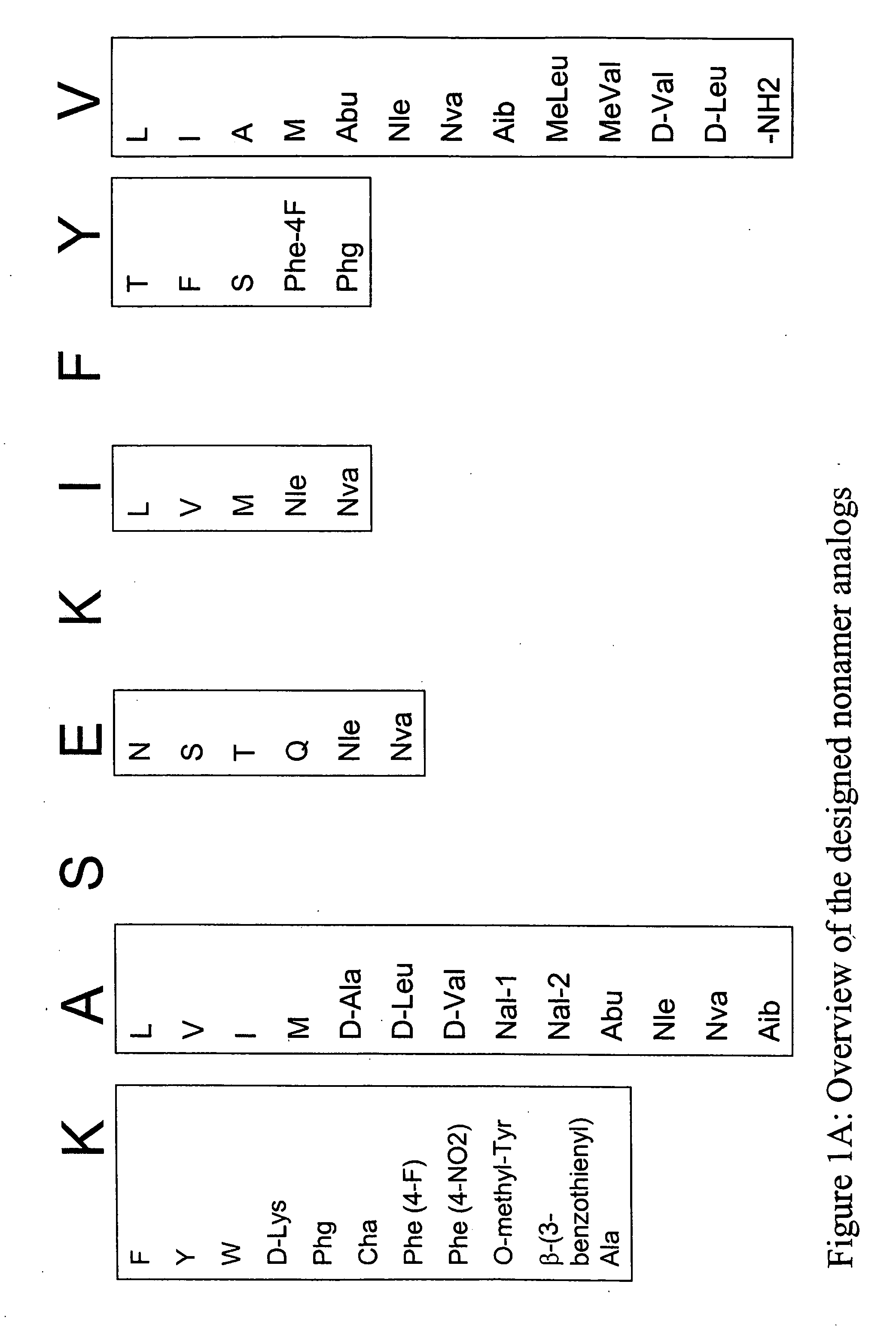
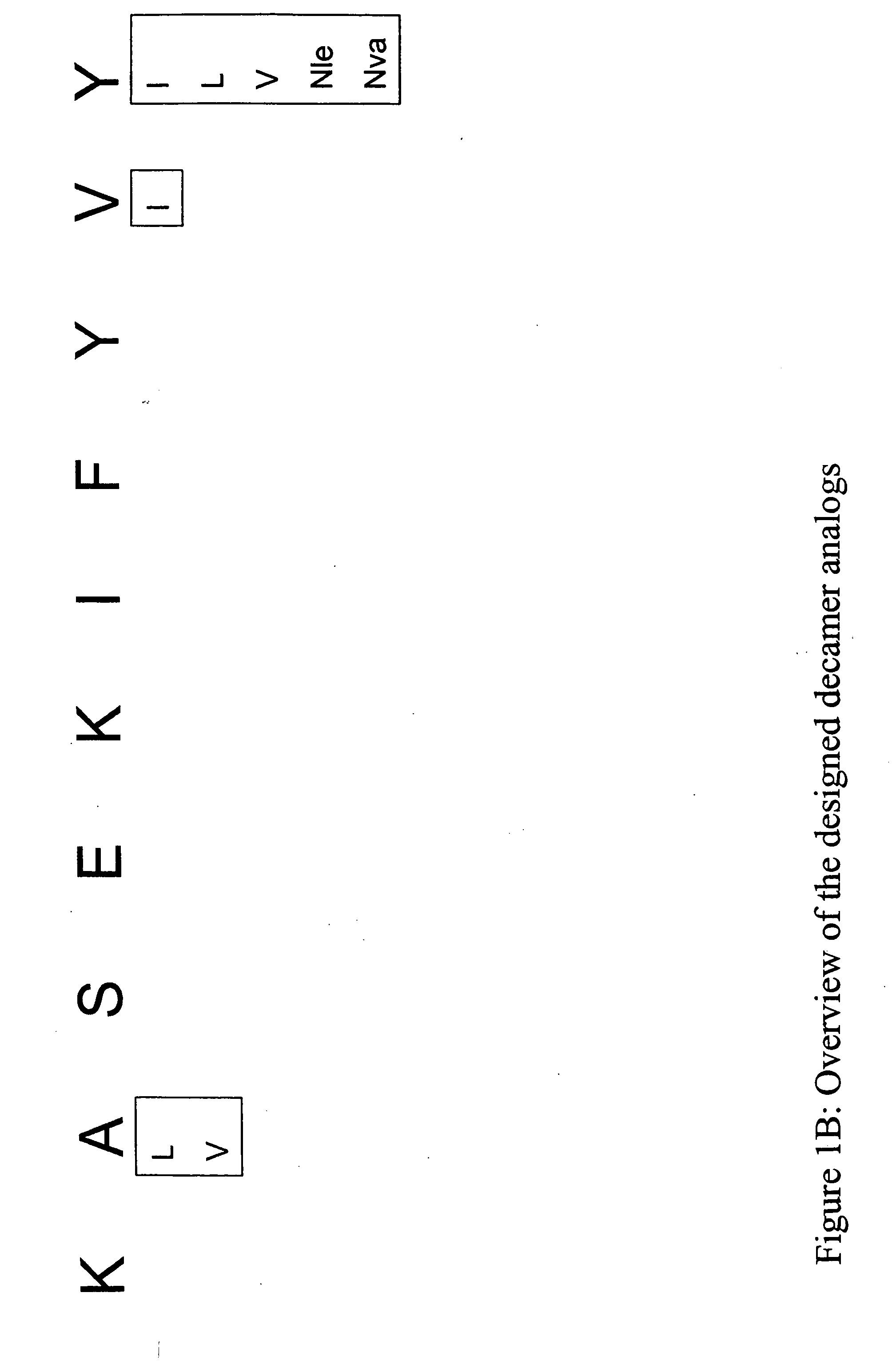
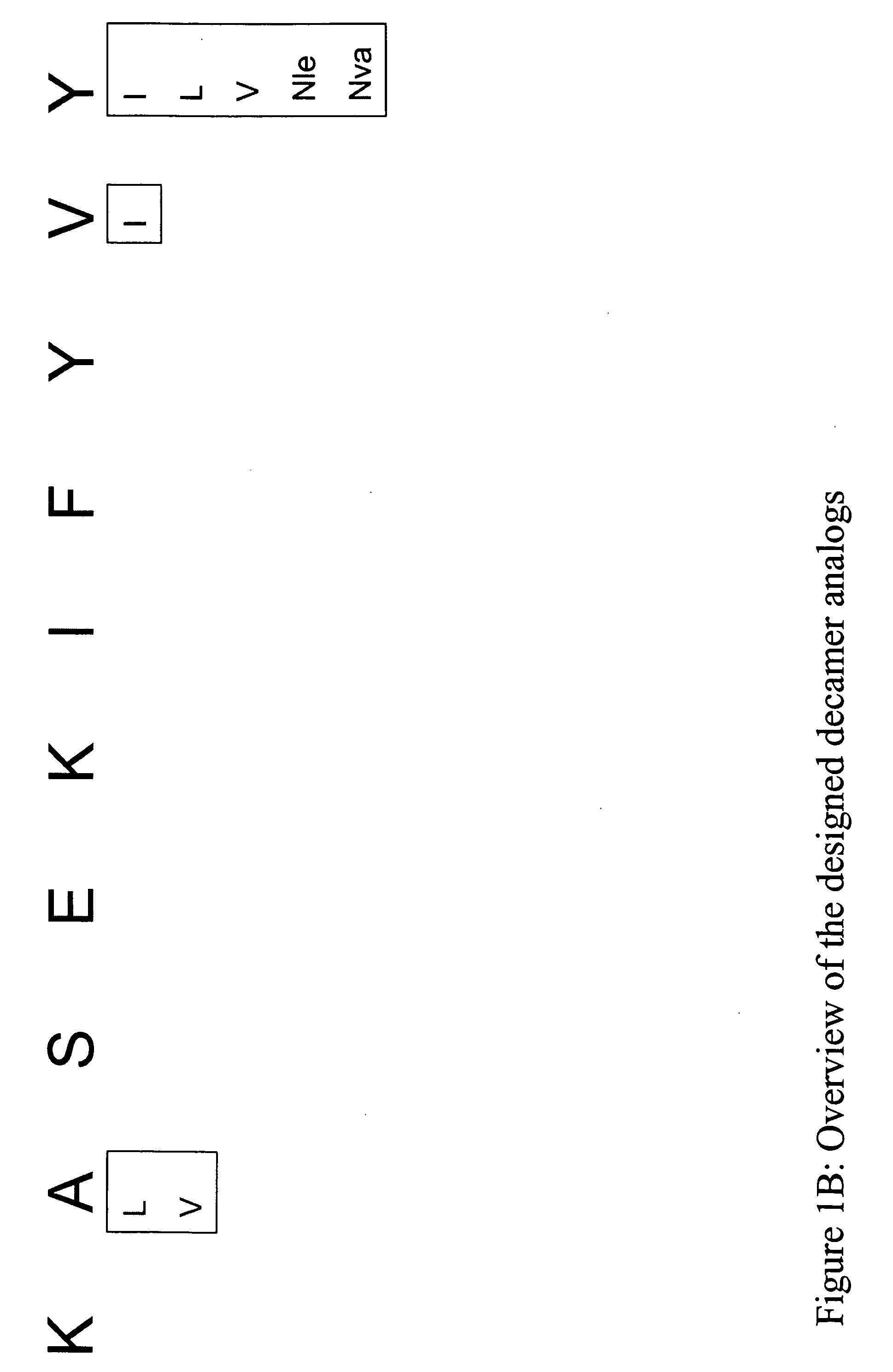
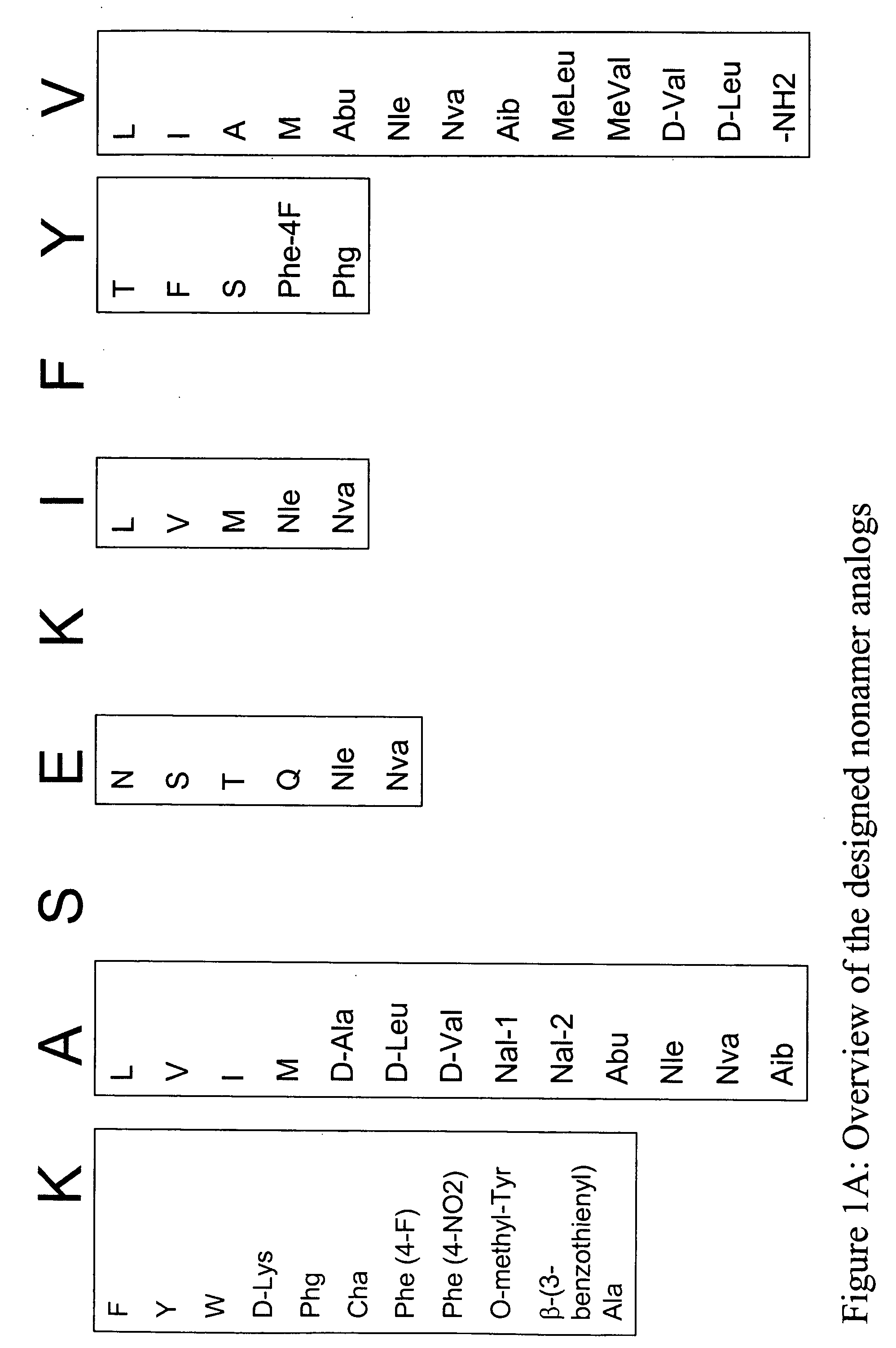
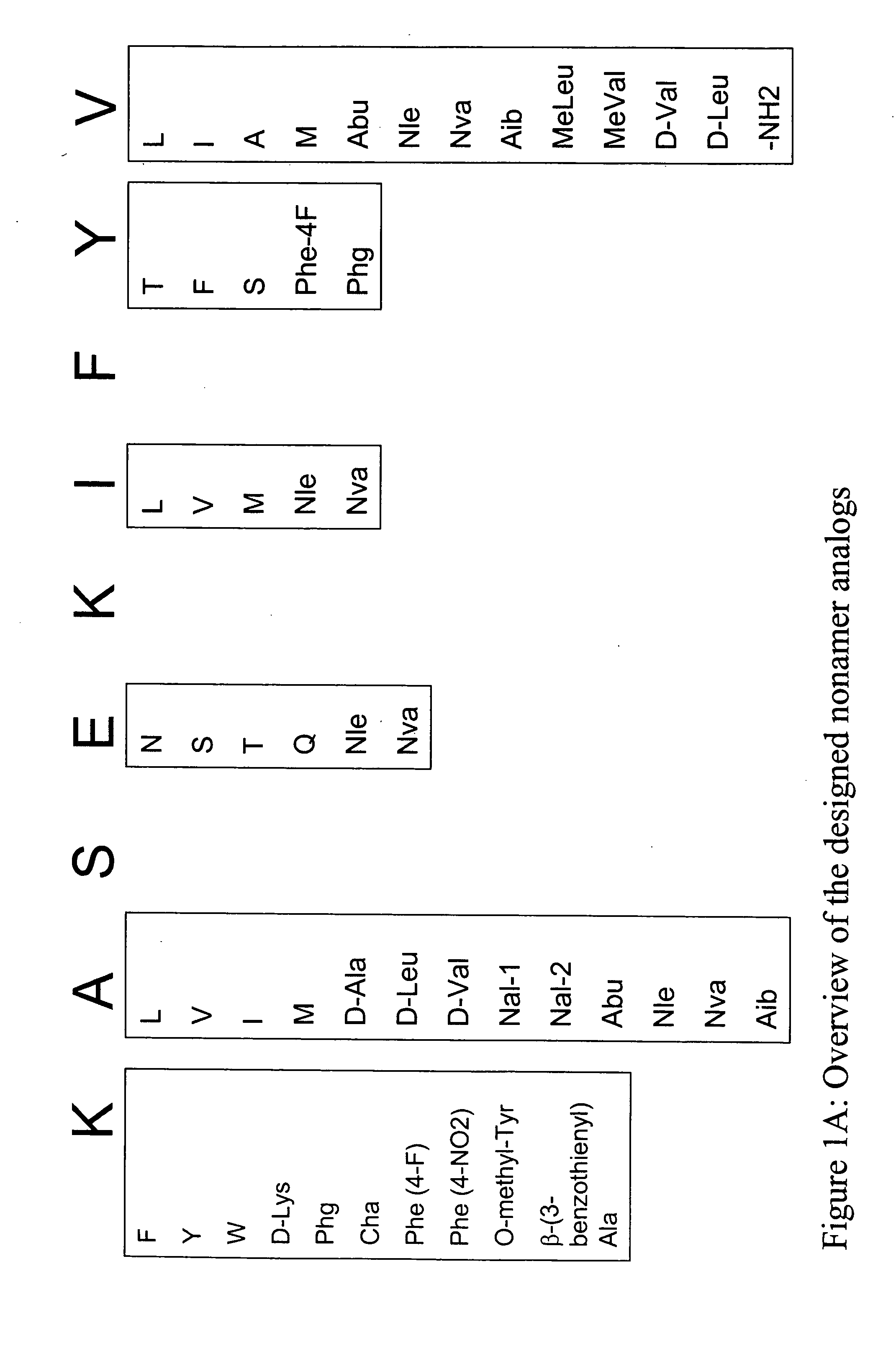
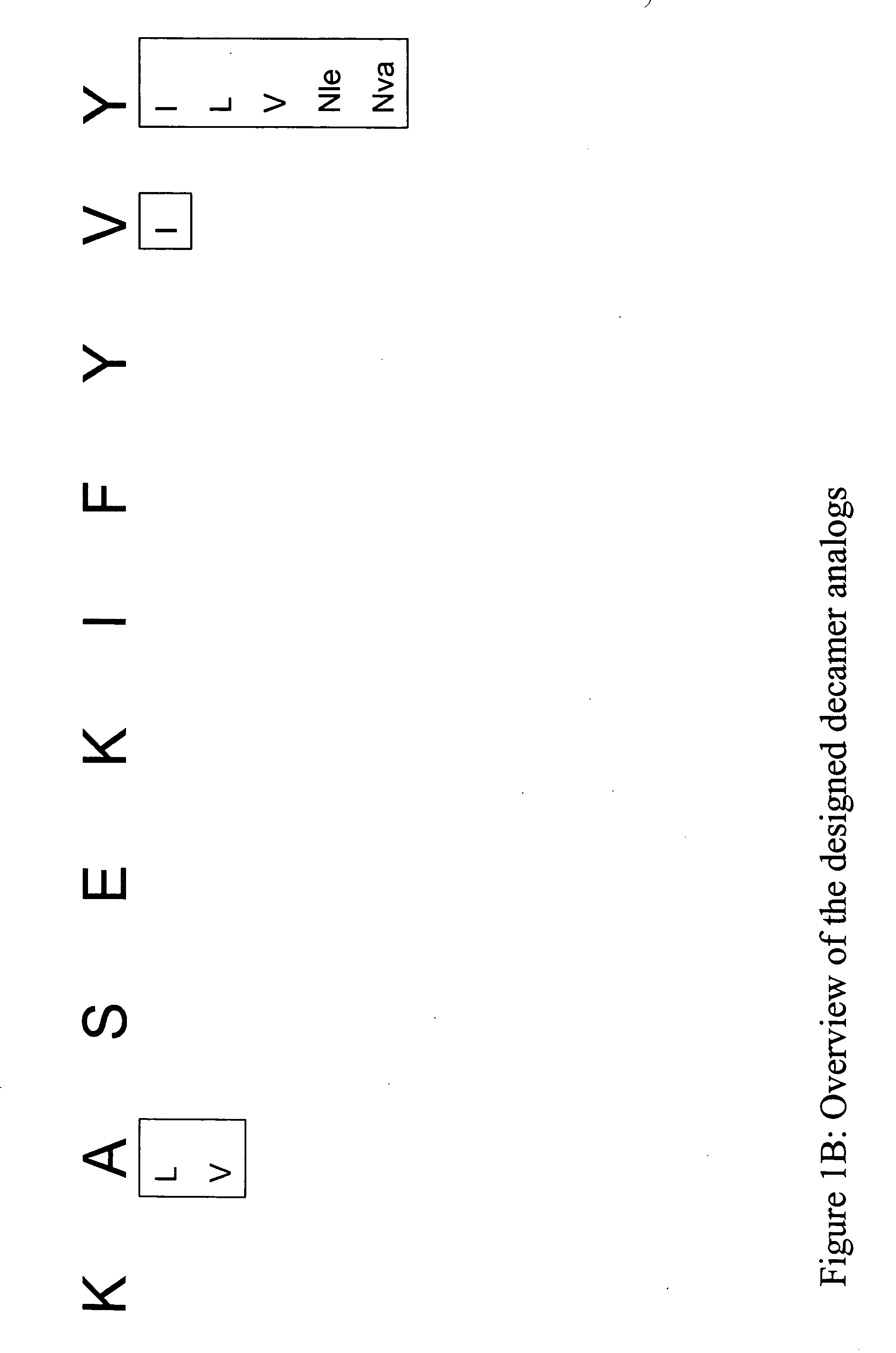
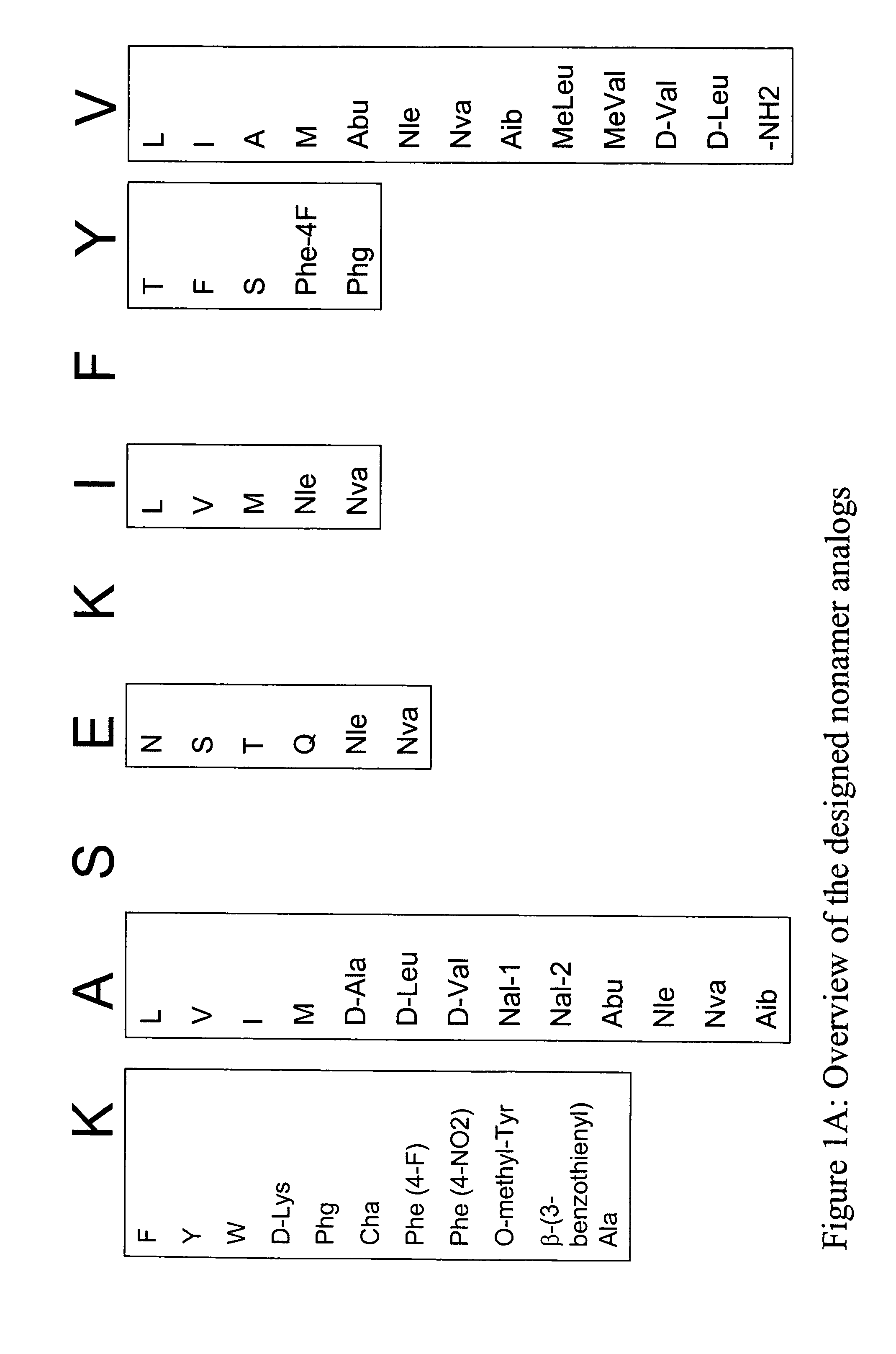
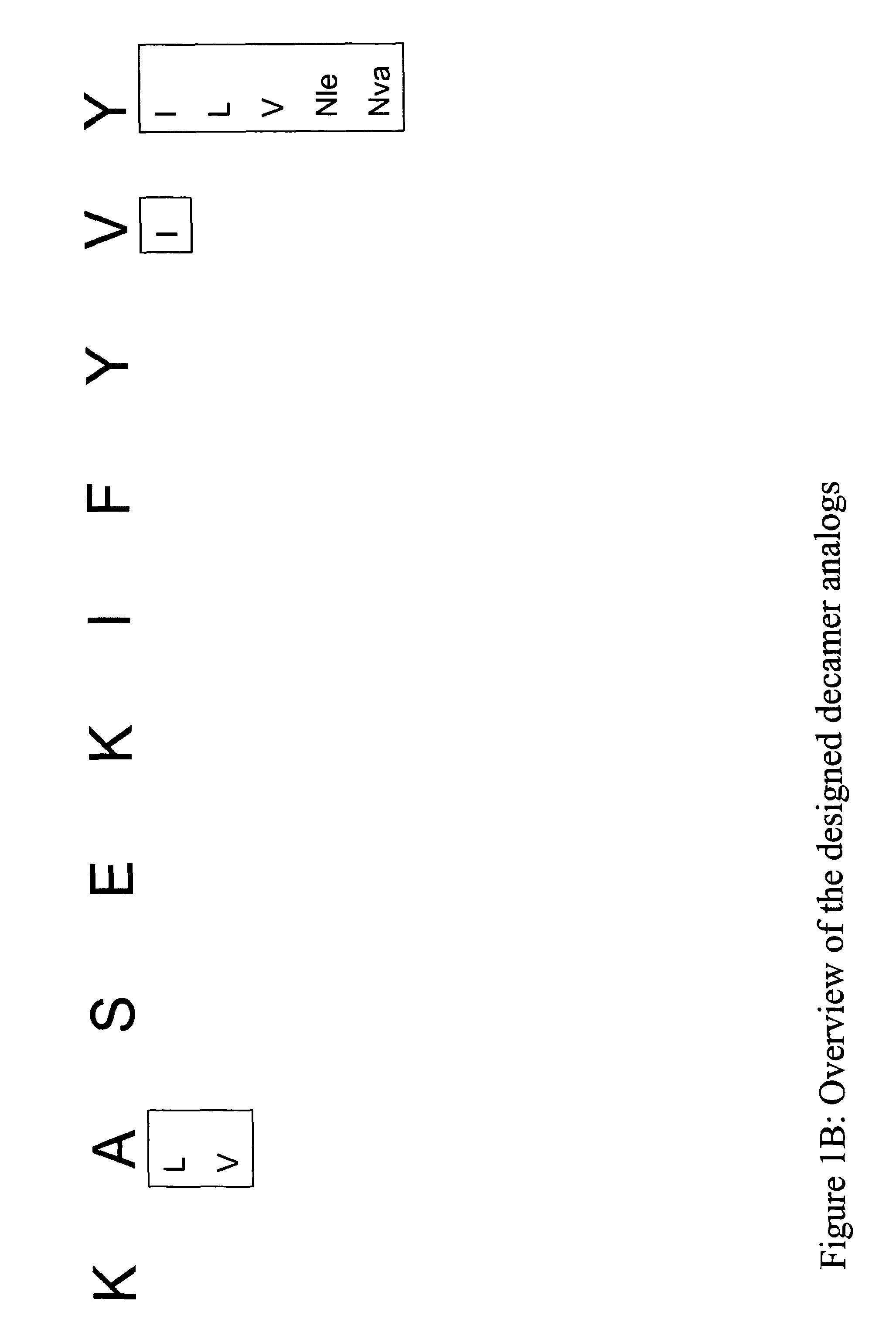
InactiveUS20060057673A1Similar and improved immunological propertyTumor rejection antigen precursorsPeptide/protein ingredientsAmino acid substitutionAmino acid
Owner:MANNKIND CORP
SSX-2 peptide analogs
InactiveUS20060063913A1Tumor rejection antigen precursorsPeptide/protein ingredientsAmino acid substitutionAmino acid
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
Orally administered small peptides synergize statin activity
InactiveUS7148197B2Readily taken up and deliveredMany symptomOrganic active ingredientsPeptide/protein ingredientsThreonineTyrosine
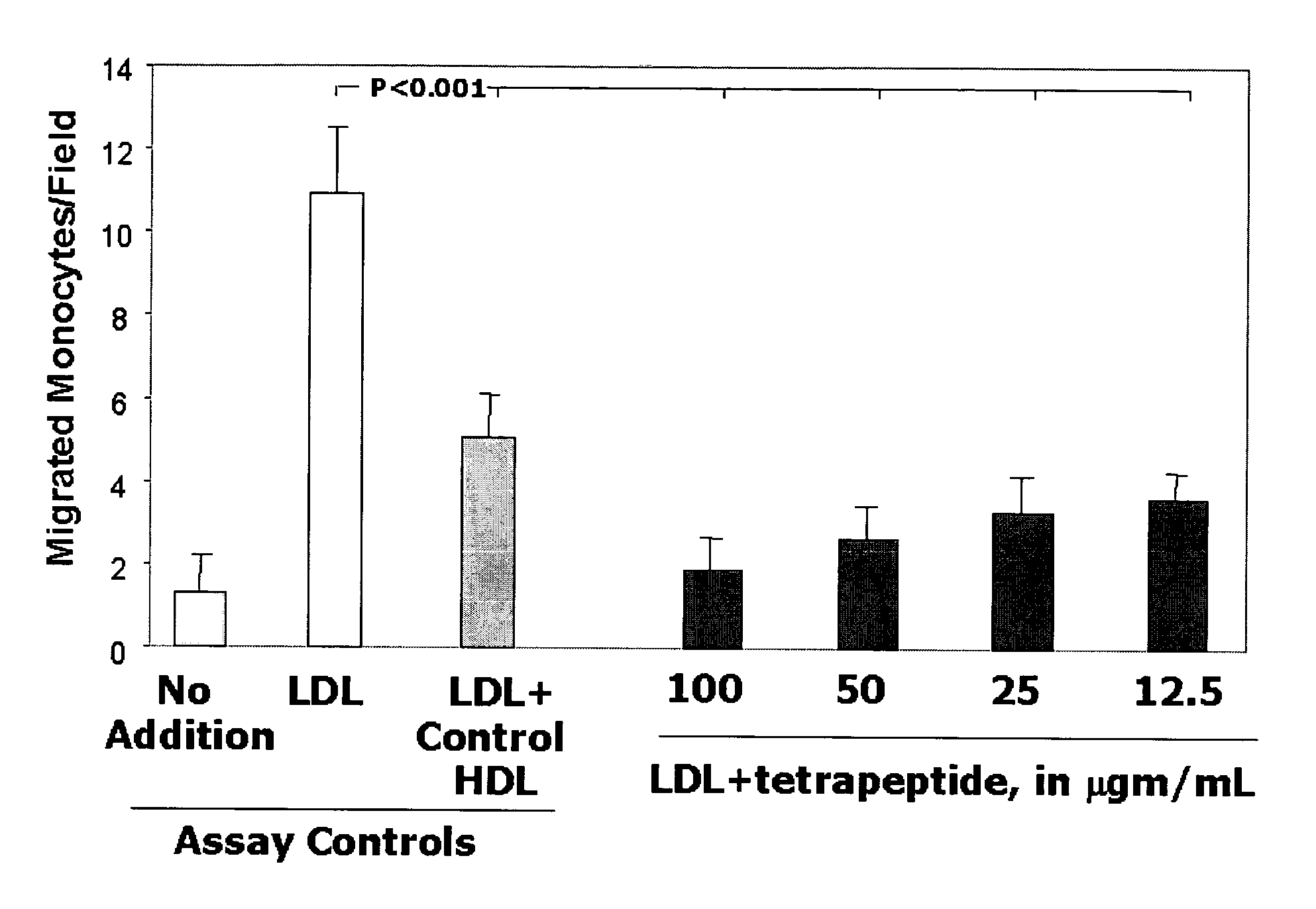
This invention provides novel peptides for the treatment of atherosclerosis. In certain embodiments the peptide is X1-X2-X3-X4 where X1 and X4 are independently selected from the group consisting of alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), tryptophan (Trp), methionine (Met), serine (Ser) bearing a hydrophobic protecting group, beta-naphthyl alanine, alpha-naphthyl alanine, norleucine, cyclohexylalanine, threonine (Thr) bearing a hydrophobic protecting group, tyrosine (Tyr) bearing a hydrophobic protecting group, lysine (Lys) bearing a hydrophobic protecting group, arginine (Arg) bearing a hydrophobic protecting group, ornithine (Orn) bearing a hydrophobic protecting group, aspartic acid (Asp) bearing a hydrophobic protecting group, cysteine (Cys) bearing a hydrophobic protecting group, and glutamic acid (Glu) bearing a hydrophobic protecting group; X2 and X3 are independently selected from the group consisting of Asp, Arg, and Glu; and the peptide converts pro-inflammatory HDL to anti-inflammatory HDL or makes anti-inflammatory HDL more anti-inflammatory.
Owner:RGT UNIV OF CALIFORNIA +1
Prevention of incorporation of non-standard amino acids into protein
ActiveUS20070009995A1Cellular level is reducedReducing, or substantially eliminating, endogenous cellular levels of norleucineBacteriaPeptide/protein ingredientsBeta-methylnorleucinePhenylalanine dehydrogenase
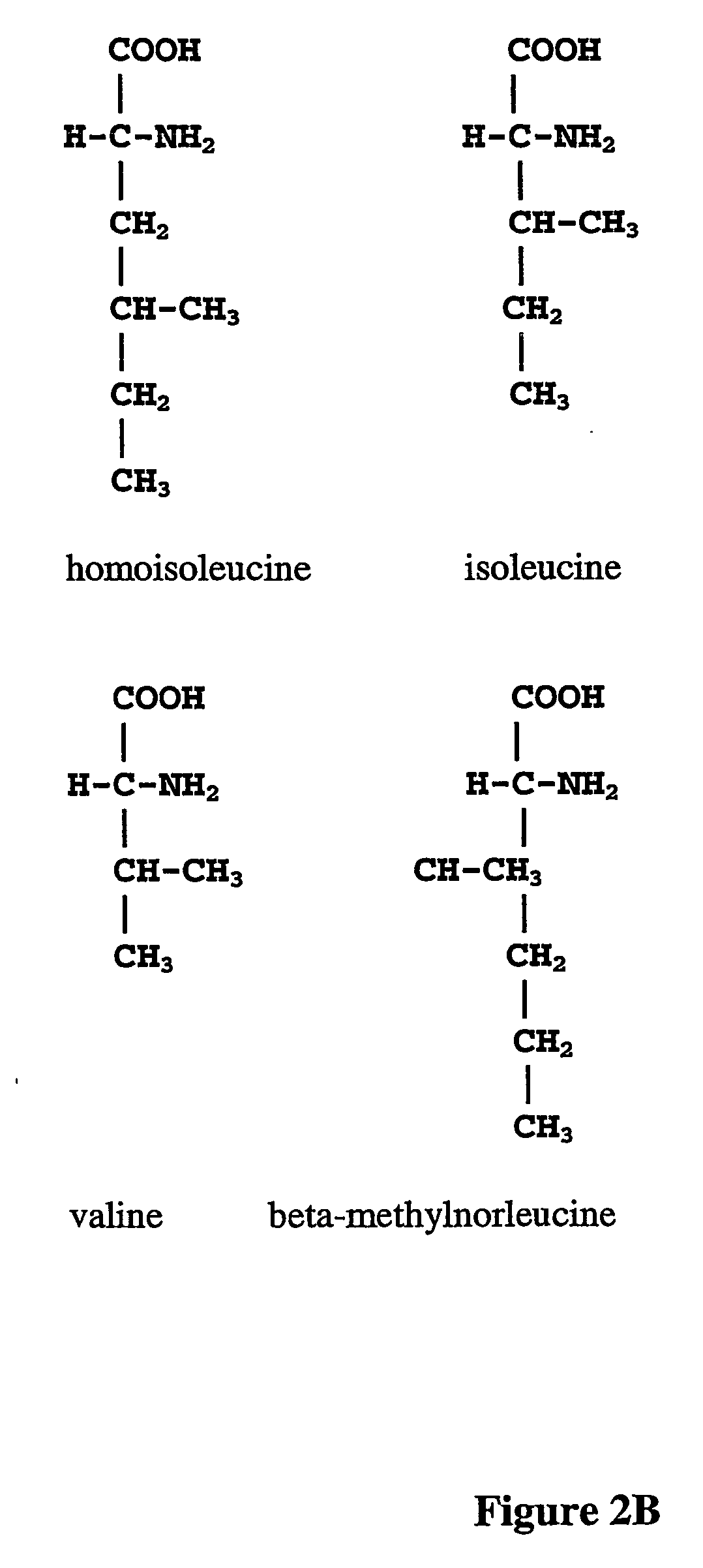
The instant invention is drawn to the methods and compositions necessary to provide recombinant proteins with a substantially reduced or eliminated content of norleucine or other non-standard amino acids. Various embodiments of the invention provide for the substantial elimination of the incorporation of non-standard amino acids into recombinant proteins by the co-expression or enhanced expression of a protein (or the enzymatically active portion thereof) capable of degrading norleucine or other non-standard amino acids, including norvaline, beta-methylnorleucine, and homoisoleucine. In certain particular embodiments of the invention, the norleucine is degraded by a glutamate dehydrogenase, a leucine dehydrogenase, a valine dehydrogenase, a phenylalanine dehydrogenase, a glutamate / leucine / phenylalanine / valine dehydrogenase, or an opine dehydrogenase. Also provided are the cells and DNA constructs for carrying out these methods.
Owner:MONSANTO TECH LLC
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS20040058415A1High yieldRapid and predictable approachFungiBacteriaDihydrofolic acidAminoacid analog
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
Use of amino acids for treatment of various conditions
InactiveUS20060094785A1Effective treatmentImprove toleranceOrganic active ingredientsBiocidePhysiologyAlloisoleucine
A method of treating a patient for a condition characterized by symptoms that can be alleviated by interfering with or supplementing the activity of endogenous ligands on the a2S subunit of a voltage gated calcium channel, said method comprising: administering to a patient experiencing the condition an amount of one or more of L-norleucine, L-isoleucine, L-alloisoleucine, L-methionine, Lleucine, 2-cyclohexylglycine, 2-phenylglycine, 2-amino-2-norbornane carboxylic acid, 1-aminocyclohexane carboxylic acid, 2-aminoheptanoic acid, 2-aminocaprylic acid, and 2-aminononanoic acid under conditions effective to treat the condition, wherein when the condition is a hot flash or a symptom of hormonal variation, the compound is not L-leucine.
Owner:UNIVERSITY OF ROCHESTER
Prame peptide analogues
InactiveUS20070060524A1Tumor rejection antigen precursorsPeptide/protein ingredientsMHC restrictionAmino acid substitution
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally, classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
Melanoma antigen peptide analogues
InactiveUS20070060518A1Peptide/protein ingredientsGenetic material ingredientsAntigenMelanoma antigen
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally, classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
PSMA peptide analogues
InactiveUS20070049533A1Tumor rejection antigen precursorsPeptide/protein ingredientsAmino acid substitutionPSMA Peptide
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally, classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
Methods and compositions for preventing norleucine misincorporation into proteins
Owner:GENENTECH INC
PSMA peptide analogues
InactiveUS7511118B2Tumor rejection antigen precursorsPeptide/protein ingredientsMHC restrictionAmino acid substitution
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally, classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
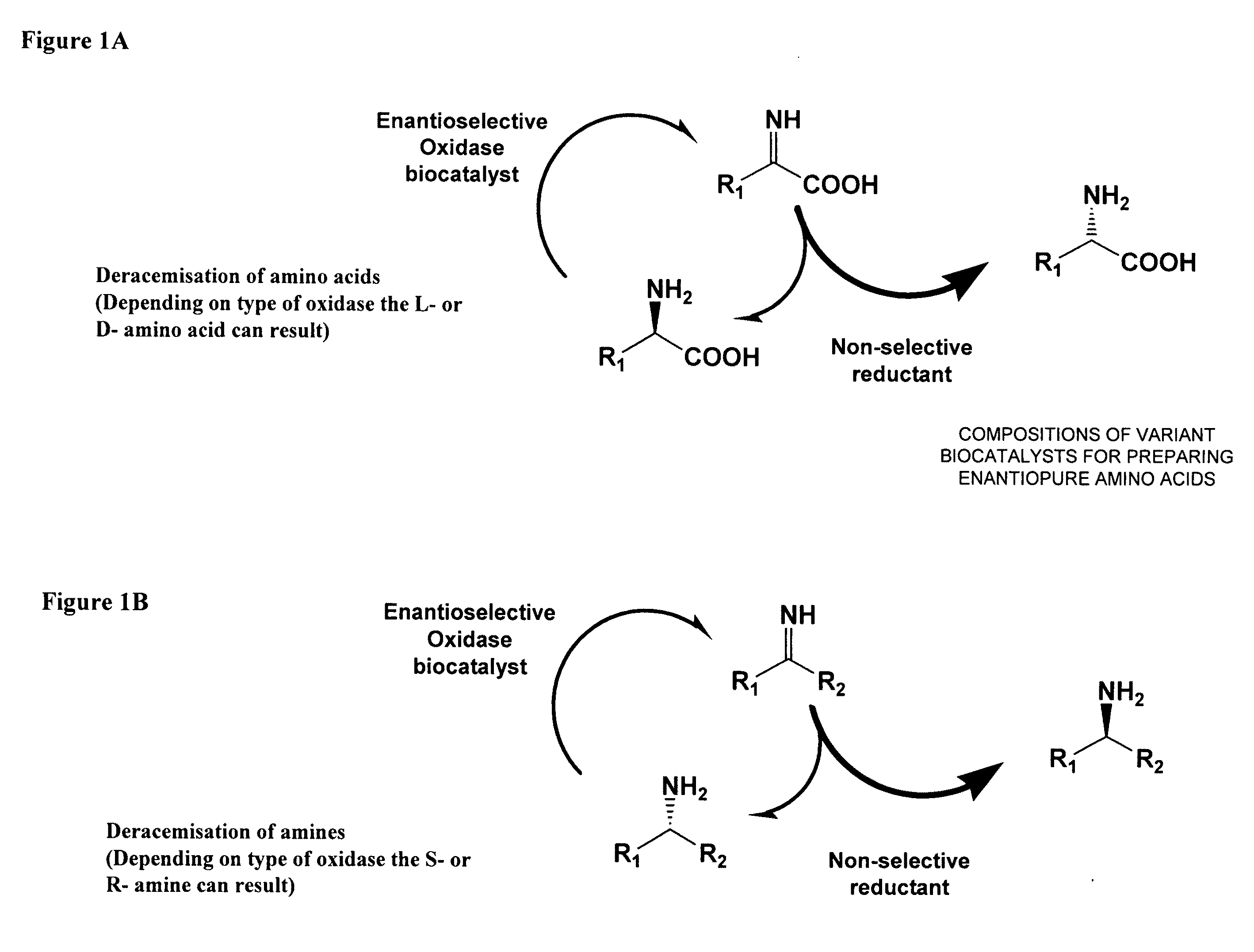
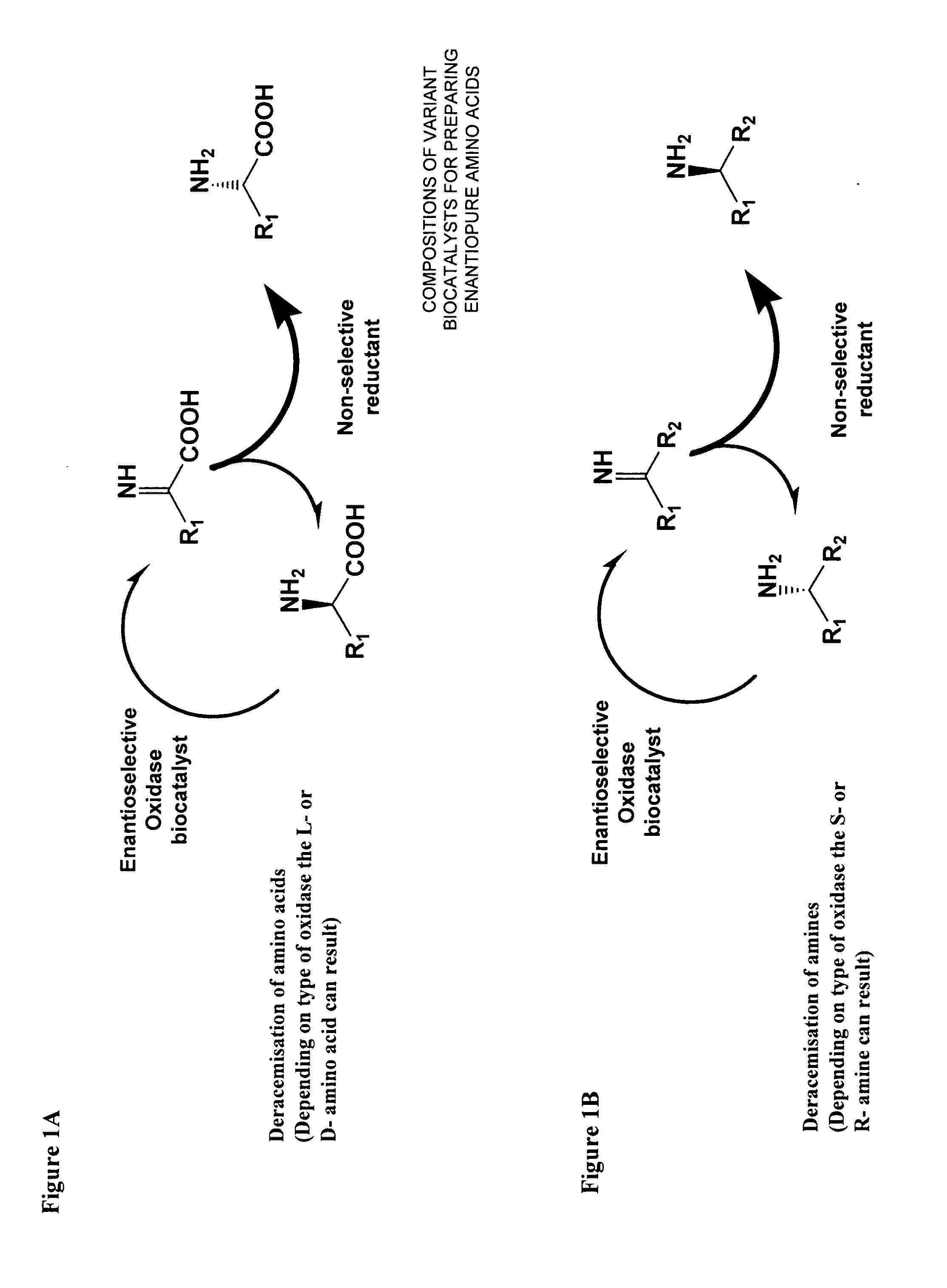
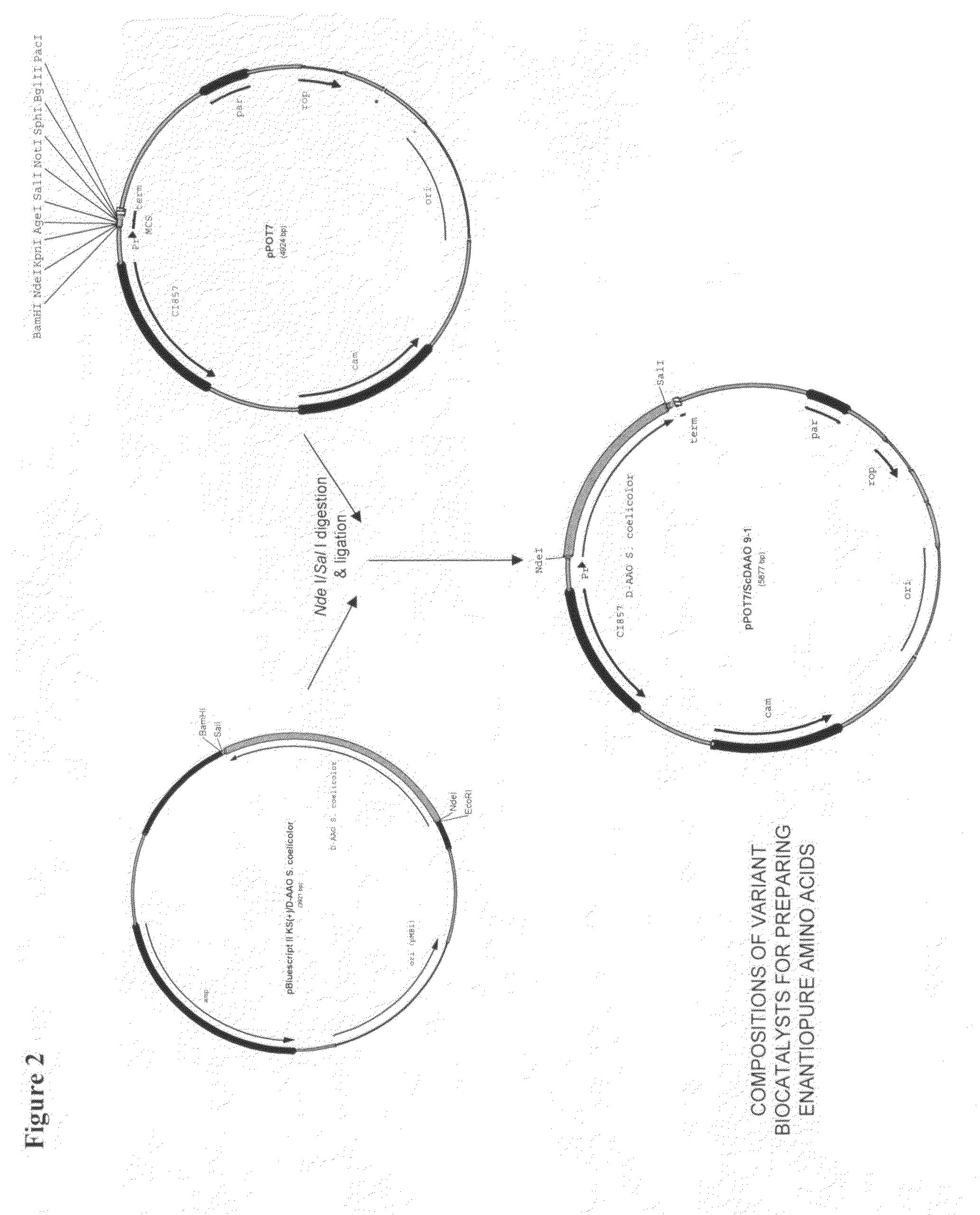
Compositions of variant biocatalysts for preparing enantiopure amino acids
InactiveUS20110059503A1Increased biocatalytic activityHigh activityOxidoreductasesFermentationPenicillamineEnantio selectivity
A composition of variant biocatalysts, specifically variants of D-amino acid oxidases, with improved biocatalytic activity towards D-amino acid substrates such as, but not limited to, D-tert-leucine, D-norvaline, D-2-aminobutyrate, D-alanine, D-isoleucine, D-valine, D-methionine, D-hydroxyadamantlyglycine, D-penicillamine, or D-norleucine is disclosed. Further disclosed is a method of preparing enantioselective amino acids using variant D-amino acid oxidases of the present invention.
Owner:RICHMOND CHEM CORP
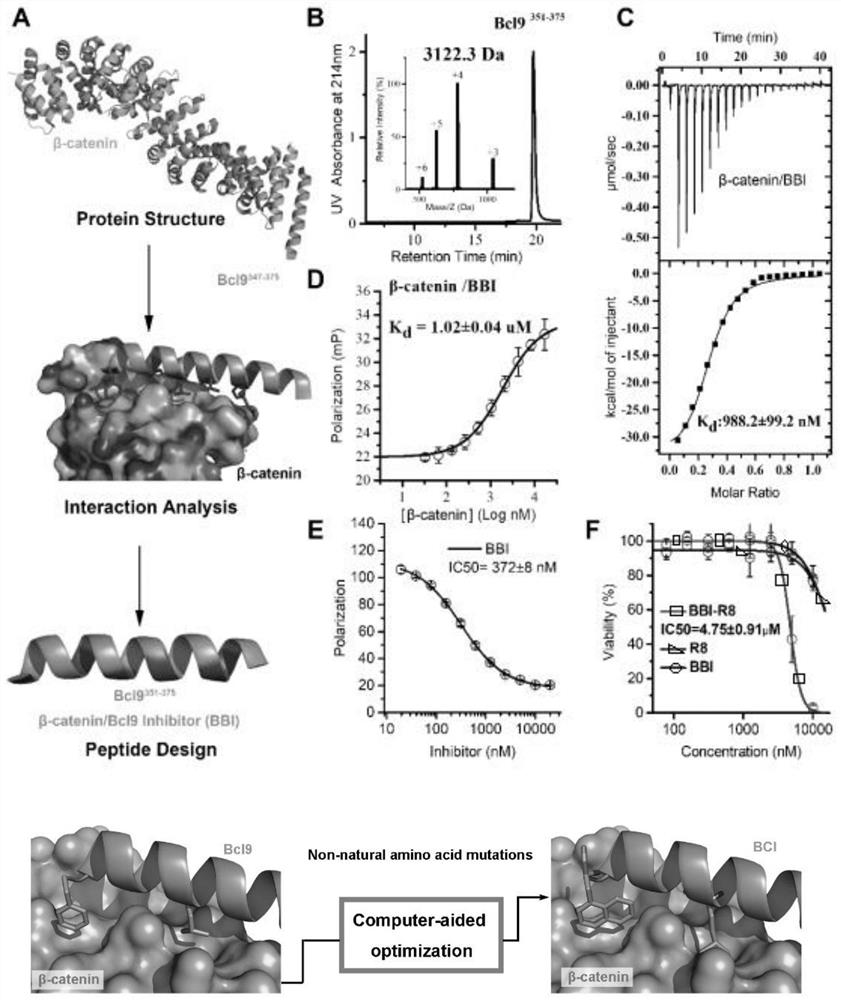
Polypeptide specifically combined with beta-catenin protein with high affinity, and application and synthesis method of polypeptide
ActiveCN111909242AThe synthesis method is simple and easy to obtainInhibit onPeptide/protein ingredientsPeptidesDiseaseCancer cell
The invention discloses a polypeptide specifically combined with beta-catenin protein with high affinity, and application and a synthesis method of the polypeptide. The amino acid sequence of the polypeptide is LEHRERSLQT(X1)RDIQRML(X2)P, wherein X1 is leucine, norleucine or homoleucine, and X2 is phenylalanine, 1-naphthyl alanine, 2-naphthyl alanine, 2-anthryl alanine or 9-anthryl alanine. The polypeptide is used for inhibiting growth of cancer cells. By the polypeptide, various tumor treatment targets can be achieved. The polypeptide inhibits opening of a beta-catenin protein-mediated Wnt signal pathway by inhibiting mutual combination of the beta-catenin protein and BCL9 in the cancer cells, so that growth of tumors is inhibited, self-apoptosis of the cells is induced, and the tumor disease treatment targets are achieved. The synthesis method of the polypeptide is simple and easy to implement, and the final product has high yield efficiency, has mass production potential, and has great drug clinical transformation potential.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Cyclic peptide for treating cancer
The present disclosure generally relates to a circularized peptide for treating cancer. An cyclic peptide is disclosed that has an amino acid sequence selected from Lys-X5-Glu-X1-X2-Gln-Met-Glu-Asp-Asp-X3-X4 (SEQ ID NO: 3), (SEQ ID NO: 4), Lys-Gly-X6-Val-Leu-Gln-Met-X7-X8-X9-Leu-Val (SEQ ID NO: 5), Lys-X5-Glu-X1-X2-Gln-X12-Glu-Asp-Asp-X3-X4 (SEQ ID NO: 9), and X10-X5-X6-Val-Leu-Gln-Met-Glu-Asp-X9-X3-X4 (SEQ ID NO: 10). The amino acids X1, X2, X3, and X4 can be each independently valine, leucine, isoleucine, or alanine; X5 can be glycine, alanine, leucine, isoleucine, or valine; X6, X7, X8, and X9 can be each independently glutamic acid or asparagine; X10 can be lysine or arginine; X11 can be methionine or cysteine; and X12 can be methionine or norleucine. The cyclic peptide can have the amino acid sequence Lys-Gly-Glu-Val-Leu-Gln-Met-Glu-Asp-Asp-Leu-Val (SEQ ID NO: 1).
Owner:SAINT LEO UNIVERSITY
Mutant enzyme of glutamate dehydrogenase and construction method thereof
The invention discloses mutant enzyme of glutamate dehydrogenase and a construction method thereof. The mutant enzyme has an amino acid sequence shown as SEQ ID NO: 1. The mutant gene is well expressed in a heterologous host, namely Escherichia coli; and expressed proteins are one kind of dehydrogenases which can catalyze various amino acids. The method mainly comprises the following steps of: (1) obtaining a glutamate dehydrogenase gene gdh and constructing a recombinant plasmid; (2) constructing a mutant strain; (3) constructing an expression strain; and (4) expressing and purifying. Compared with the prior art, the generated engineering bacteria not only can catalyze glutamic acid, but also can catalyze methionine and norleucine.
Owner:ANHUI NORMAL UNIV
Methods and compositions for preventing norleucine misincorporation into proteins
ActiveUS20160186227A1Reducing and preventing misincorporationReducing or preventing norleucine misincorporationBacteriaImmunoglobulins against growth factorsMicrobiologyMicrobial host
The present invention relates to methods and compositions for preventing incorporation of norleucine into proteins during recombinant protein production in bacteria. The present invention also provides microorganism host cells and nucleic acid molecules for use with the methods and compositions provided herein.
Owner:GENENTECH INC
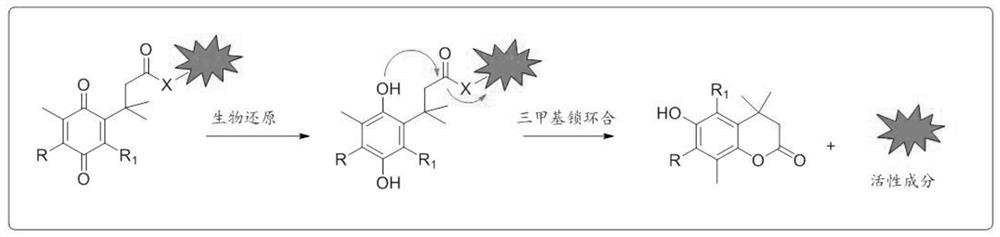
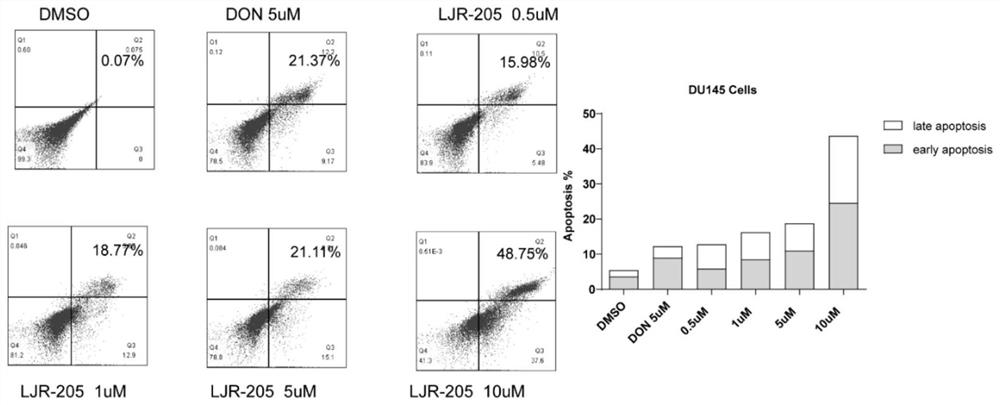
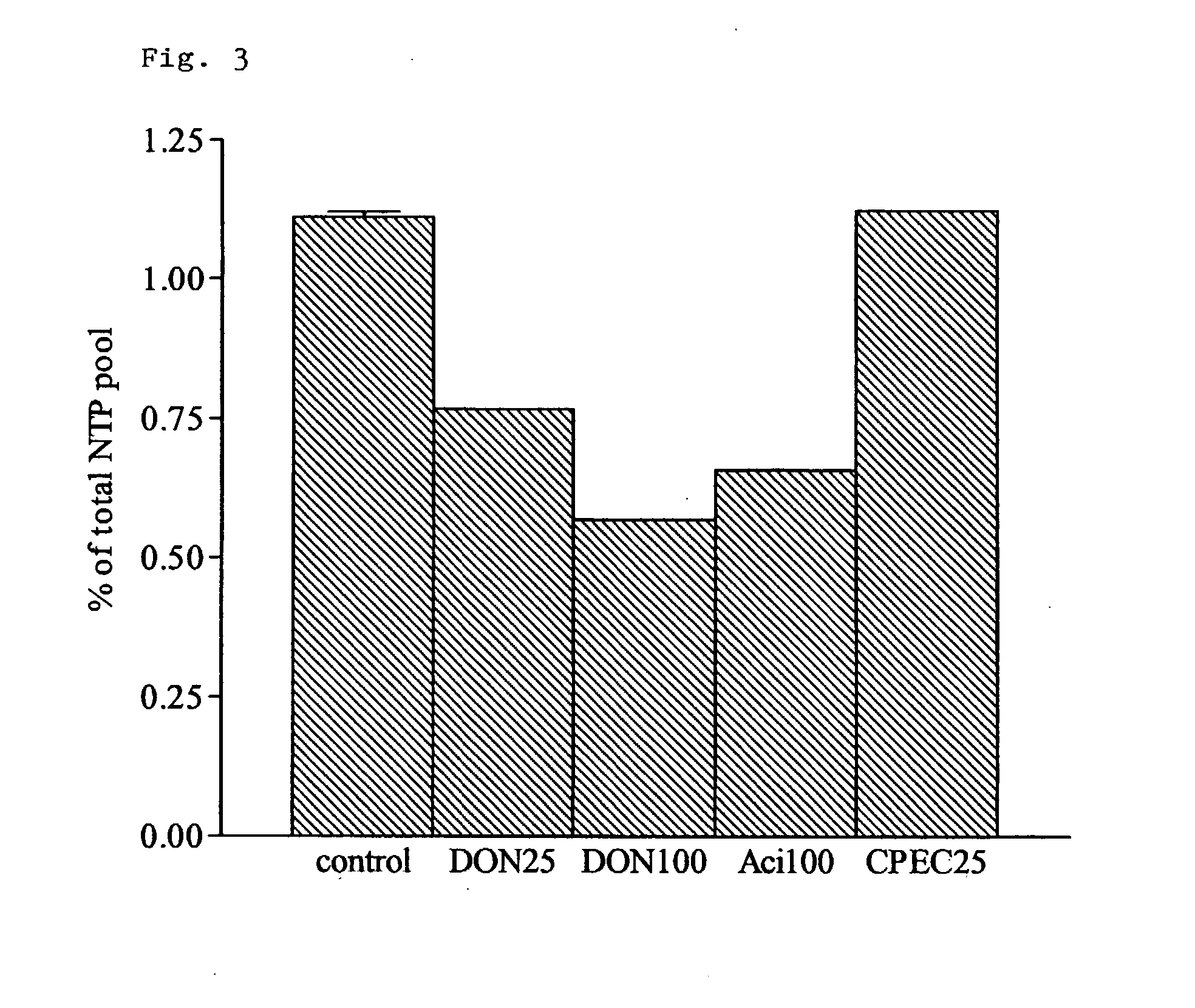
Application of 6-diazo-5-oxo-L-leucine in preparation of drugs for preventing and treating psoriasis
InactiveCN112807297ASuppress inflammatory immune responseRelieve symptomsOrganic active ingredientsAntipyreticLocal immunityPharmaceutical drug
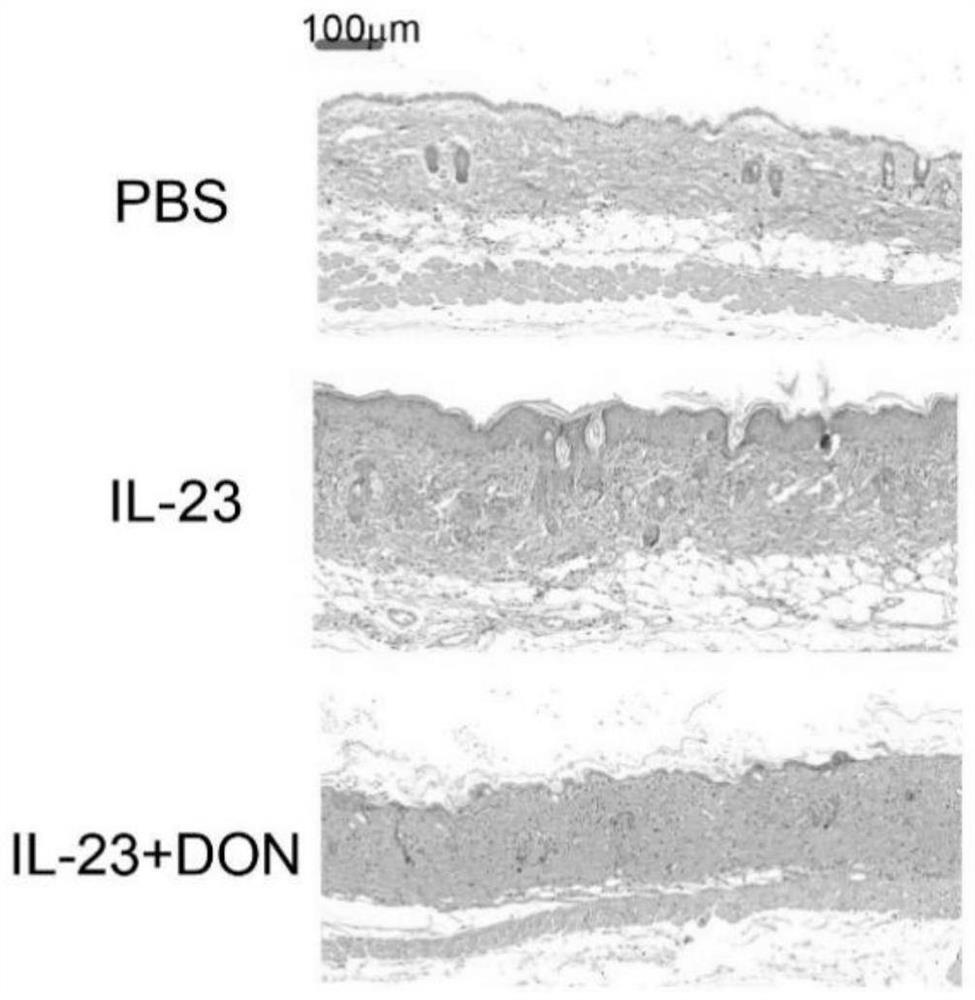
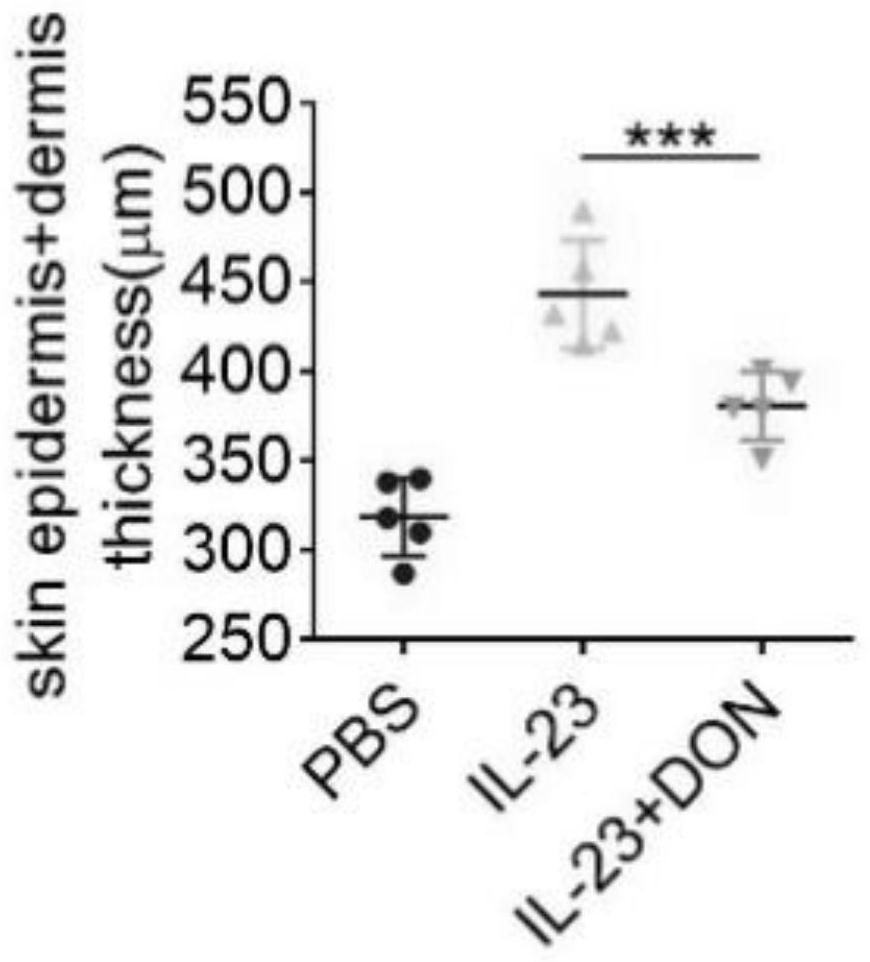
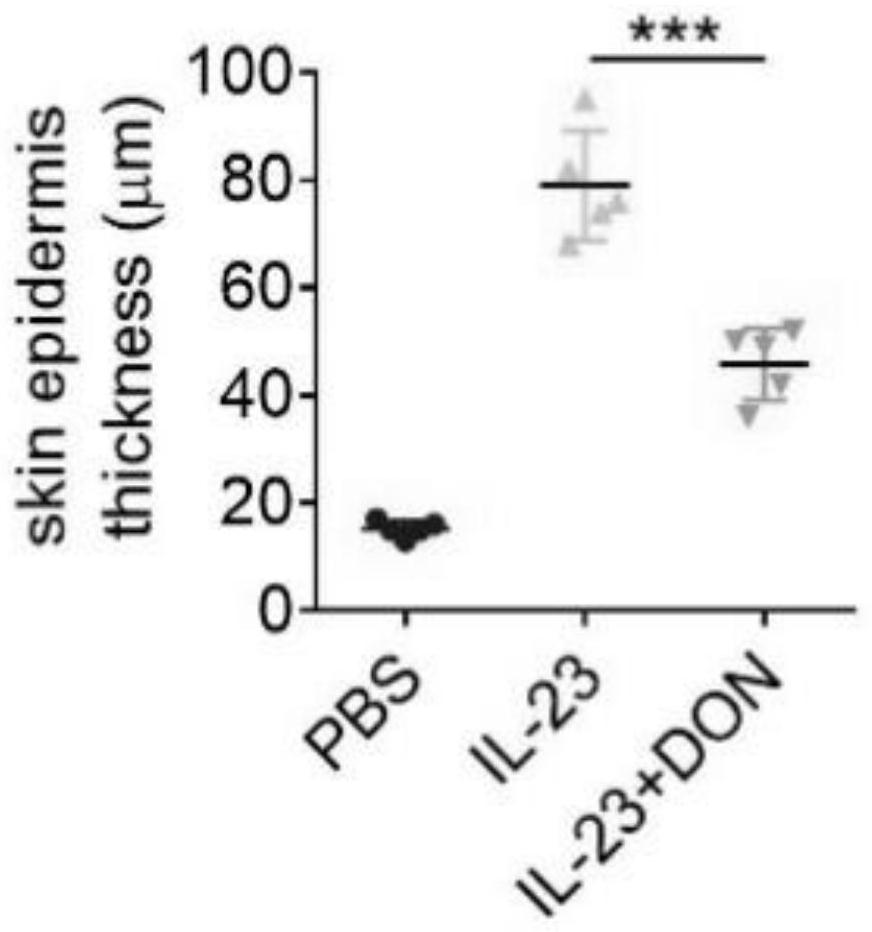
The invention discloses application of 6-diazo-5-oxo-L-leucine in preparation of drugs for preventing and treating psoriasis, and relates to the technical field of medicine. Experimental comparisons discover that the skin thickening at the psoriasis lesion after DON metabolic intervention is obviously reduced, the infiltration of immune cells is reduced, and DON can effectively inhibit inflammatory local immune response; at the same time, DON alleviates the spleen swelling phenomenon of psoriasis, and also effectively inhibits the inflammatory immune response of psoriasis; and by detecting the expression of proinflammatory factors at the skin inflammation site of psoriasis, DON also inhibits the expression of such genes, and DON effectively alleviates the symptoms of psoriasis.
Owner:深圳市福田区风湿病专科医院
Mini-gastrin analogue, in particular for use in CCK2 receptor positive tumour diagnosis and/or treatment
ActiveUS10130724B2Weak affinityEasy to oxidizePeptide/protein ingredientsIsotope introduction to peptides/proteinsDiseaseLow affinity
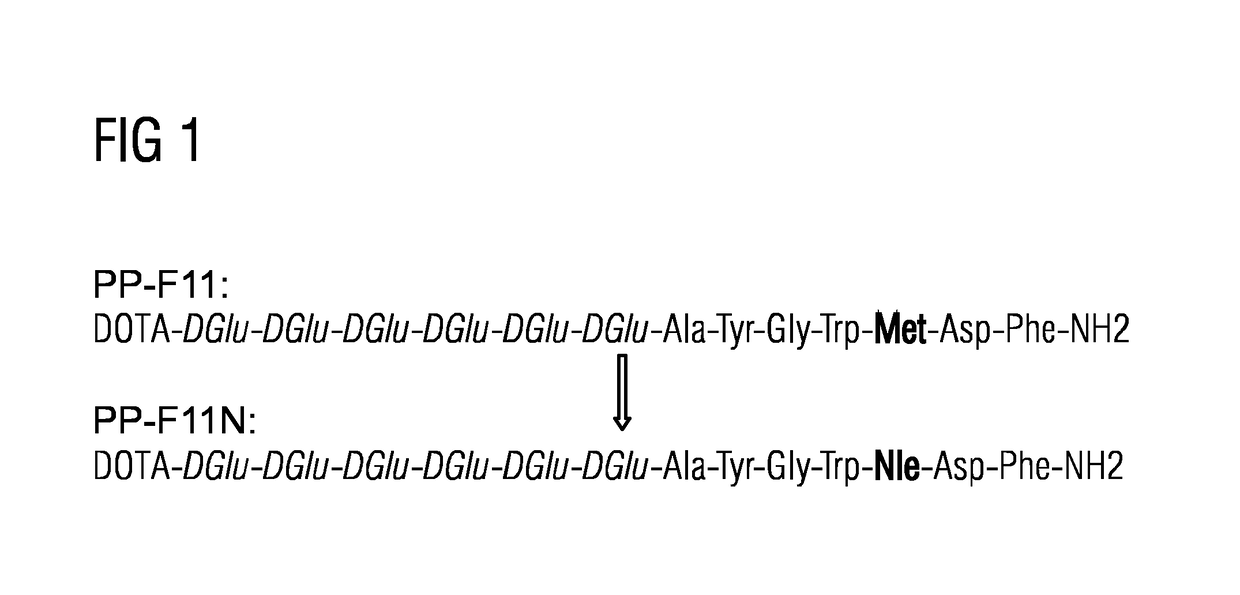
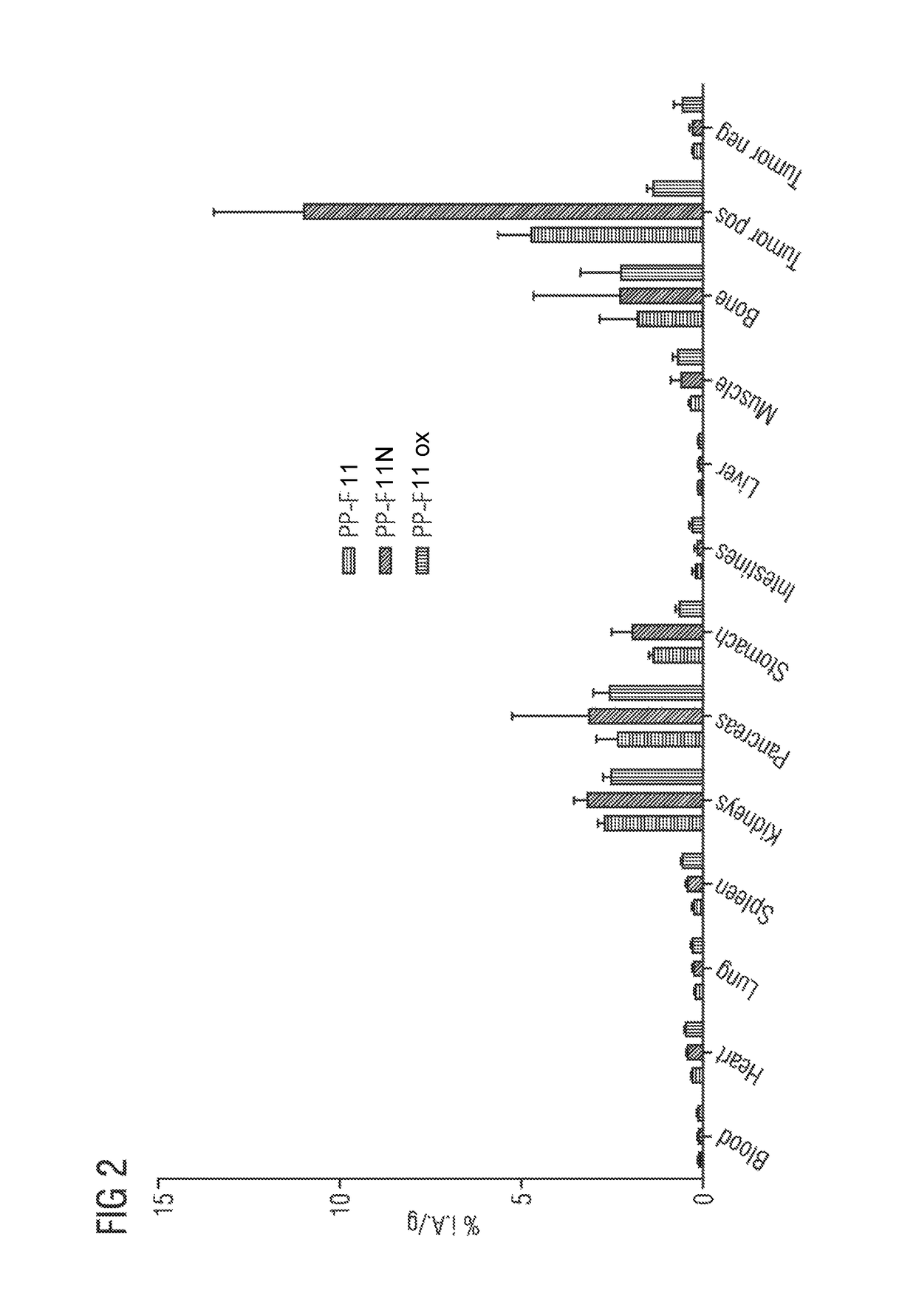
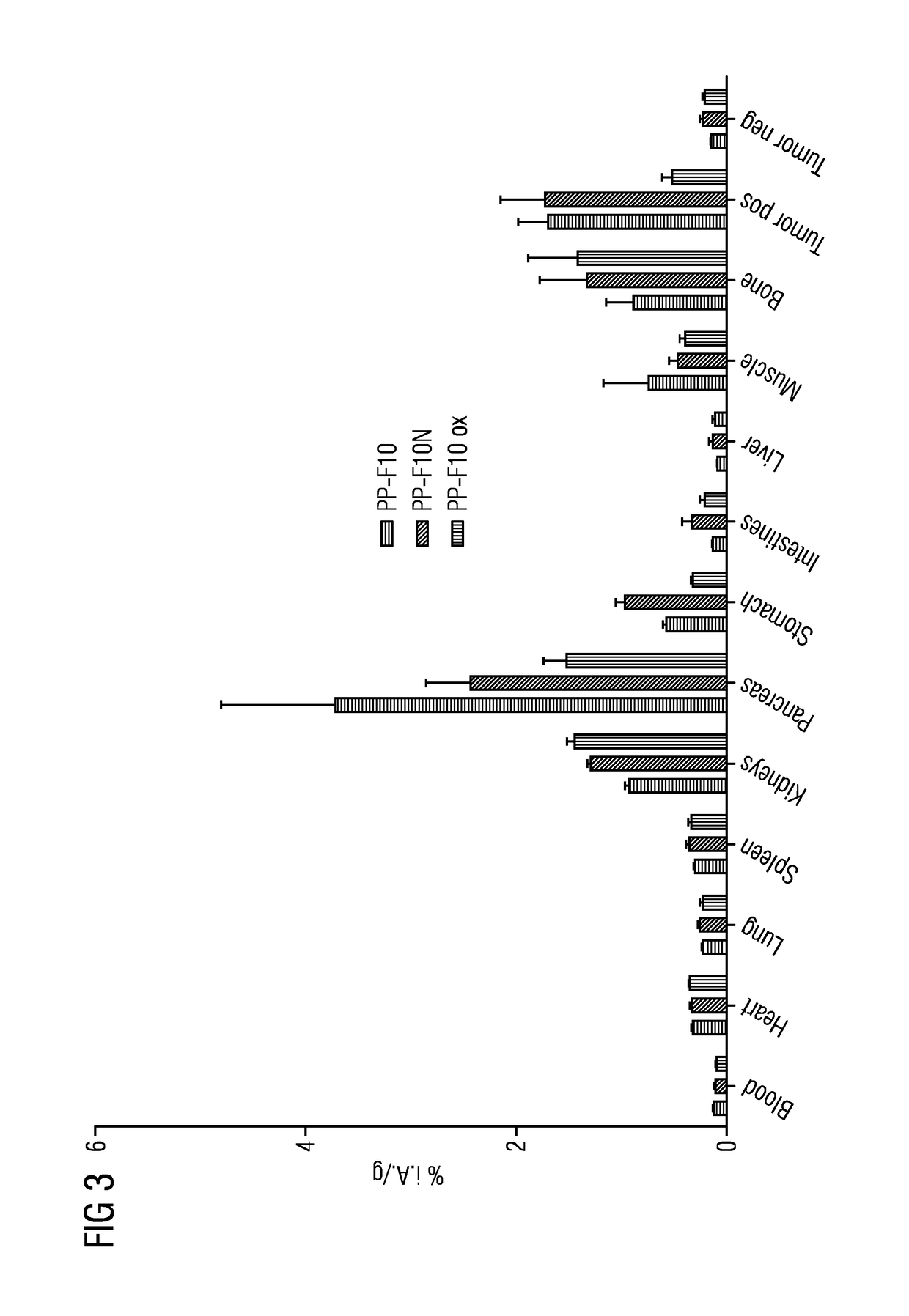
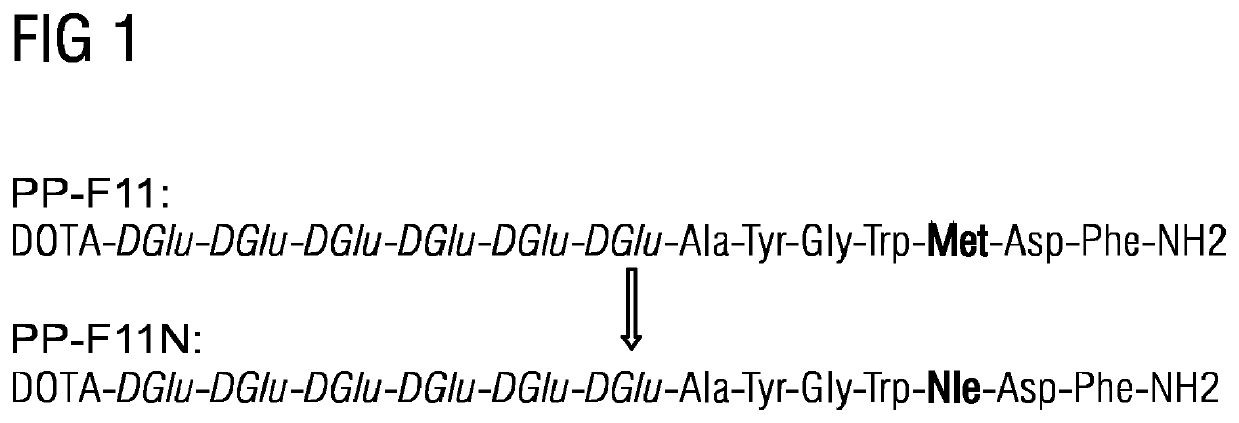
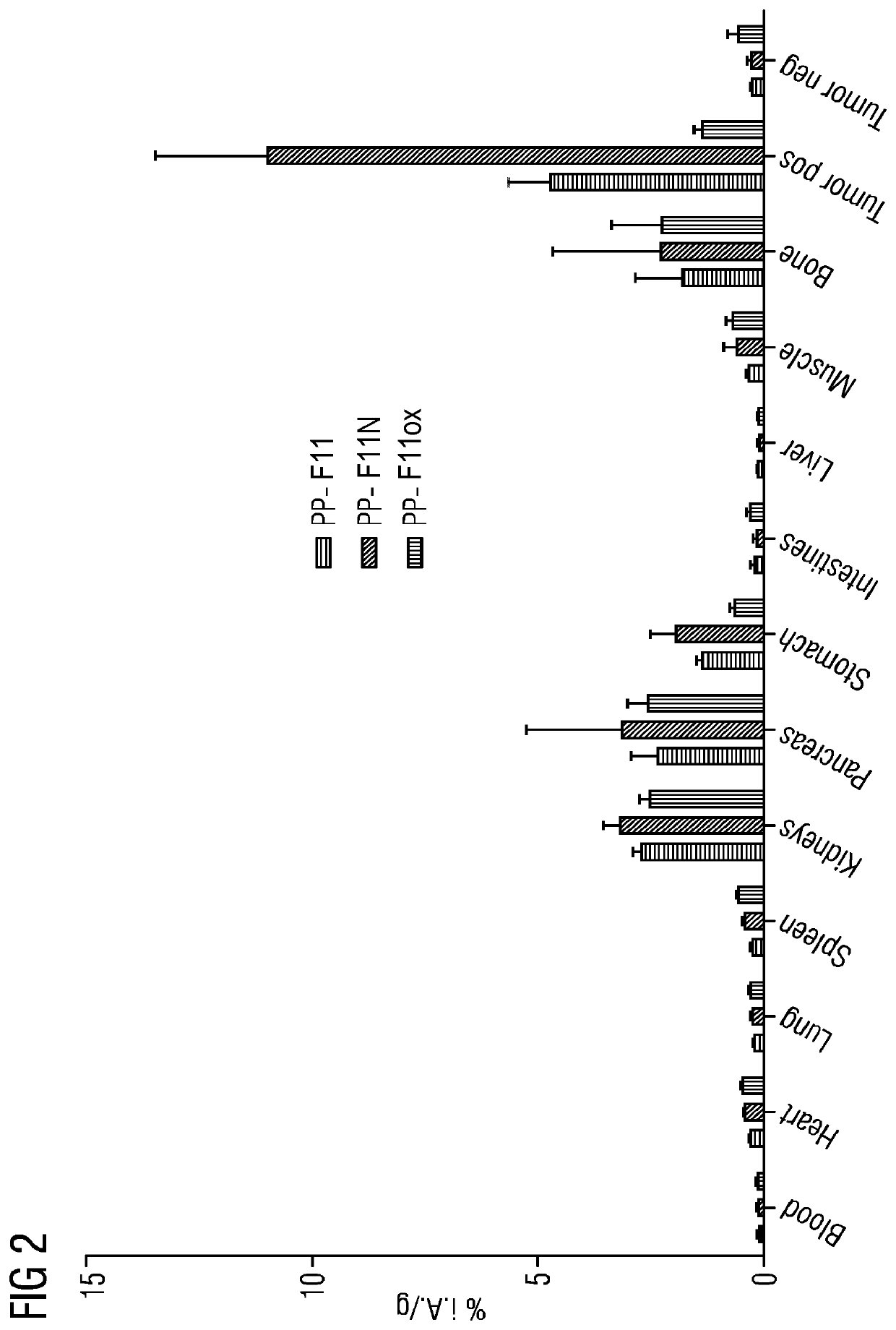
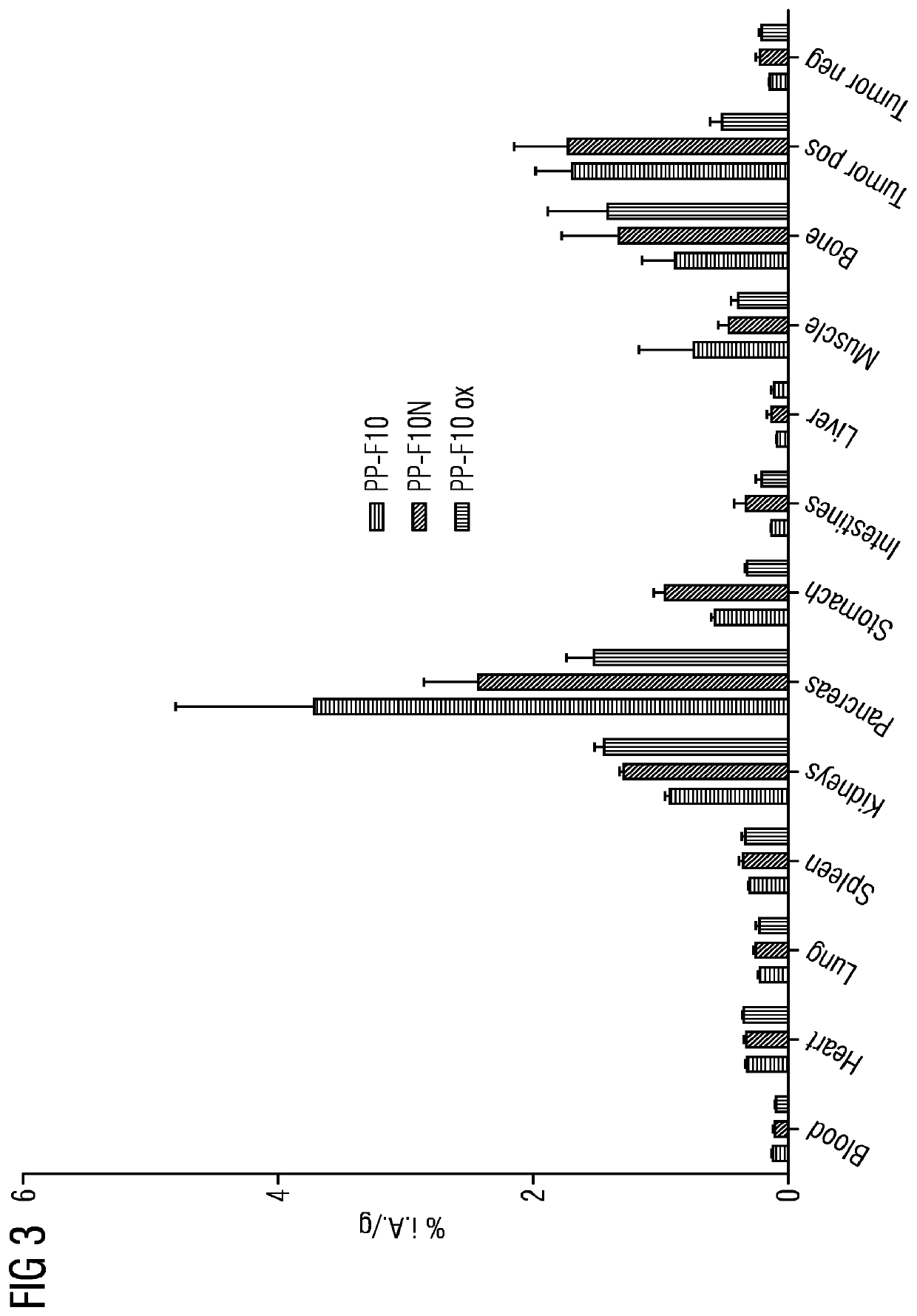
A gastrin analog shows high uptake in CCK-2 receptor positive tumors and simultaneously a very low accumulation in the kidneys. This is achieved by a mini-gastrin analog PP-F11 having the formula: PP-F11-X-DGlu-DGlu-DGlu-DGlu-DGlu-DGlu-Ala-Tyr-Gly-Trp-Y-Asp-Phe-NH2, wherein Y is an amino acid replacing methionine and X is a chemical group attached to the peptide for diagnostic and / or therapeutic intervention at CCK-2 receptor relevant diseases. Very suitable compounds with respect to a high tumor to kidney ratio are mini-gastrin analogs with six D-glutamic acids or six glutamines. These compounds still possess a methionine which can be oxidized easily which is a disadvantage for clinical application under GMP due to the forms which may occur. The elimination of the methionine leads to a lower affinity to oxidation which in general favors the tumor-kidney-ratio. Ideally, the methionine is replaced by norleucine. This PP-F11N mini gastrin exhibits currently the best tumor-kidney-ratio and is the most promising candidate.
Owner:PAUL SCHERRER INSTITUT
Method and device
InactiveUS20050085624A1Peptide/protein ingredientsPeptide preparation methodsLipid formationPrenylation
A method of modification of a protein or polypeptide in the presence of a modifying composition capable of providing at least one modification wherein a liquid phase comprising the protein or polypeptide is brought into contact with a solid phase capable of immobilizing the protein or polypeptide and the solid phase carrying the immobilized protein is brought at least once into contact with a liquid phase comprising the composition capable of modifying the protein or polypeptide and modification reaction(s) are allowed to occur. The liquid phase comprising the protein or polypeptide may be a liquid extract of eukaryote or prokaryote cells. The modification may be a acylation, phosphorylation, dephosphorylation, SUMOylation, ubiquitinylation, carboxymethylation, formylation, acetylation, deacetylation, gamma carboxyglutamic acid, norleucine, amidation, deamidation, carboxylation, carboxyamylation, sulfation, methylation, demethylation, hydroxylation, ADP-ribosylation, maturation, adenylation, O-linked glycosylation, N-linked glycosylation, methonine oxidation, and addition of lipid (prenylation).
Owner:BIRSE DARCY
Prevention of incorporation of non-standard amino acids into protein
ActiveUS8603781B2Reducing, or substantially eliminating, endogenous cellular levels of norleucineLower Level RequirementsBacteriaPeptide/protein ingredientsBeta-methylnorleucinePhenylalanine dehydrogenase
The instant invention is drawn to the methods and compositions necessary to provide recombinant proteins with a substantially reduced or eliminated content of norleucine or other non-standard amino acids. Various embodiments of the invention provide for the substantial elimination of the incorporation of non-standard amino acids into recombinant proteins by the co-expression or enhanced expression of a protein (or the enzymatically active portion thereof) capable of degrading norleucine or other non-standard amino acids, including norvaline, beta-methylnorleucine, and homoisoleucine. In certain particular embodiments of the invention, the norleucine is degraded by a glutamate dehydrogenase, a leucine dehydrogenase, a valine dehydrogenase, a phenylalanine dehydrogenase, a glutamate / leucine / phenylalanine / valine dehydrogenase, or an opine dehydrogenase. Also provided are the cells and DNA constructs for carrying out these methods.
Owner:MONSANTO TECH LLC
Skin care composition
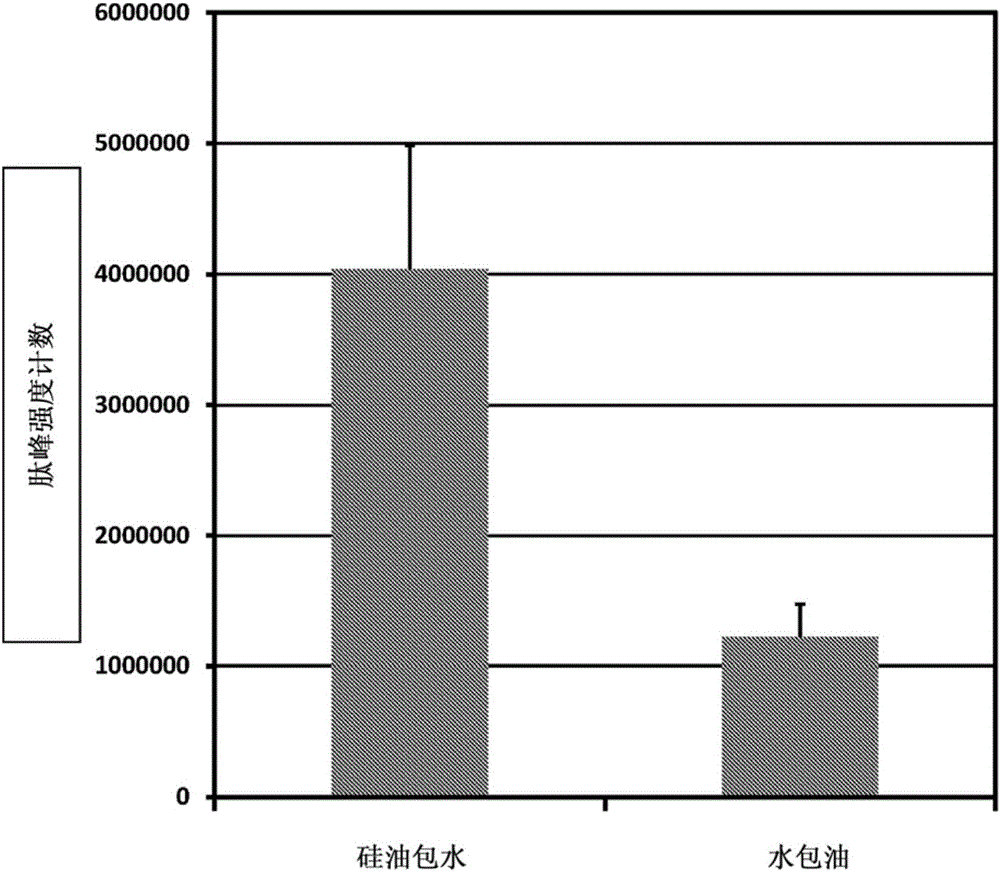
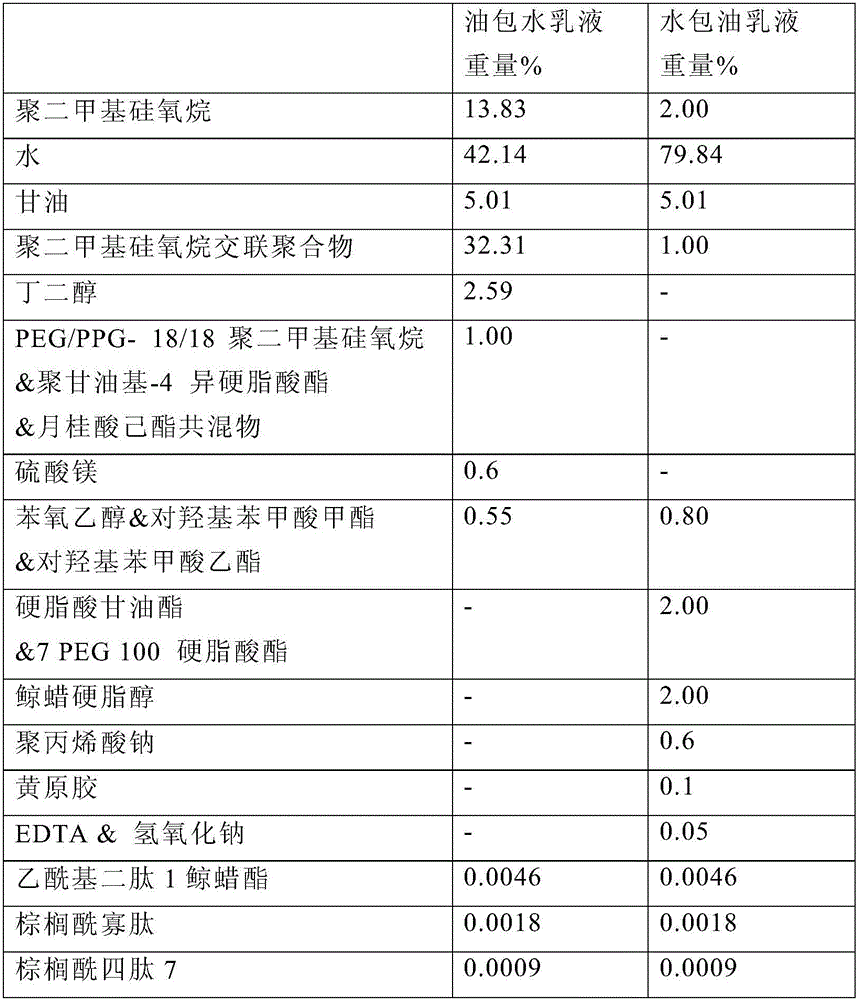
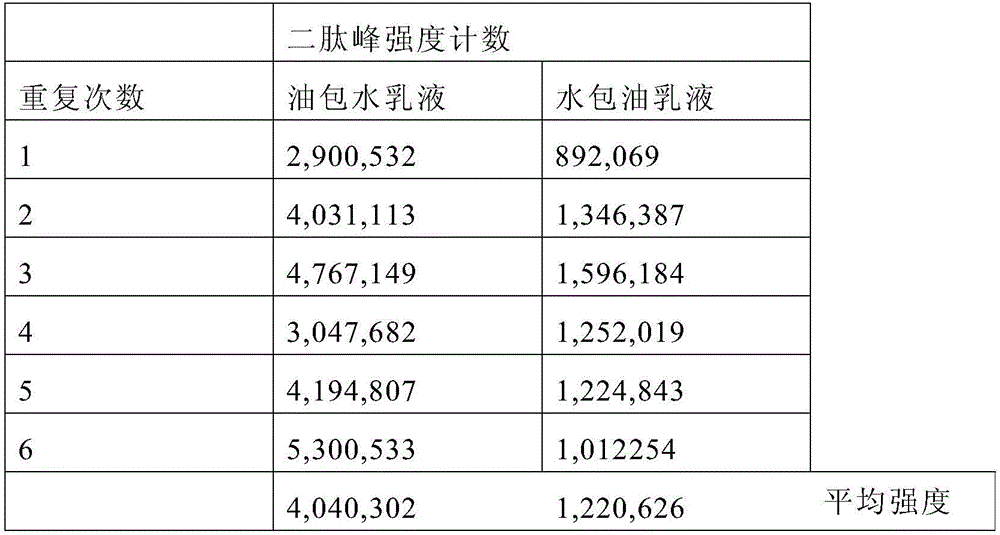
A water-in-oil emulsion comprising less than 60% water, and wherein said emulsion comprises an oil phase and a water phase, and wherein the water phase comprises a dipeptide selected from the group consisting of acetyl dipeptide (1) cetyl ester, acetyl dipeptide (3) aminohexanoate, azelaoyl bisdipeptide (10), coumaroyl dipeptide (3), dicetyl dipeptide (9), dipeptide diamino butyroyl benzylamide diacetate, dipeptide (1), dipeptide (10), dipeptide (11), dipeptide (12), dipeptide (15), dipeptide (16), dipeptide (17), dipeptide (18), dipeptide (19), dipeptide (2), dipeptide (20), dipeptide (3), dipeptide (4), dipeptide (5), dipeptide (6), dipeptide (7), dipeptide (8), dipeptide (8) HCL, dipeptide (9), hexanoyl dipeptide (3) norleucine acetate, methyl undecylenoyl dipeptide (16), nicotinoyl dipeptide (22), nicotinoyl dipeptide (23), nicotinoyl dipeptide (24), nicotinoyl dipeptide (26), oleoyl dipeptide (15), palmitoyl dipeptide (10), palmitoyl dipeptide (13), palmitoyl dipeptidel (7), palmitoyl dipeptide (5) diaminobutyroyl hydroxythreonine, palmitoyl dipeptide (5) diaminohydroxybutyrate, palmitoyl dipeptide (7) and mixtures thereof.
Owner:BOOTS PURE DRUG CO LTD
NQO1 activated 6-diazo-5-oxo-L-n-leucine prodrug as well as preparation method and application thereof
The invention discloses an NQO1 activated type 6-diazo-5-oxo-L-n-leucine prodrug as well as a preparation method and application of thereof. NQO1 is highly expressed in most tumor cells, so a tumor targeting effect can be realized by introducing a quinonyl acid group activated by the NQO1, DON prodrugs can be efficiently and rapidly released in the tumor cells, and the purpose of inhibiting tumor proliferation is achieved. The prodrug shows high affinity to NQO1, and can rapidly, efficiently and directionally release a glutamine metabolism antagonist DON in tumor cells highly expressed by NQO1, so that the toxic and side effects of the drug are reduced, the targeting efficiency of tumor treatment is improved, and a new thought is provided for development of antitumor drugs.
Owner:CHINA PHARM UNIV
Medicament for the treatment of diseases caused by parasitic protozoa
The present invention relates to the use of an inhibitor of CTP synthetase, such as a glutamine analogue, and a substance capable of suppressing toxic effects thereof in vivo, in the manufacture of a medicament for the treatment of a disease caused by a parasitic protozoa. More specifically, said substance capable of suppressing toxic effects may be a nucleobase, such as a purine base or a nucleoside, while the glutamine analogue advantageously is 6-diazo-5-oxo-L-norleucine (DON). The invention also relates to a pharmaceutical composition as such for the treatment and / or prevention of a disease caused by a parasitic protozoa, wherein the disease is selected from the group consisting of malaria, leishmaniasis and trypanosomiasis, e.g. American trypanosomiasis (Chaga's disease), or African trypanosomiasis (African sleeping sickness).
Owner:HOFER +1
Mini-gastrin analogue, in particular for use in CCK2 receptor positive tumour diagnosis and/or treatment
ActiveUS10953114B2Promote accumulationAccumulation is very lowPeptide/protein ingredientsIsotope introduction to peptides/proteinsDiseaseReceptor
A gastrin analogue shows high uptake in CCK-2 receptor positive tumors and simultaneously a very low accumulation in the kidneys. This is achieved by a mini-gastrin analogue PP-F11 having the formula: PP-F11-X-DGlu-DGlu-DGlu-DGlu-DGlu-DGlu-Ala-Tyr-Gly-Trp-Y-Asp-Phe-NH2, wherein Y is an amino acid replacing methionine and X is a chemical group attached to the peptide for diagnostic and / or therapeutic intervention at CCK-2 receptor relevant diseases. Very suitable compounds with respect to a high tumor to kidney ratio are mini-gastrin analogues with six D-glutamic acids or six glutamines. These compounds still possess a methionine which can be oxidized easily which is a disadvantage for clinical application under GMP due to the forms which may occur. The elimination of the methionine leads to a lower affinity to oxidation which in general favors the tumor-kidney-ratio. Ideally, the methionine is replaced by norleucine. This PP-F11N mini gastrin exhibits currently the best tumor-kidney-ratio and is the most promising candidate.
Owner:PAUL SCHERRER INSTITUT
Feed additive, application thereof and feed with strong antibacterial activity
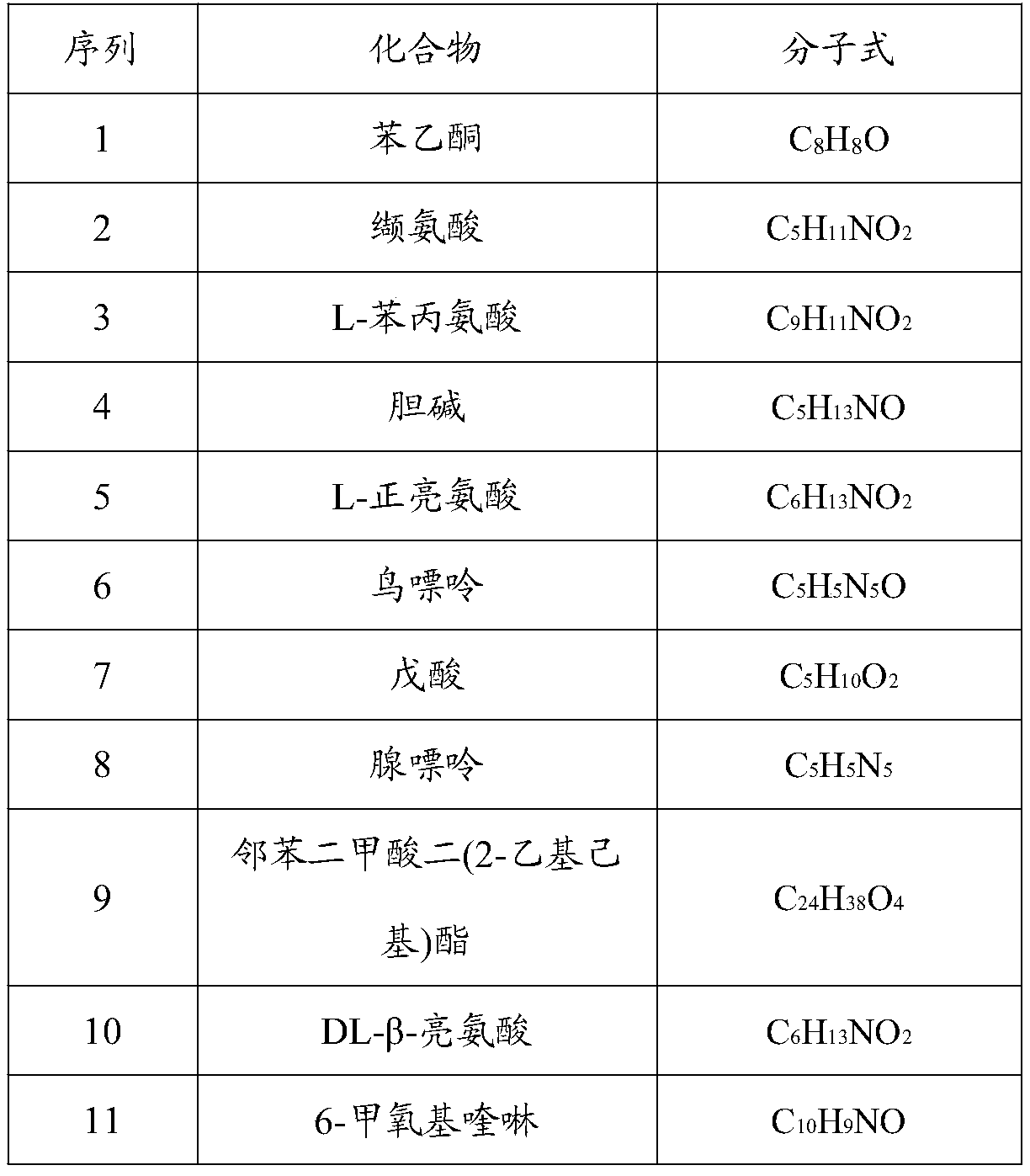
The invention relates to a feed additive, an application thereof and a feed with strong antibacterial activity, and belongs to the technical field of feeds. The feed additive disclosed by the invention is prepared from the following components: acetophenone, valine, L-phenylalanine, choline, L-leucine, guanine, valeric acid, adenine, dioctyl phthalate, DL-beta-leucine, 6-methoxyquinoline, stearicacid, erucyl amide and 4-oxoproline. The feed additive disclosed by the invention does not contain antibiotics, can efficiently inhibit bacteria, and is a safe green feed additive for livestock and poultry.
Owner:SHENZHOUSPACEBIOTECHGRP
Modified glucagon molecues and formulations with oxidation resistance and methods and kits of employing the same
PendingUS20220153803A1Improve solubilityGood treatment effectPeptide/protein ingredientsMetabolism disorderPhosphorylationPharmaceutical Substances
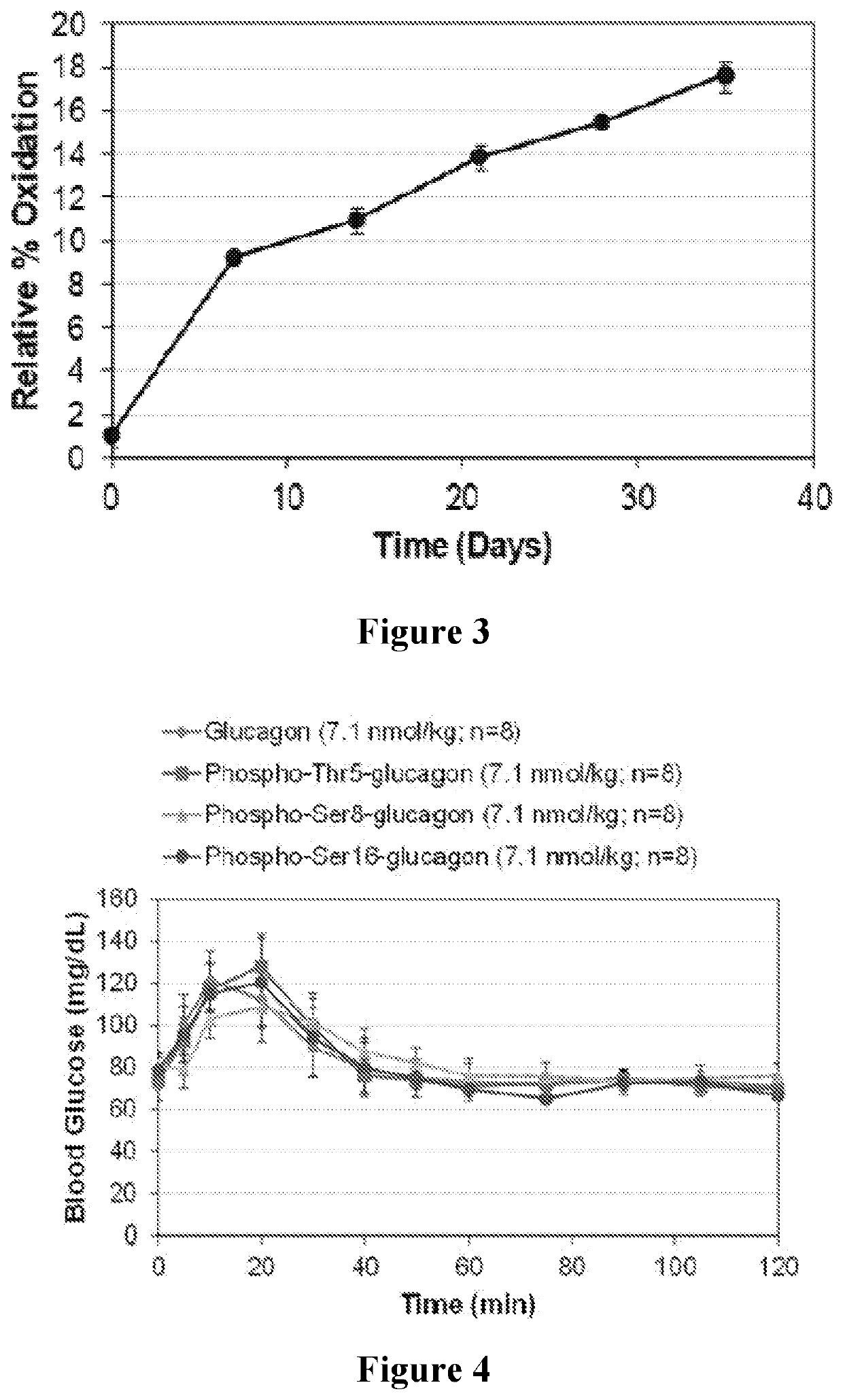
Modified glucagon molecules and buffer and / or excipient solutions are provided that result in the glucagon molecules being resistant to oxidation when stored at a substantially neutral pH. Such a modified glucagon molecule includes a substitution at position 27, with the native methionine being replaced with a methionine memetic analog, a norleucine, or an isomer of either of the foregoing. Optionally, the modified glucagon molecules may be further phosphorylated to result in enhanced solubility at a substantially neutral pH and resistance to fibrillation. Methods of using such molecules in pharmaceutical compositions and therapeutic kits are also provided.
Owner:PURDUE RES FOUND INC
Modified CCK peptides
InactiveUS20090281032A1Substantial resistance to degradationImprove biological activityPeptide/protein ingredientsMetabolism disorderGlucose disposalInsulin secretion
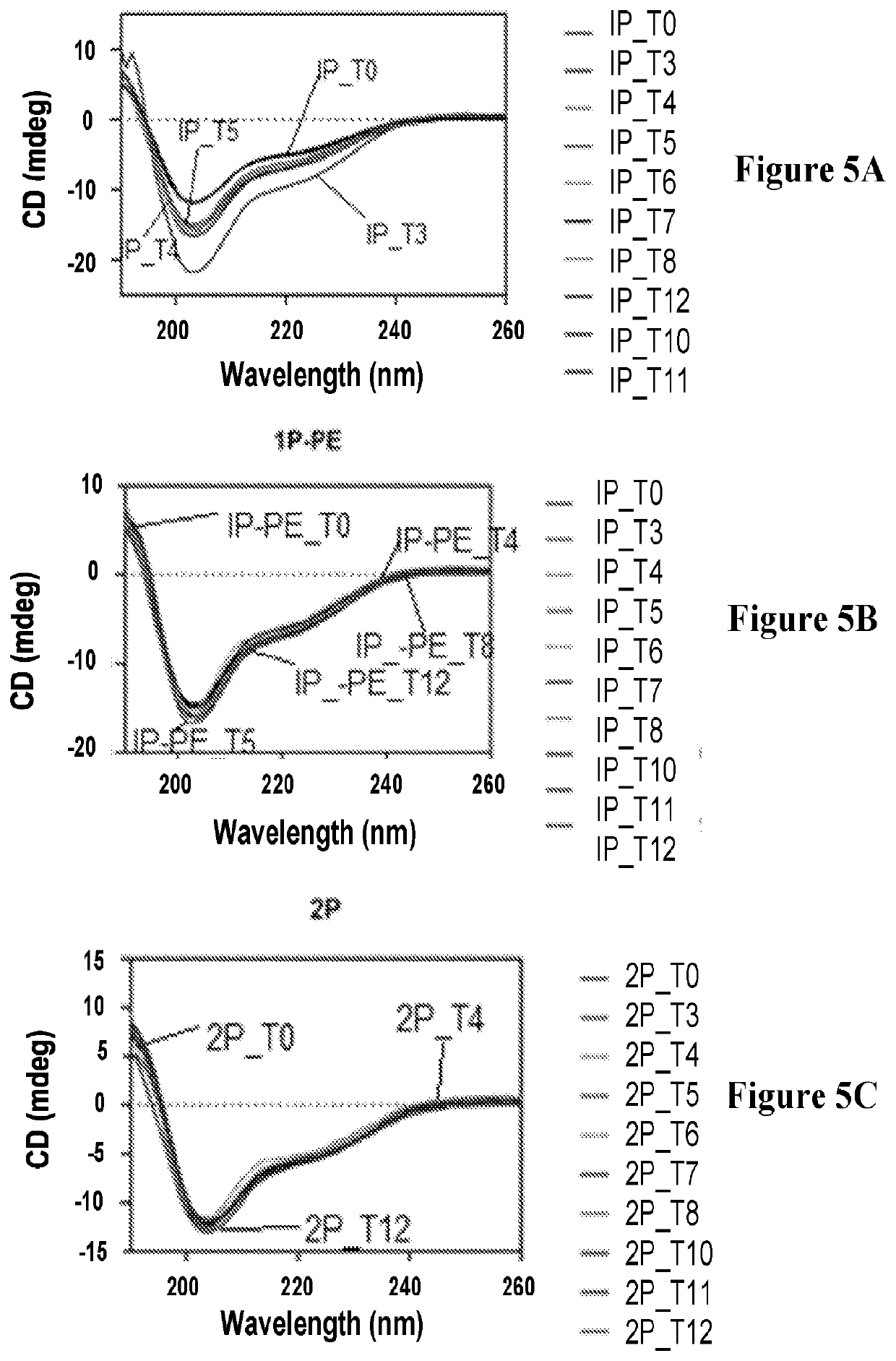
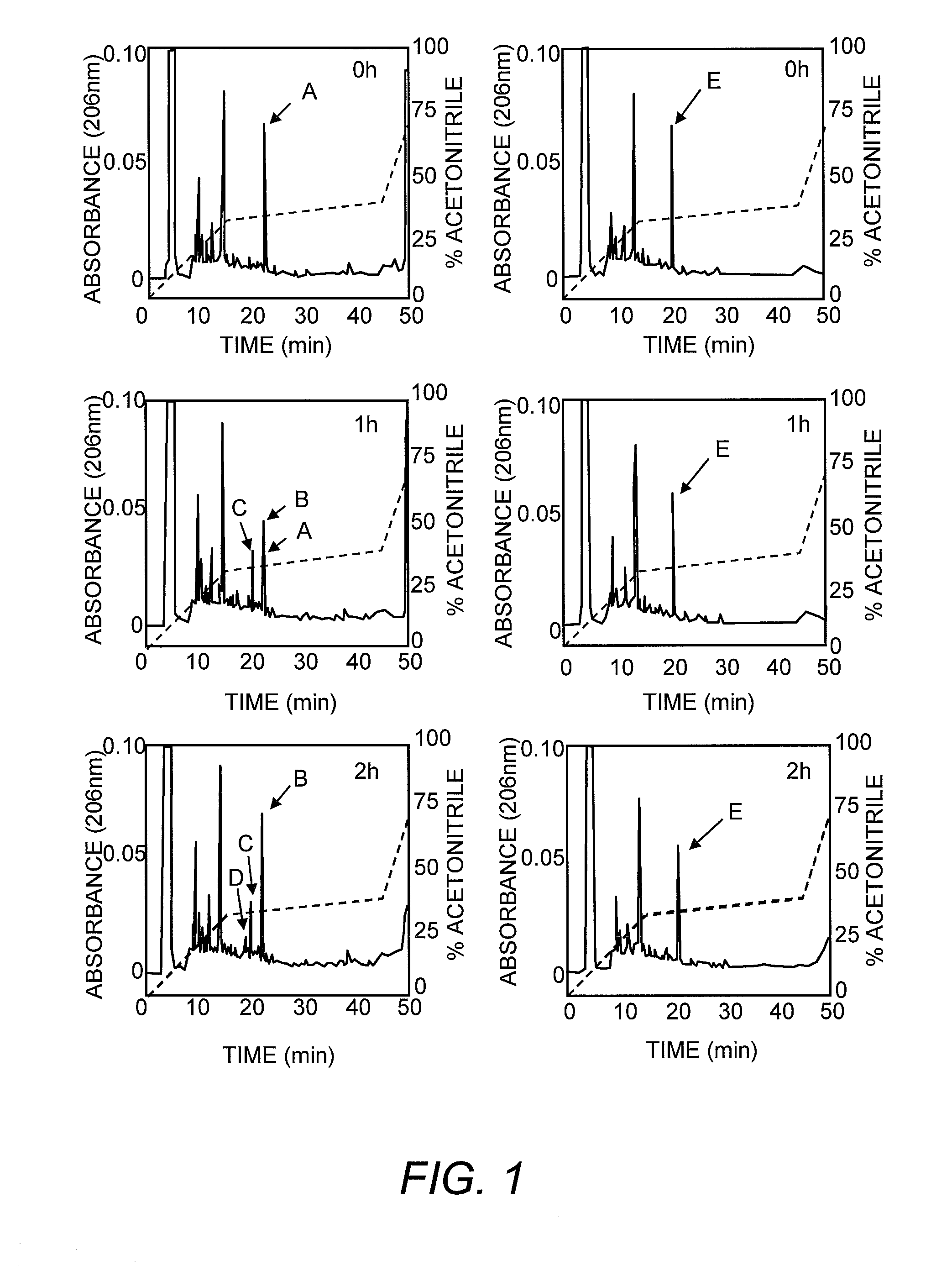
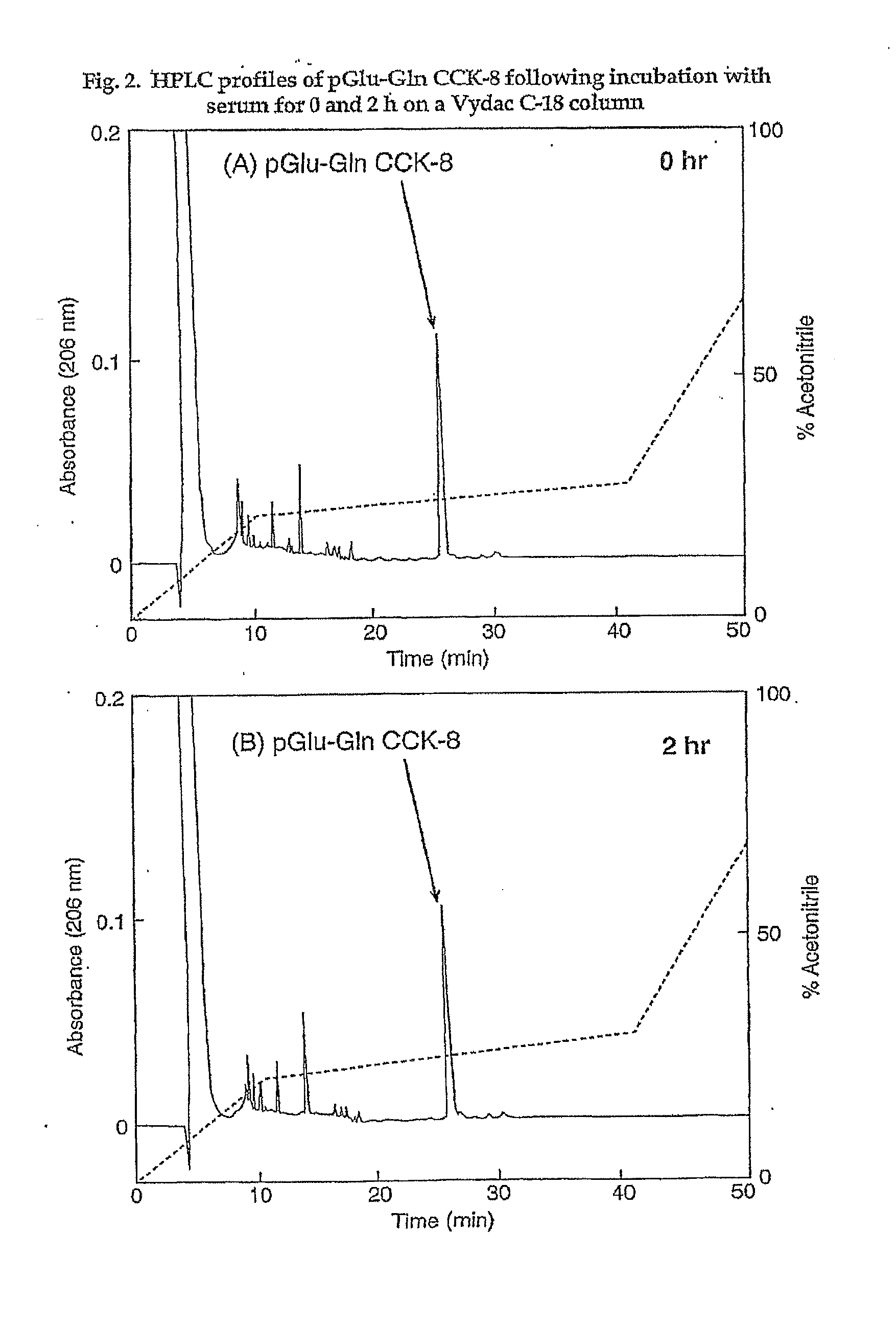
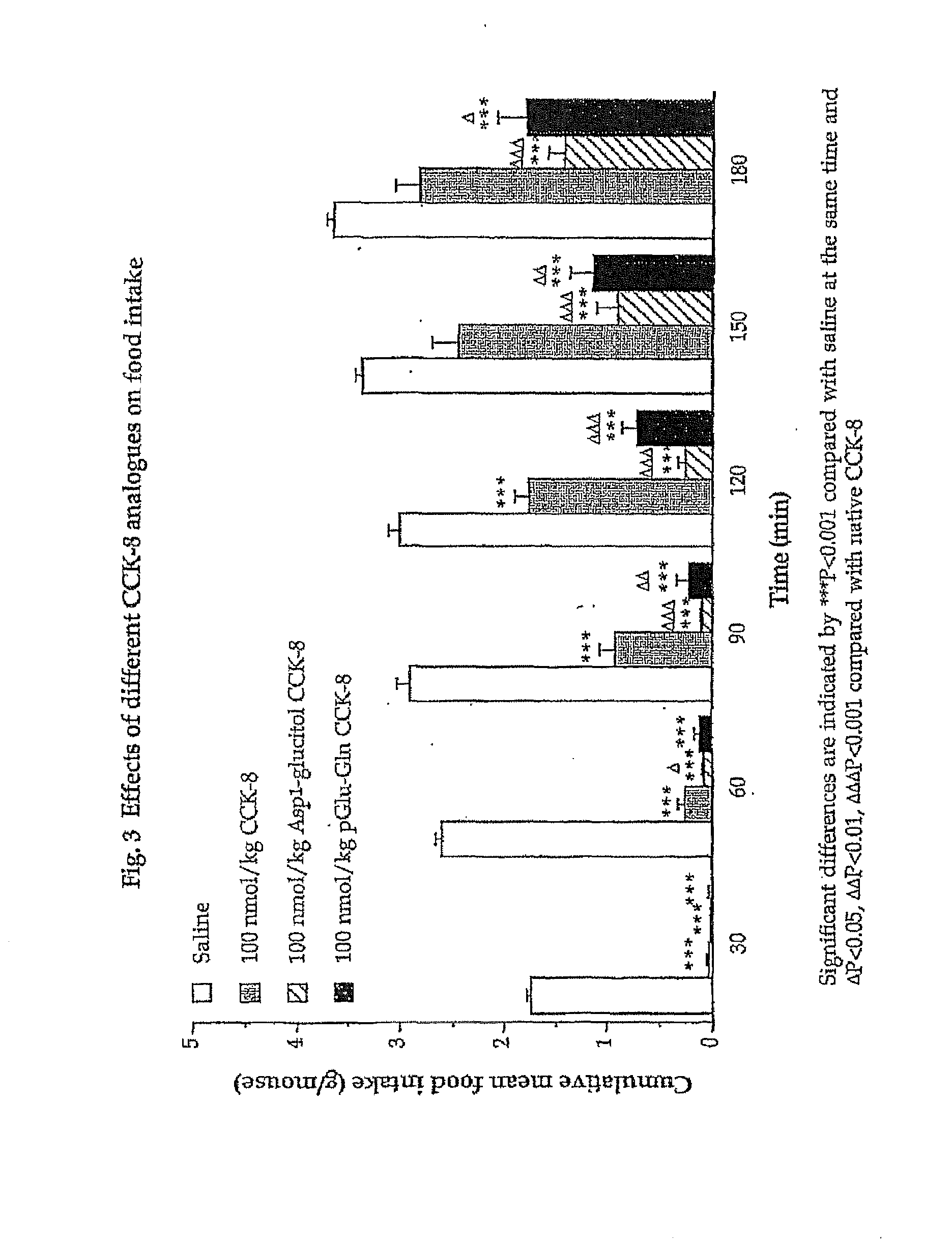
The invention concerns a peptide based on biologically active CCK-8. The peptide has improved characteristics for the treatment of at least one of obesity and type 2 diabetes and has the structure:(Z)-Asp1-Aaa2(X)-Aaa3Gly4Trp5Aaa6Asp7(Y)Aaa8K,wherein the amino acids may be either D or L amino acids; the bond between amino acid residues is either a peptide bond or a non-peptide isostere bond; Aaa2 is selected from the group comprising Tyr and Phe; when Aaa2 is Tyr, X is selected from the group comprising SO3H−, PO3H2− and a polymer moiety of the general formula —O—(CH2—O—CH2)n—H, in which n is an integer between 1 and about 22, wherein the X is covalently bound to the para phenyl oxygen of Tyr, and, when Aaa2 is Phe, X is CH2SO3Na, wherein the X is covalently bound to the para phenyl position of Phe; Aaa3 is selected from the group comprising Met, norleucine, 2-aminohexanoic acid and Thr; Aaa6 is selected from the group comprising Met, norleucine, 2-aminohexanoic acid and Phe; Aaa8 is selected from the group comprising Phe and Met; Y is covalently bound to the nitrogen of Aaa8 and is selected from the group consisting of H and CH3; K is selected from the group consisting of the hydroxyl group of Phe8, an amide covalently bound to Phe8, an ester covalently bound to Phe8, a salt of the hydroxyl group of Phe8, a salt of an amide covalently bound to Phe8, a salt of an ester covalently bound to Phe8 and a polymer moiety of the general formula —O—(CH2—O—CH2)n—H, in which n is an integer between 1 and about 22; and Z comprises at least one amino acid modification, wherein said at least one modification comprises an N-terminal extension, or an N-terminal modification, but excludes Asp1-glucitol CCK-8 where Aaa2 is Tyr and X is SO3H−.The peptides, and Asp1-glucitol CCK-8, are useful to at least one of inhibit food intake, induce satiety, stimulate insulin secretion, moderate blood glucose excursions, enhance glucose disposal and exhibit enhanced stability in plasma compared to native CCK-8
Owner:UNIVERSITY OF ULSTER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com