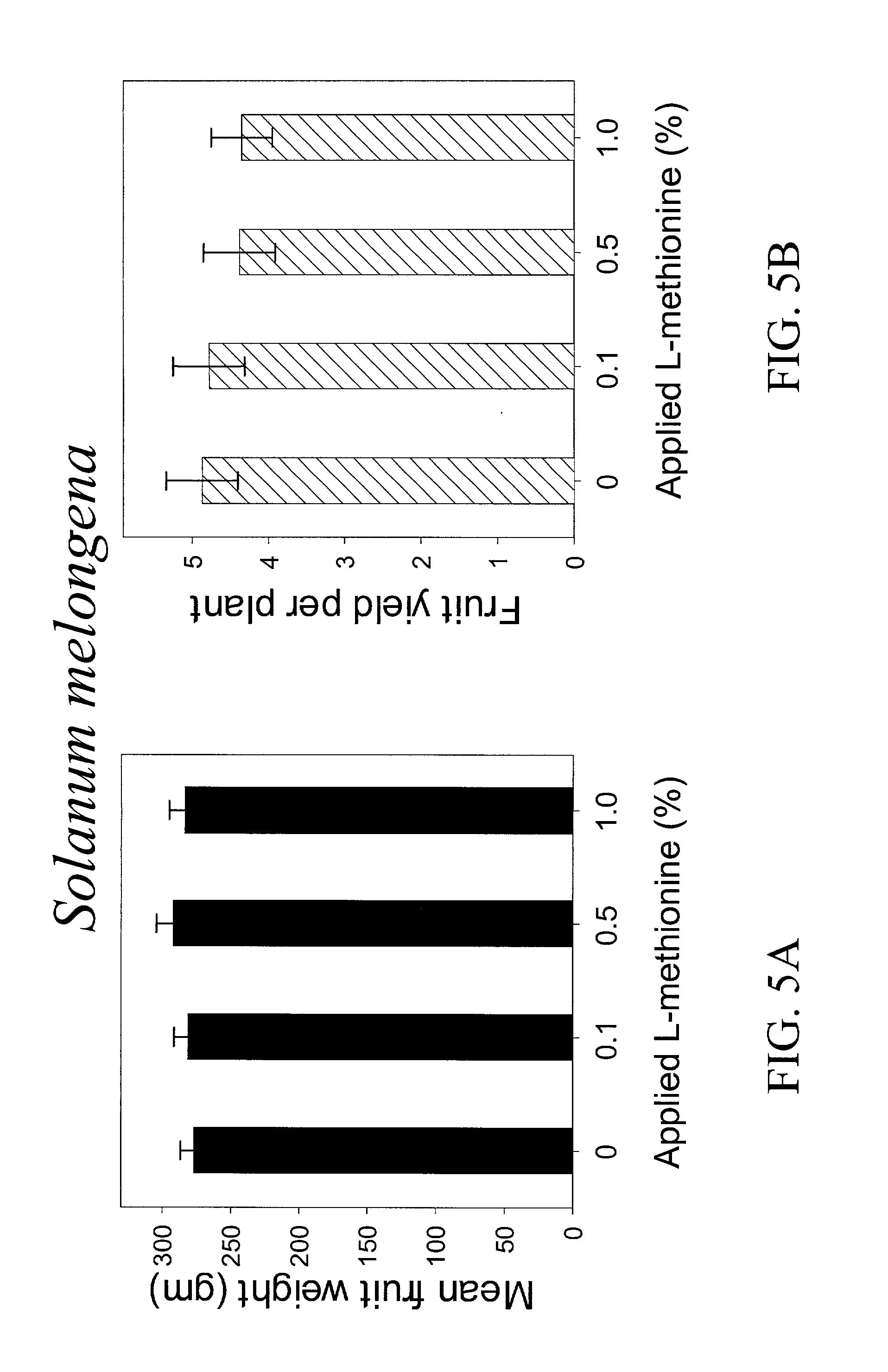
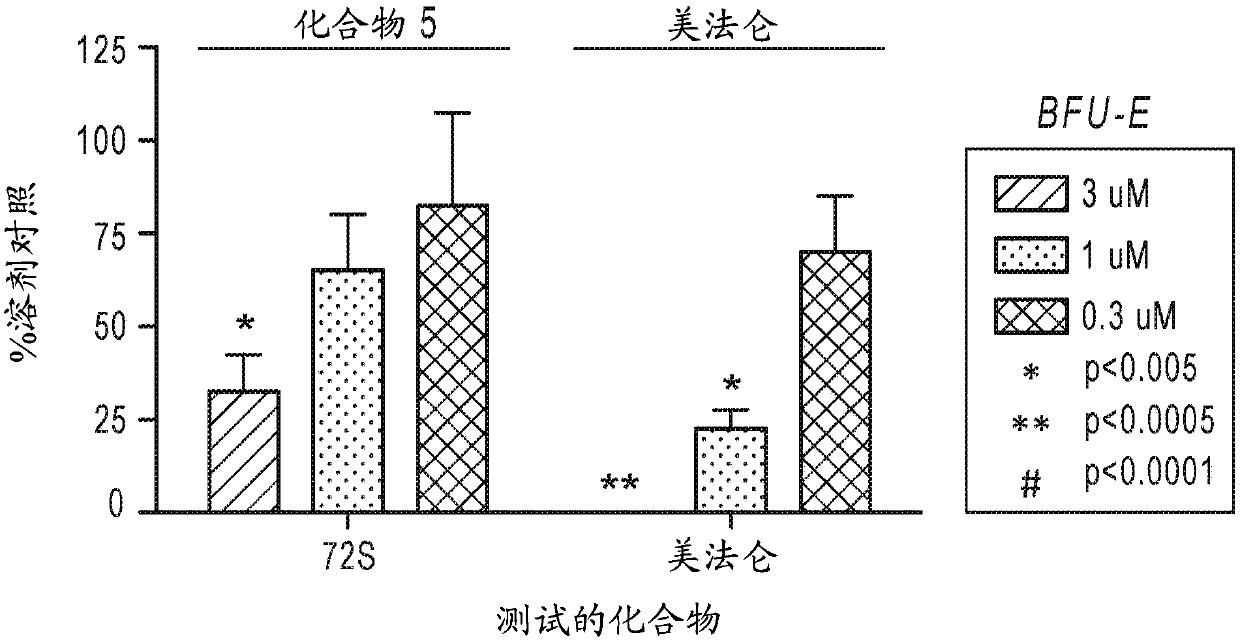
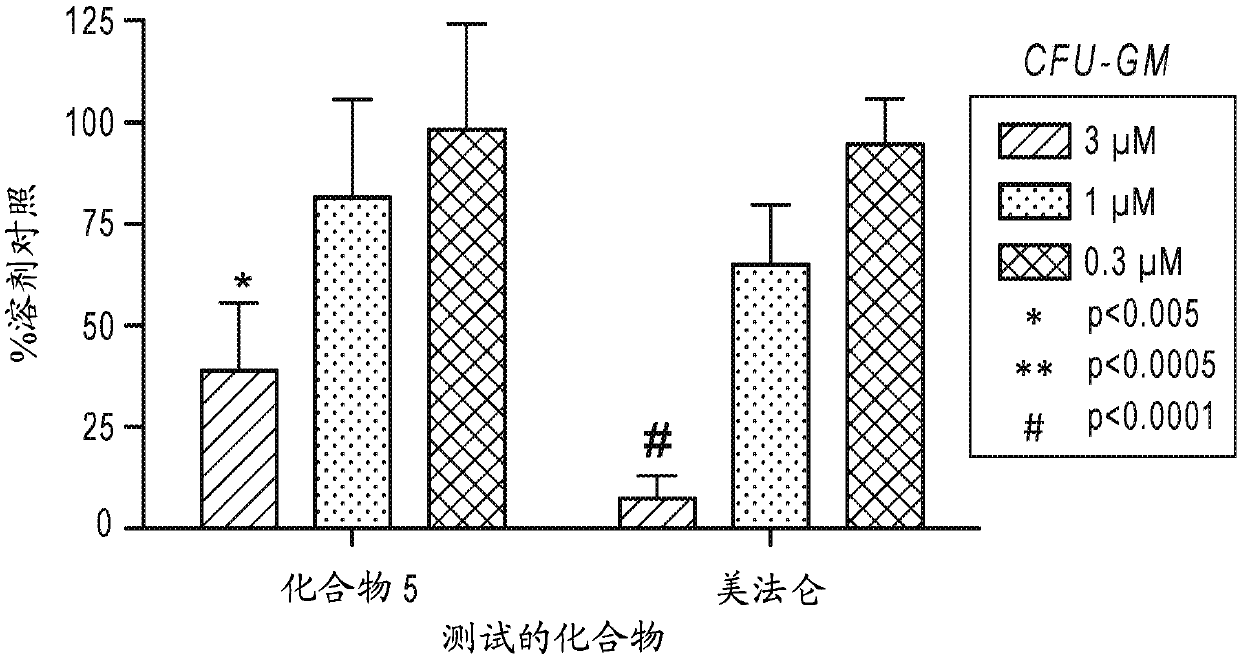
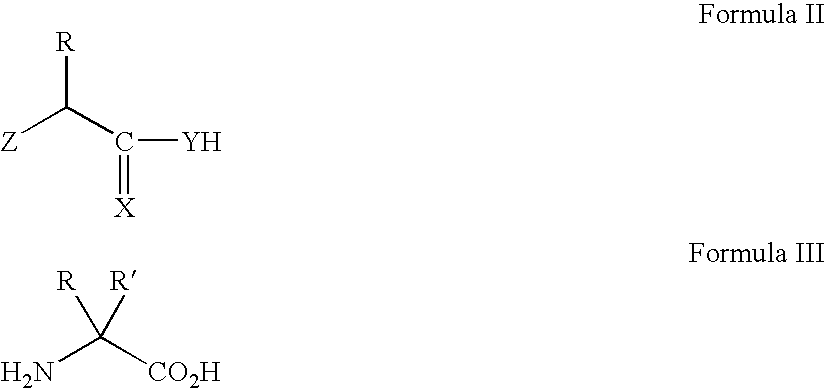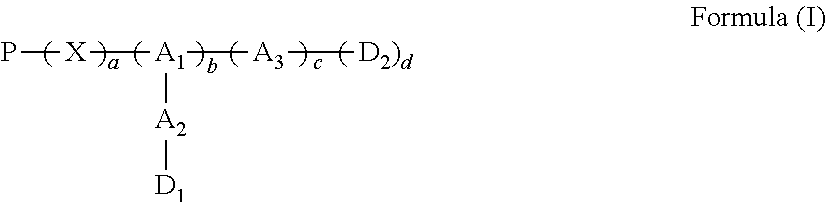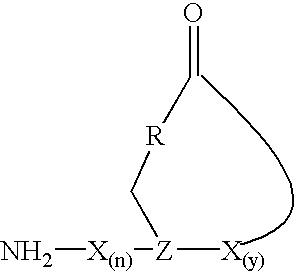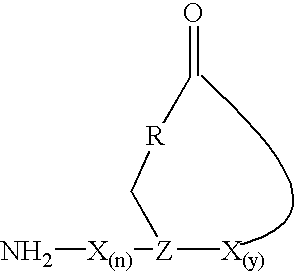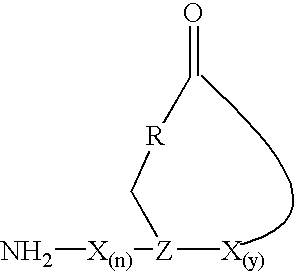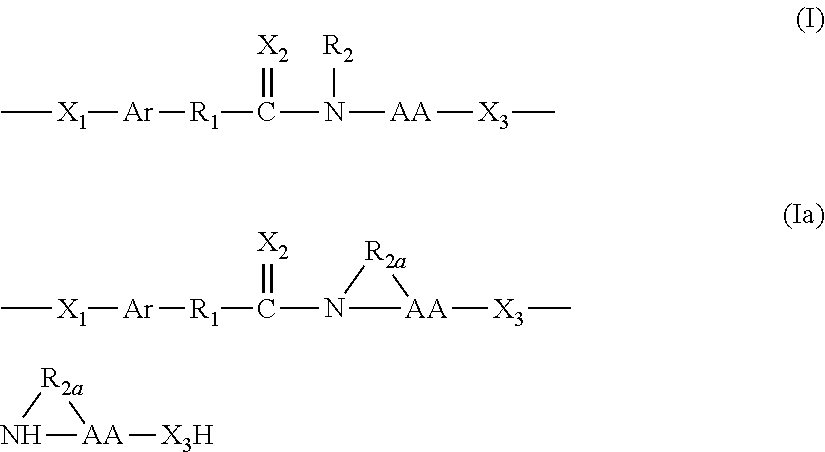Patents
Literature
73 results about "Aminoacid analog" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
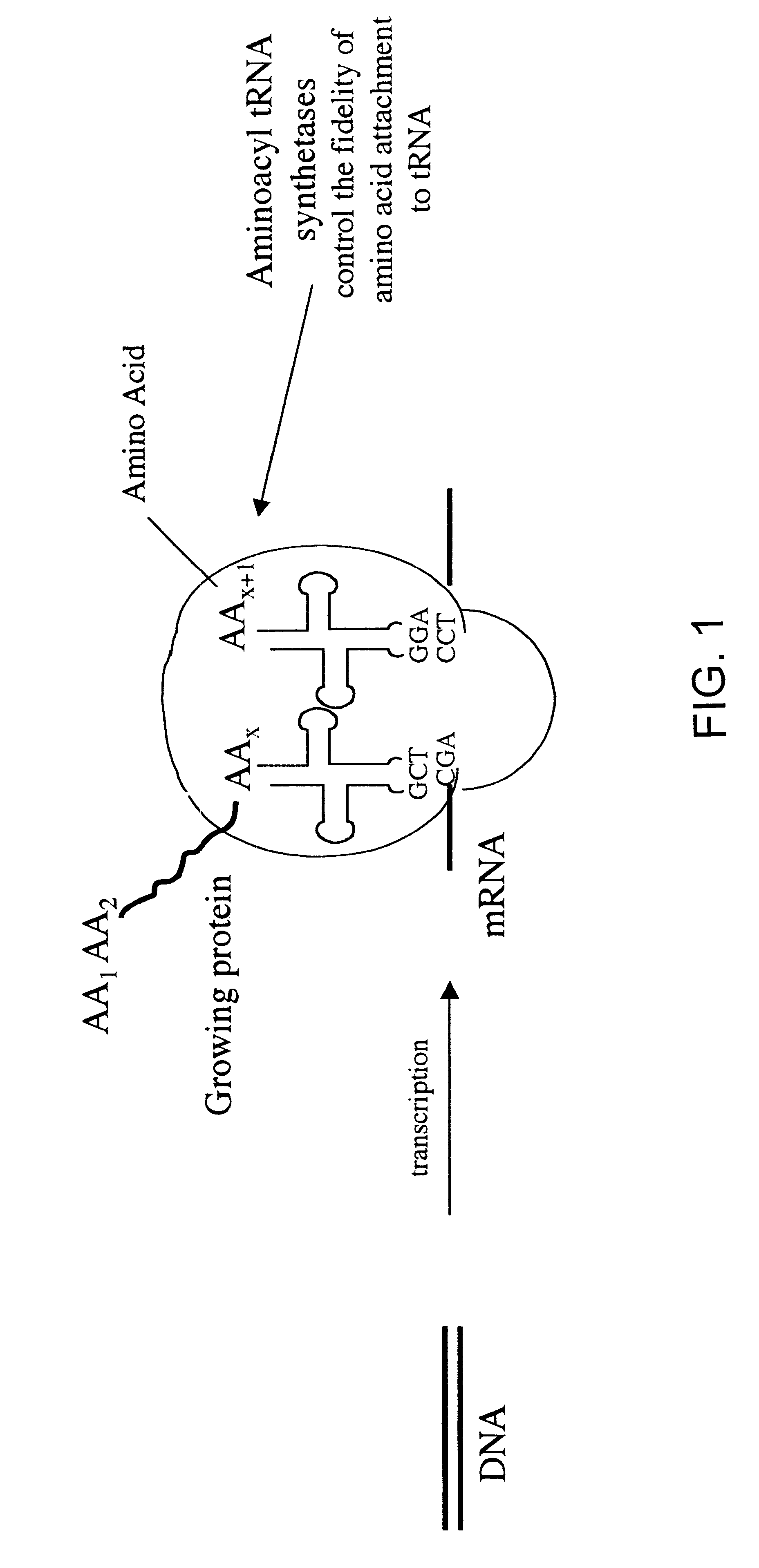
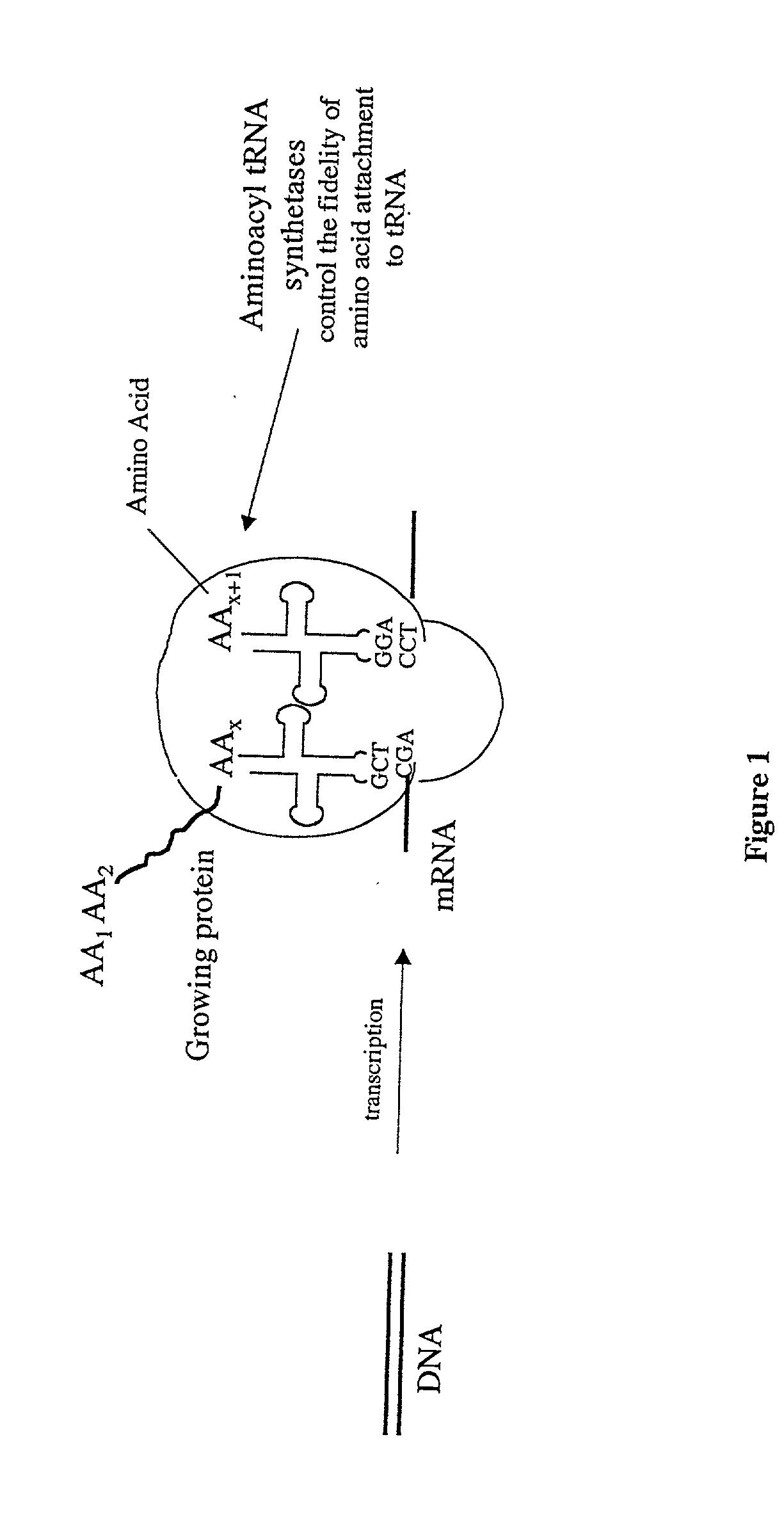
Synthetic or natural compounds which resemble naturally occurring amino acids in structure and/or function.
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS6586207B2High yieldRapid and predictable approachBacteriaOxidoreductasesMethionine biosynthesisDihydrofolate reductase
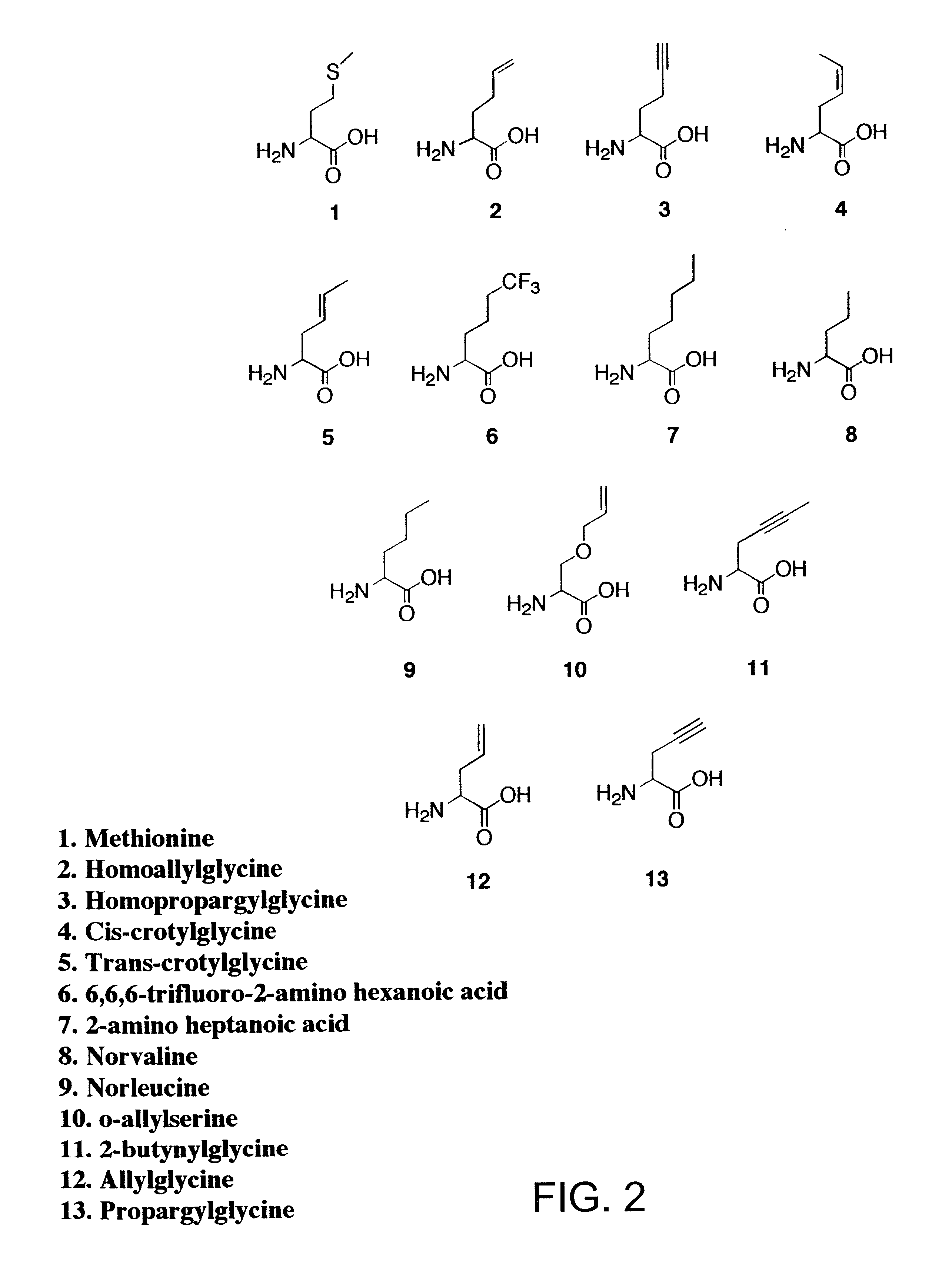
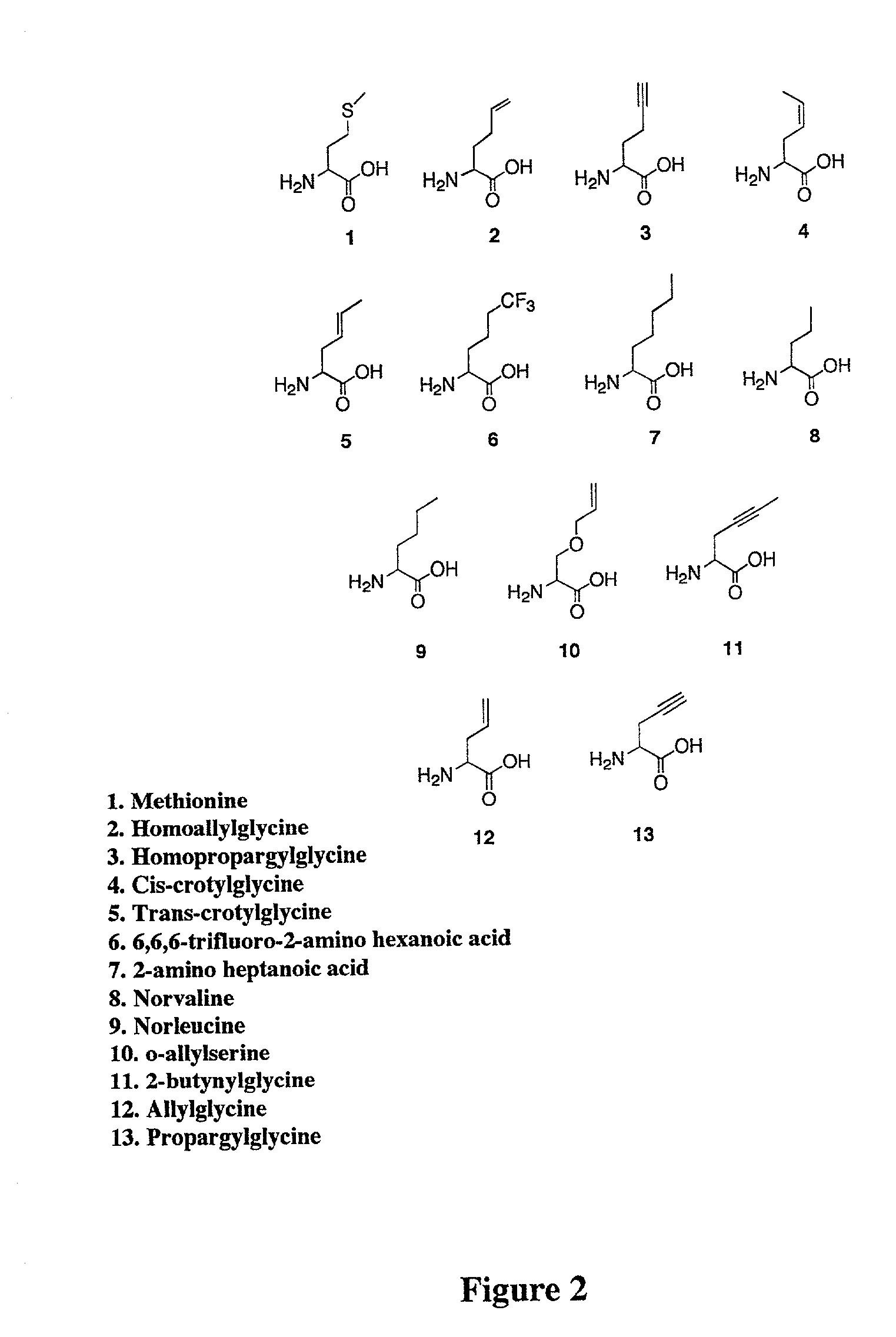
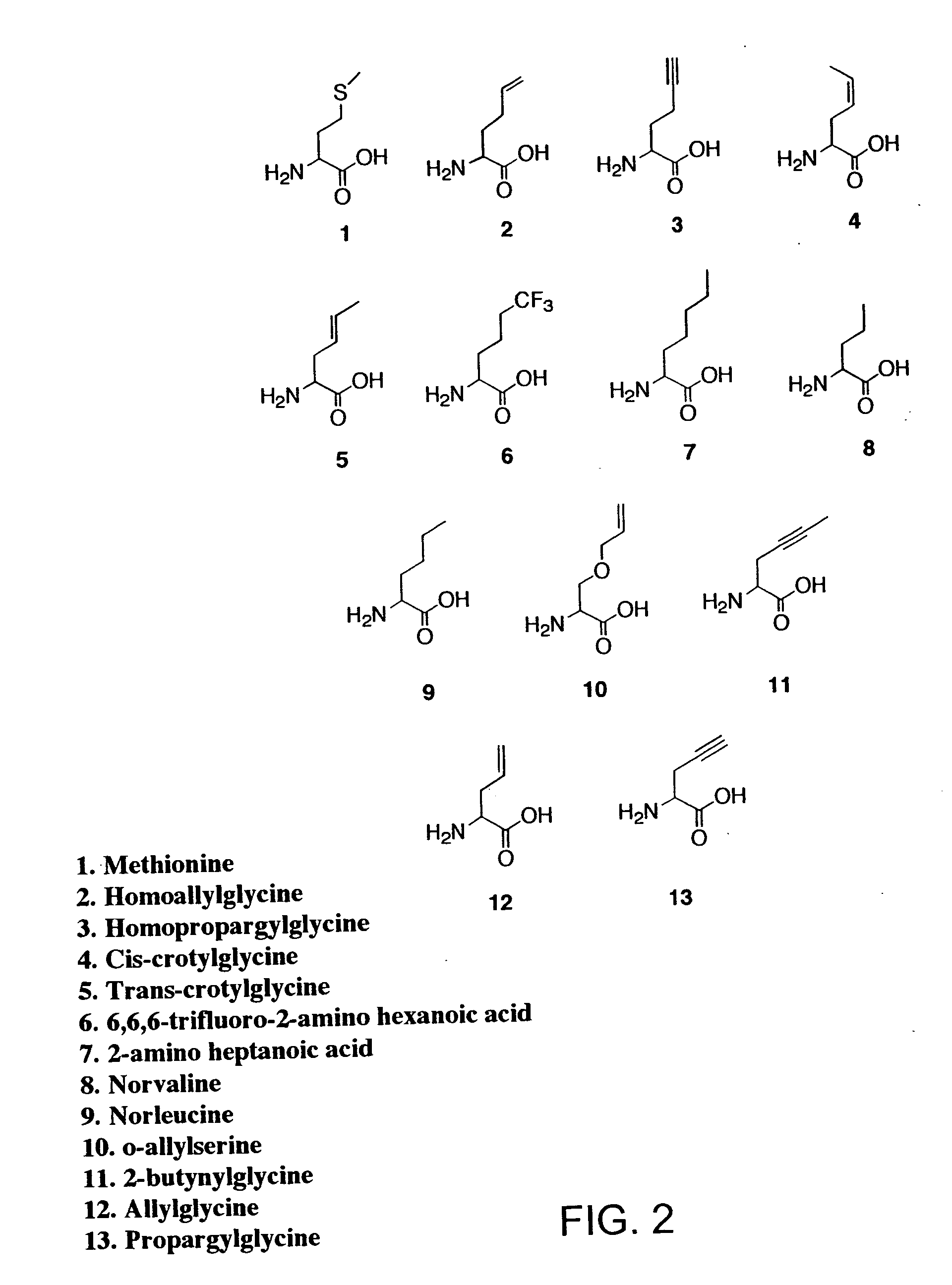
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
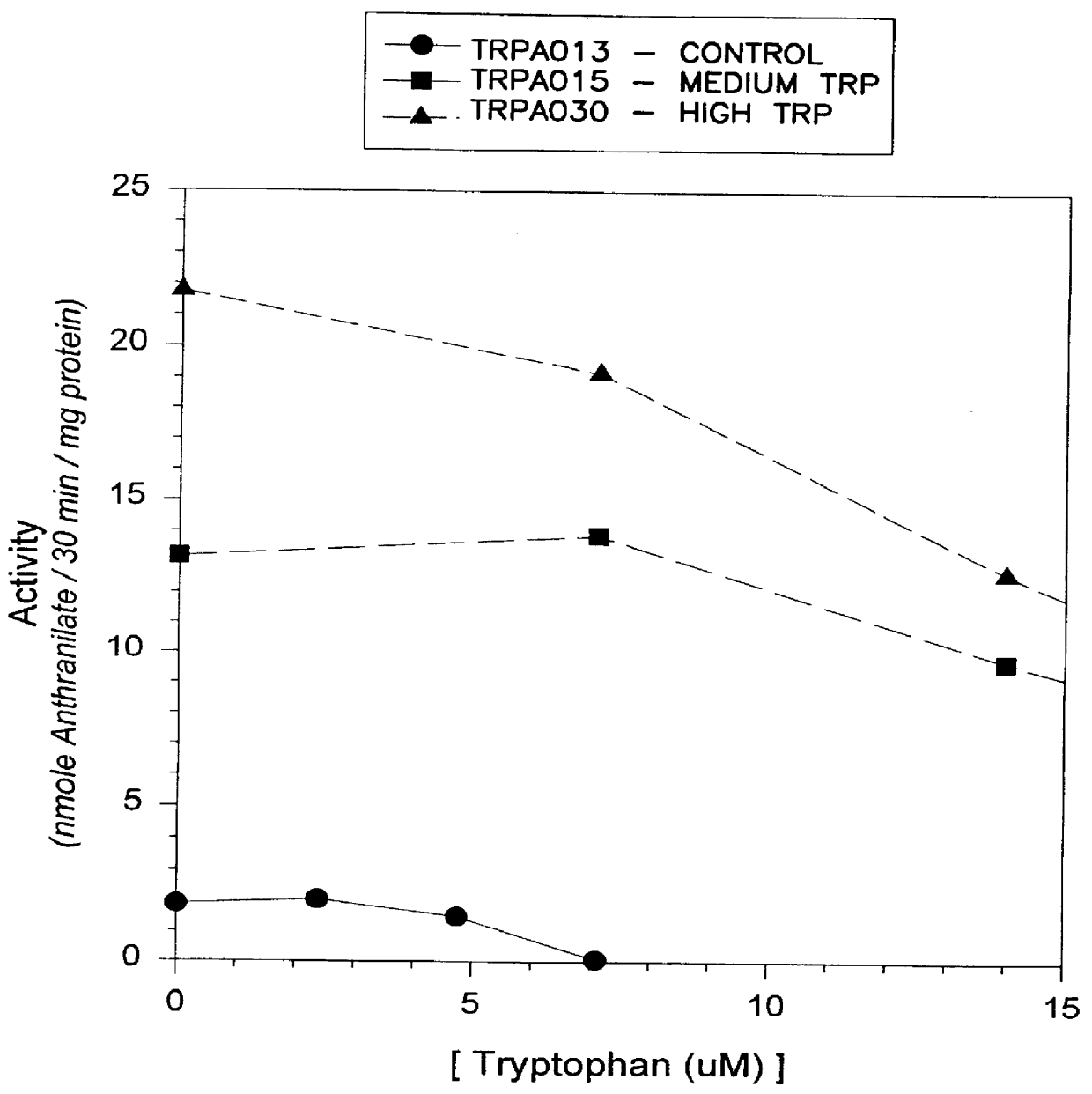
Anthranilate synthase gene and method of use thereof for conferring tryptophan overproduction
InactiveUS6118047AGrowth inhibitionNot susceptibleSugar derivativesBacteriaDNA fragmentationGMO Plants
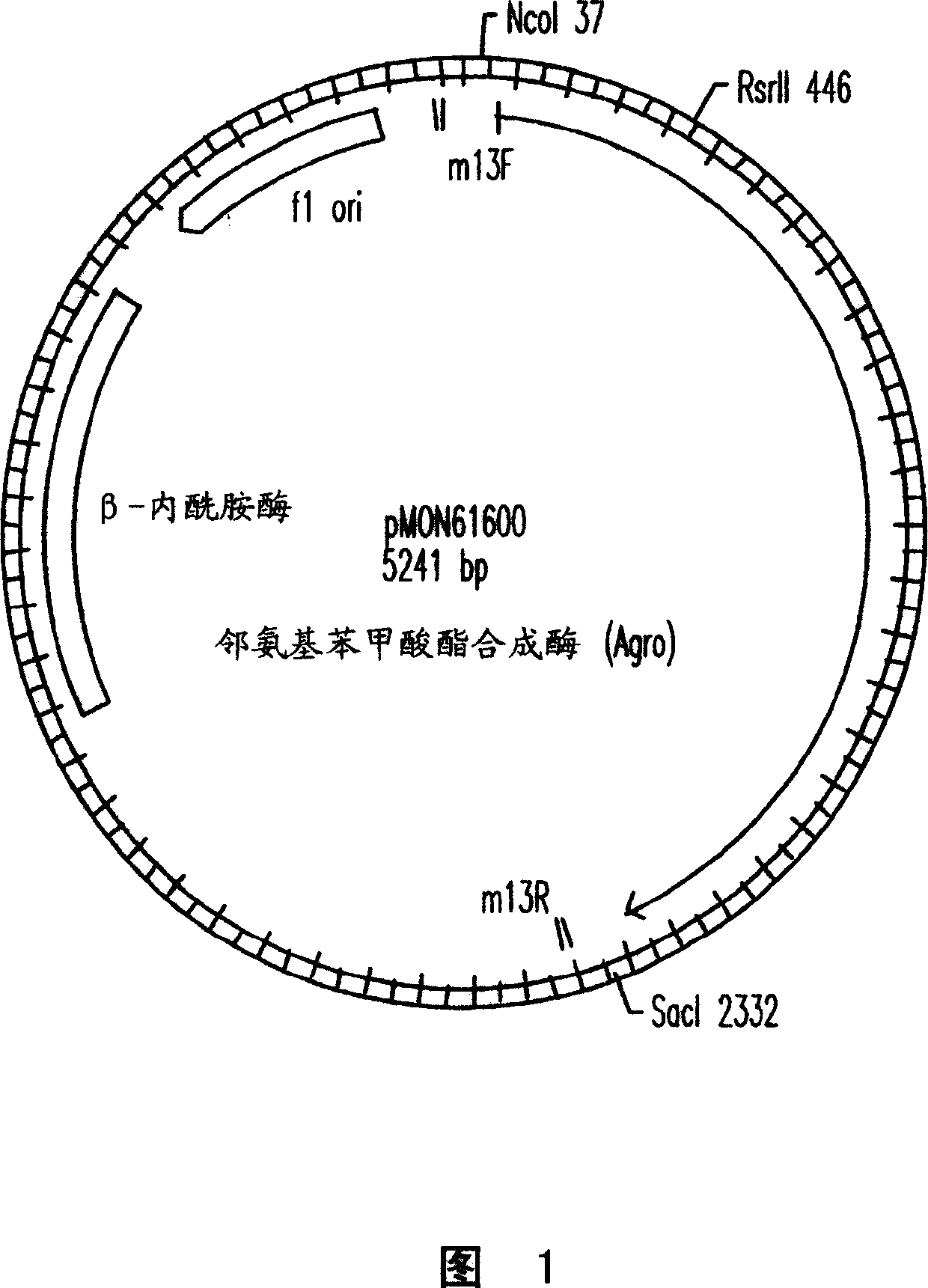
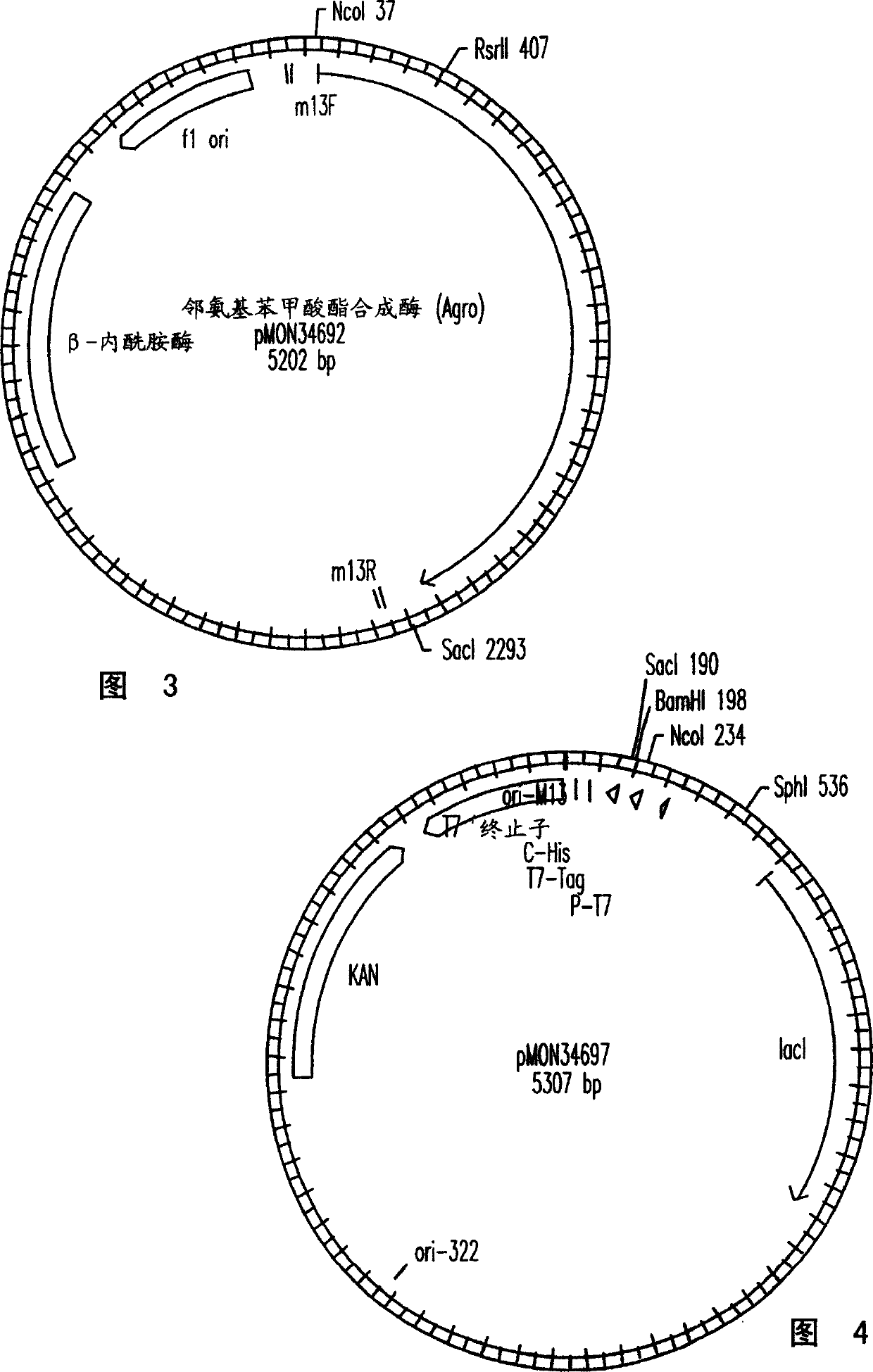
The present invention provides a method for conferring tolerance to an amino acid analog of tryptophan to a plant and / or altering the tryptophan content of a plant by introducing and expressing an isolated DNA segment encoding an anthranilate synthase in the cells of the plant. Transgenic plants transformed with an isolated DNA segment encoding an anthranilate synthase, as well as seeds and progeny derived from these plants, are also provided. The present invention also provides a cDNA sequence of an alpha and a beta subunit of a maize anthranilate synthase.
Owner:MONSANTO TECH LLC
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS20020042097A1High yieldRapid and predictable approachFungiBacteriaMethionine biosynthesisDihydrofolate reductase
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
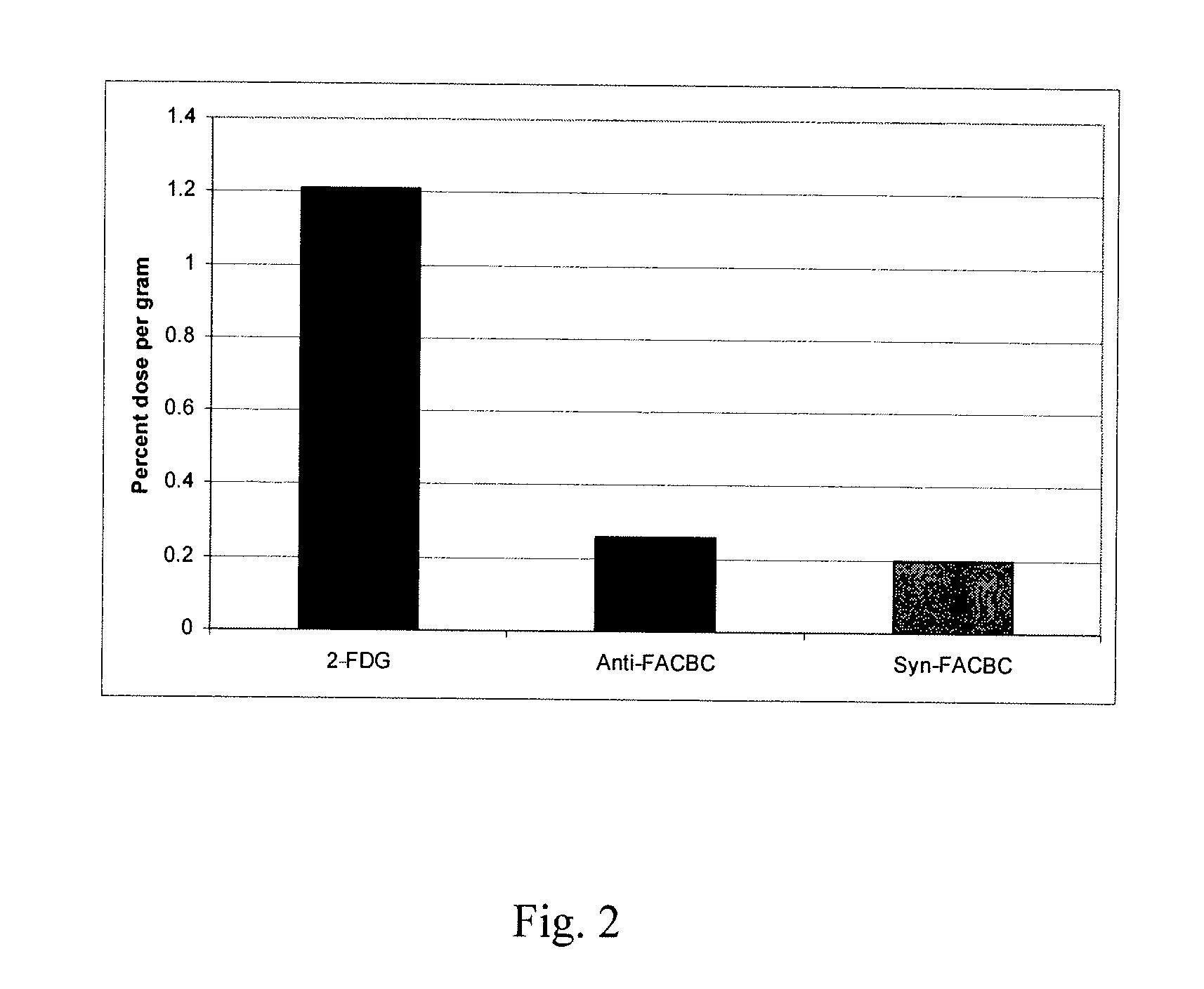
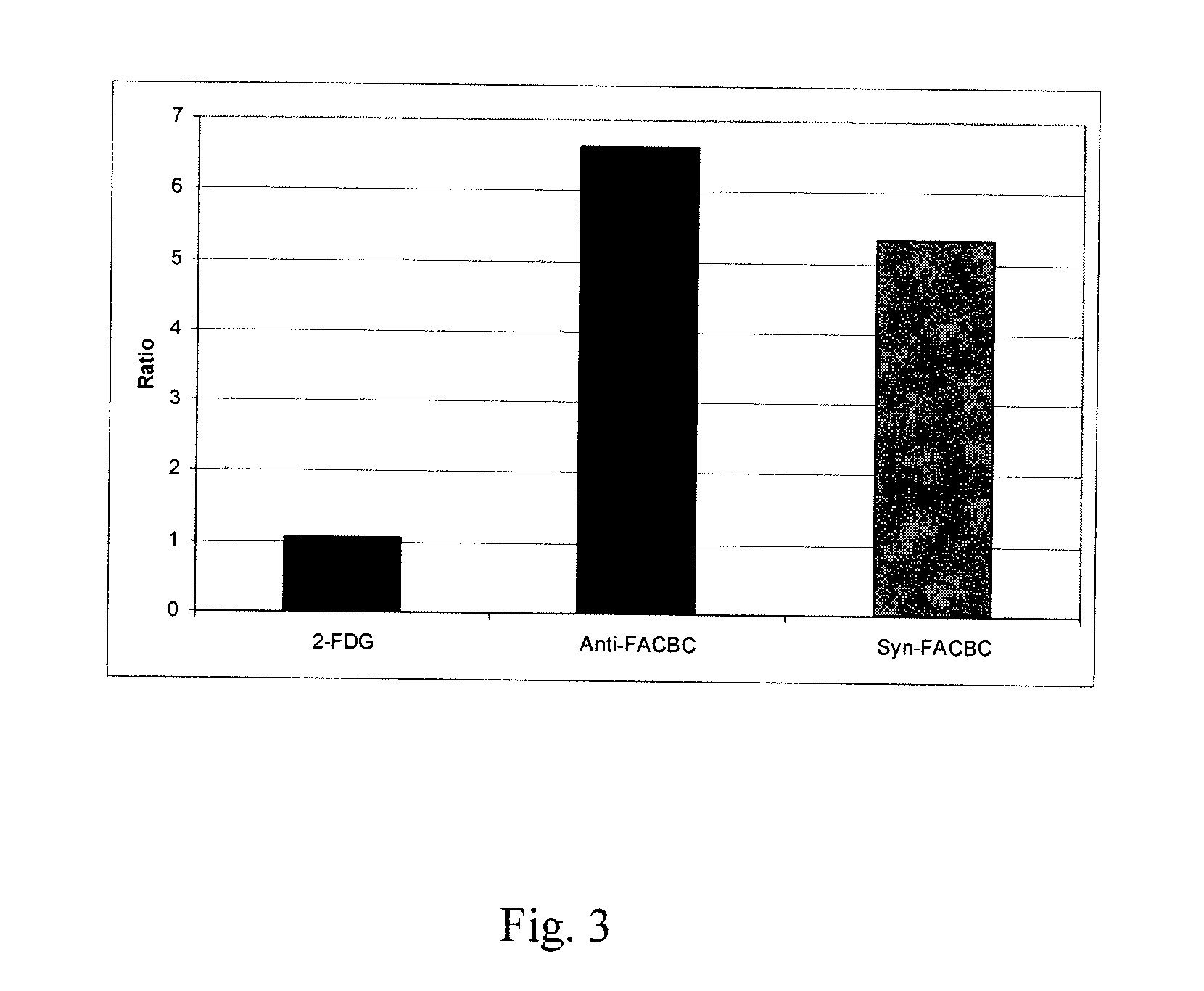
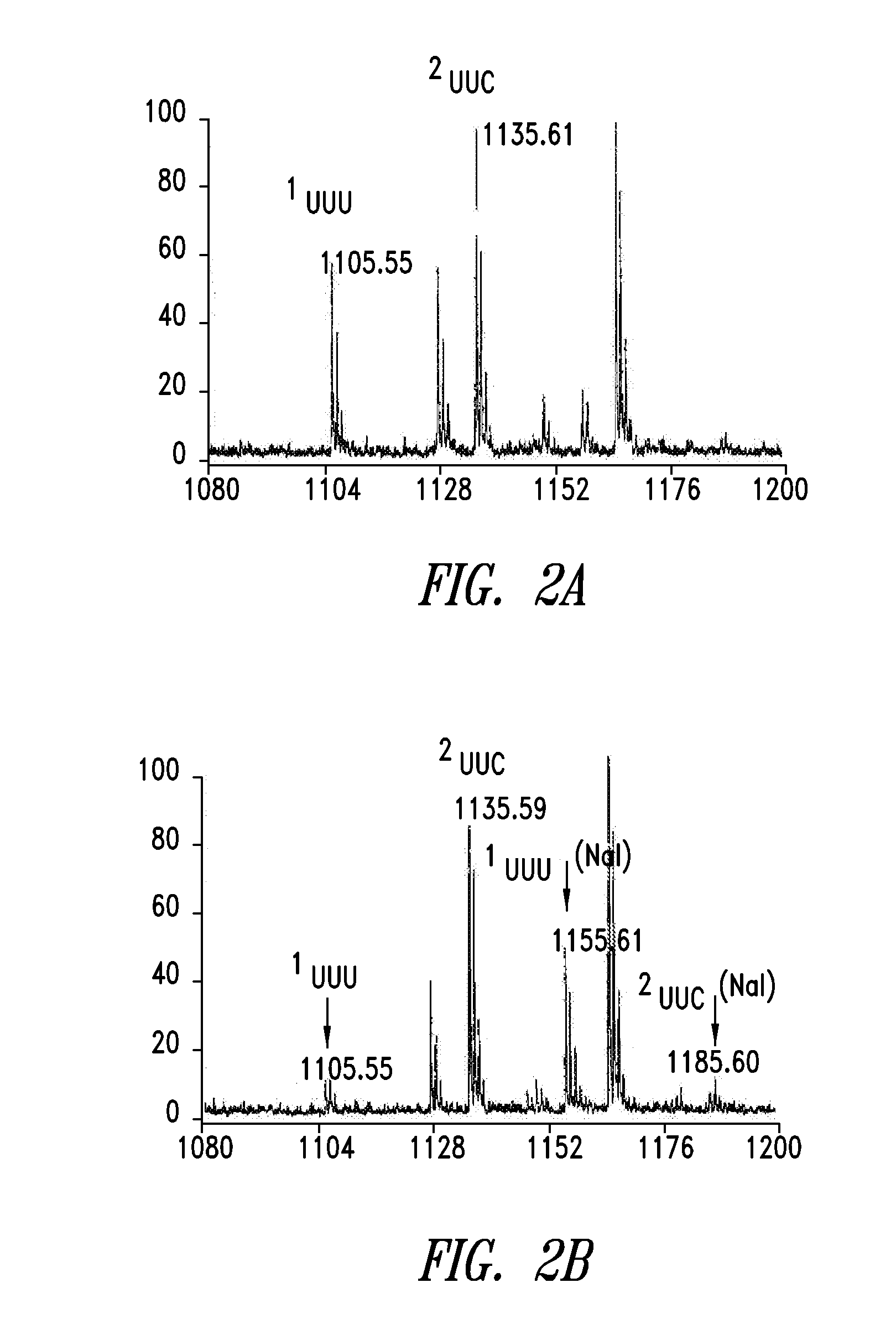
Stereoselective Synthesis of Amino Acid Analogs for Tumor Imaging
InactiveUS20060292073A1Maximum service lifeOrganic compound preparationSulfonic acid esters preparation1-amino-3-fluorocyclobutane-1-carboxylic acidCyclobutane
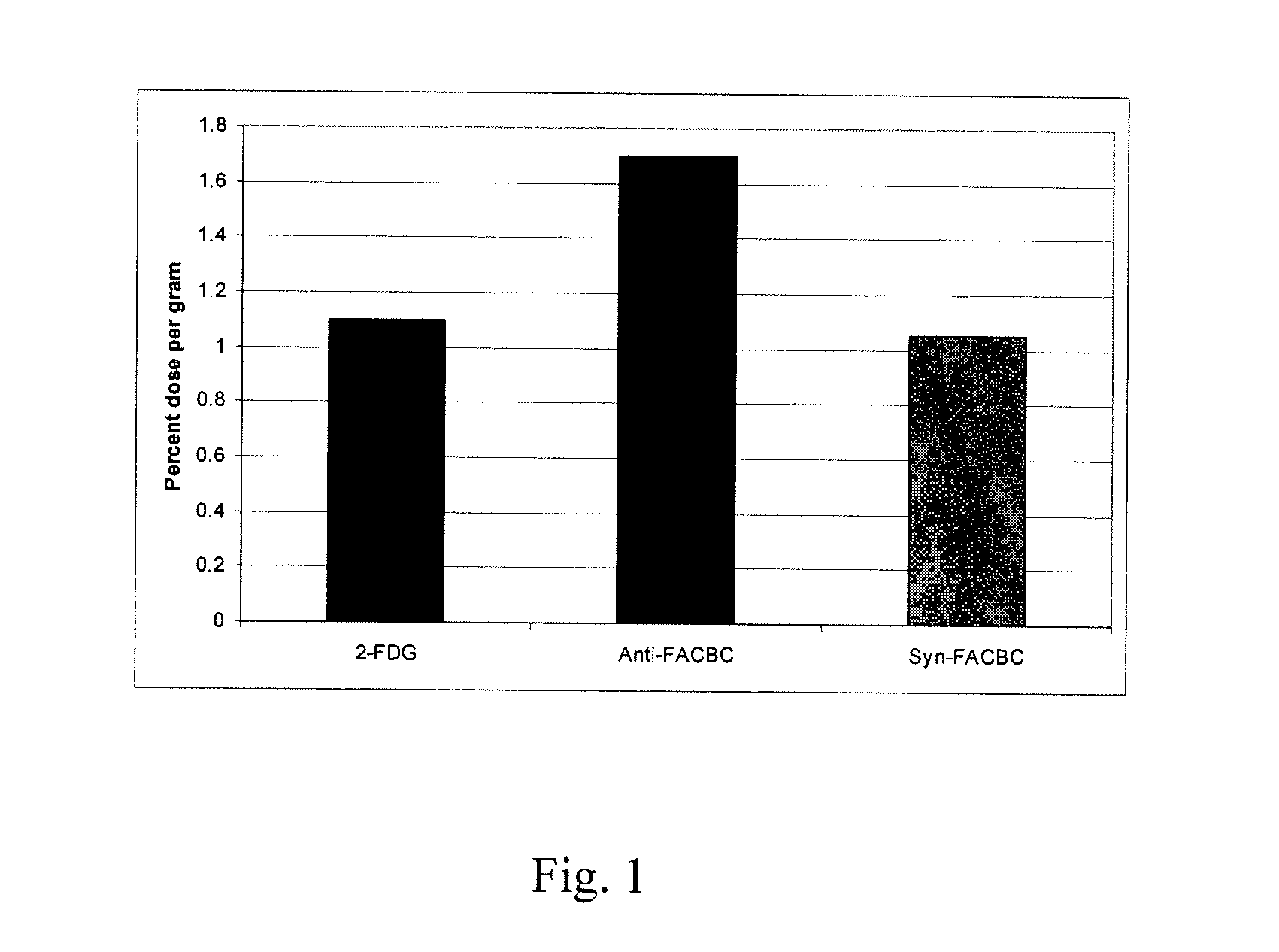
The radiolabeled non-natural amino acid 1-amino-3-cyclobutane-1-carboxylic acid (ACBC) and its analogs are candidate tumor imaging agents useful for positron emission tomography and single photon emission computed tomography due to their selective affinity for tumor cells. The present invention provides methods for stereo-selective synthesis of syn-ACBC analogs. The disclosed synthetic strategy is reliable and efficient and can be used to synthesize a gram quantity of various syn-isomers of the ACBC analogs, particularly, syn-[18F]-1-amino-3-fluorocyclobutane-1-carboxylic acid (FACBC) and syn-[123I]-1-amino-3-iodocyclobutane-1-carboxylic (IACBC) acid analogs.
Owner:EMORY UNIVERSITY
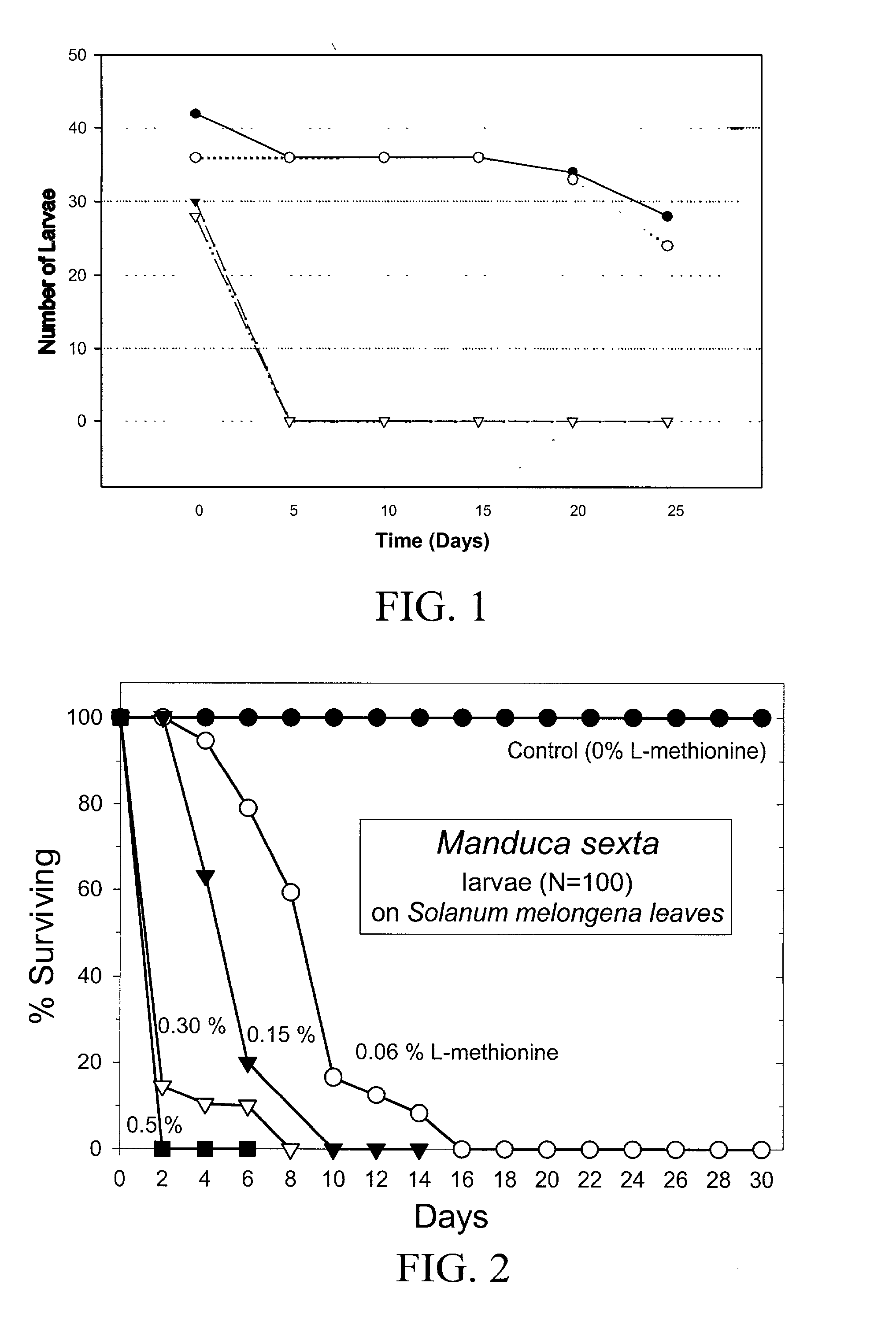
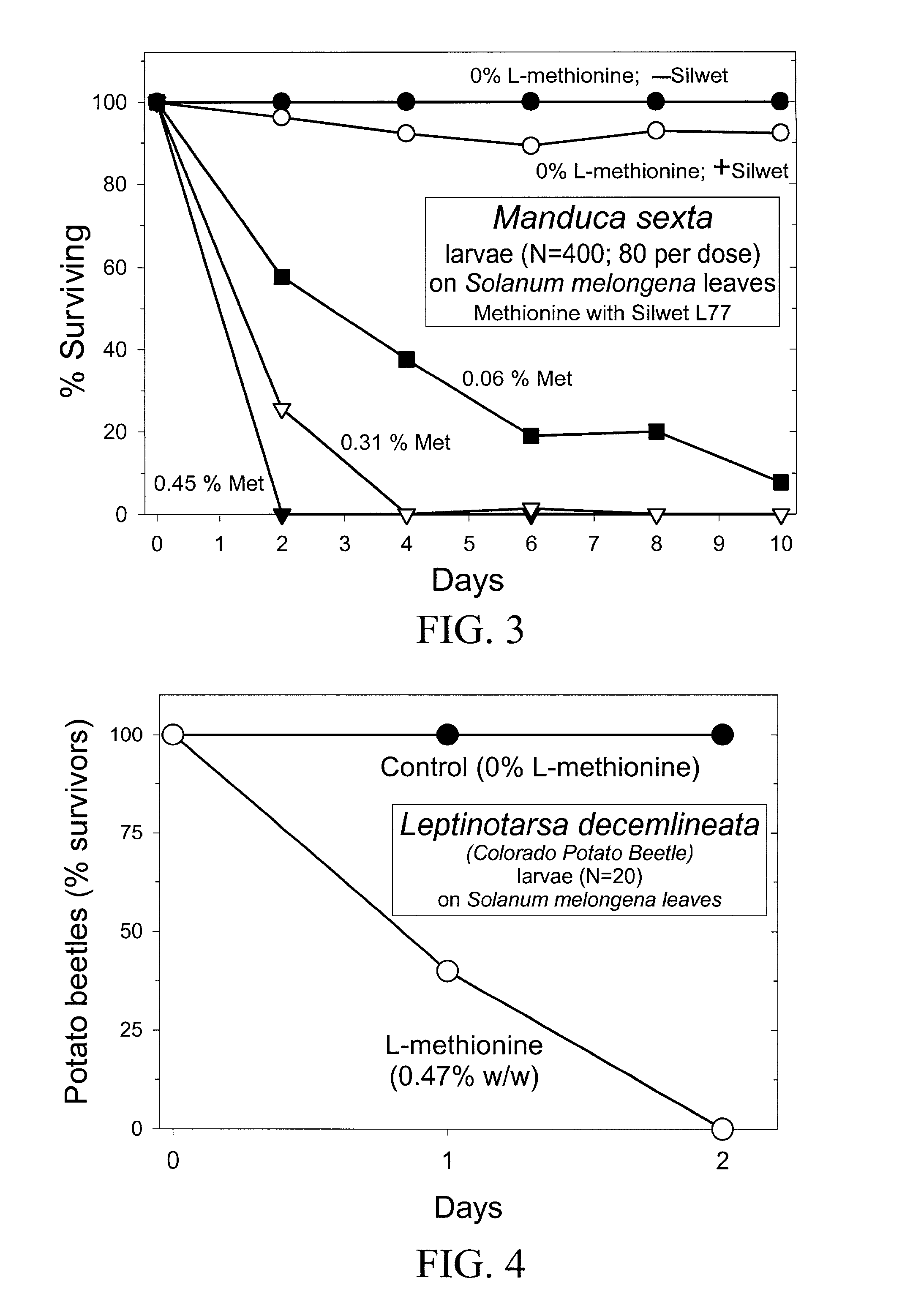
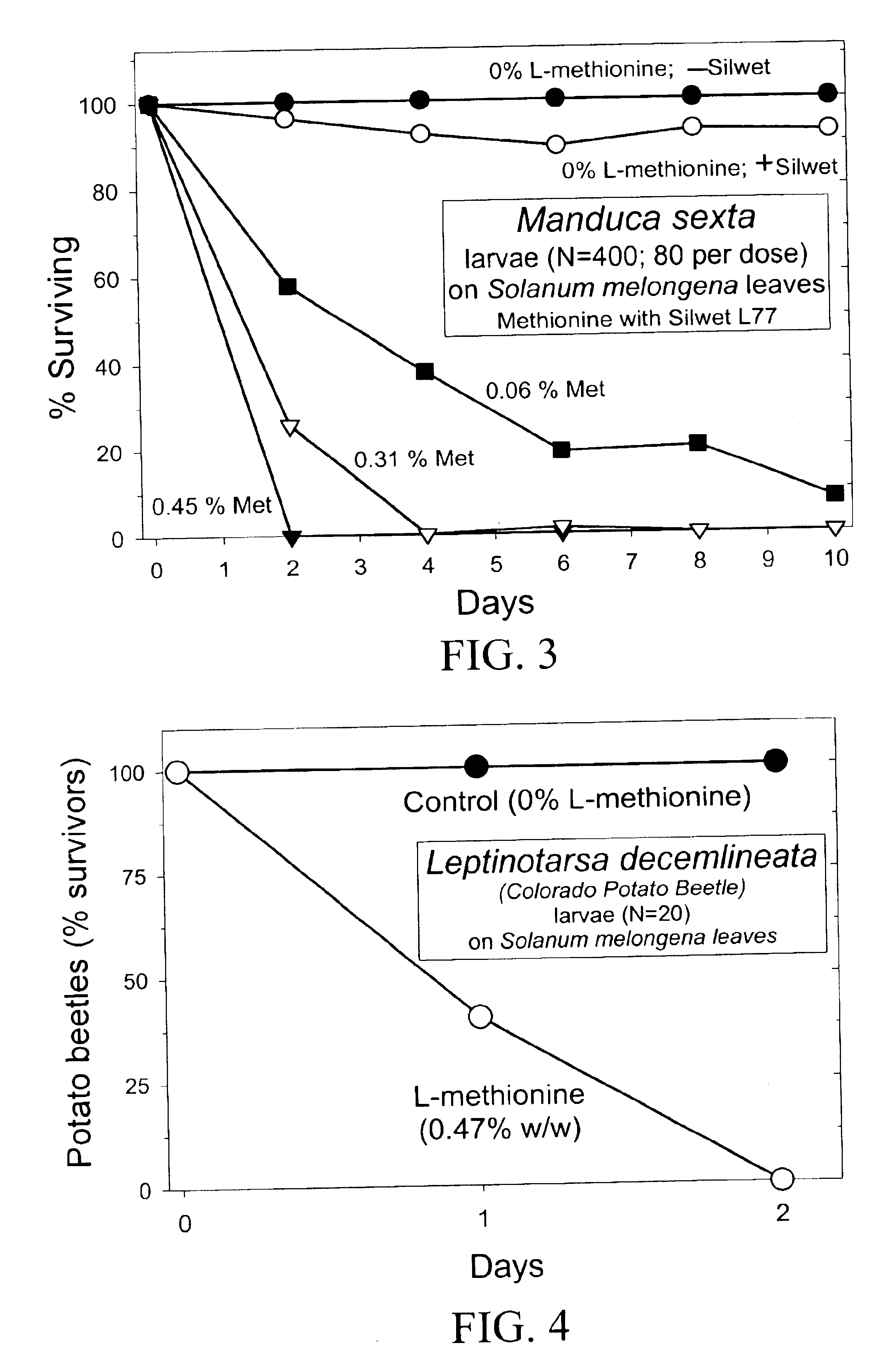
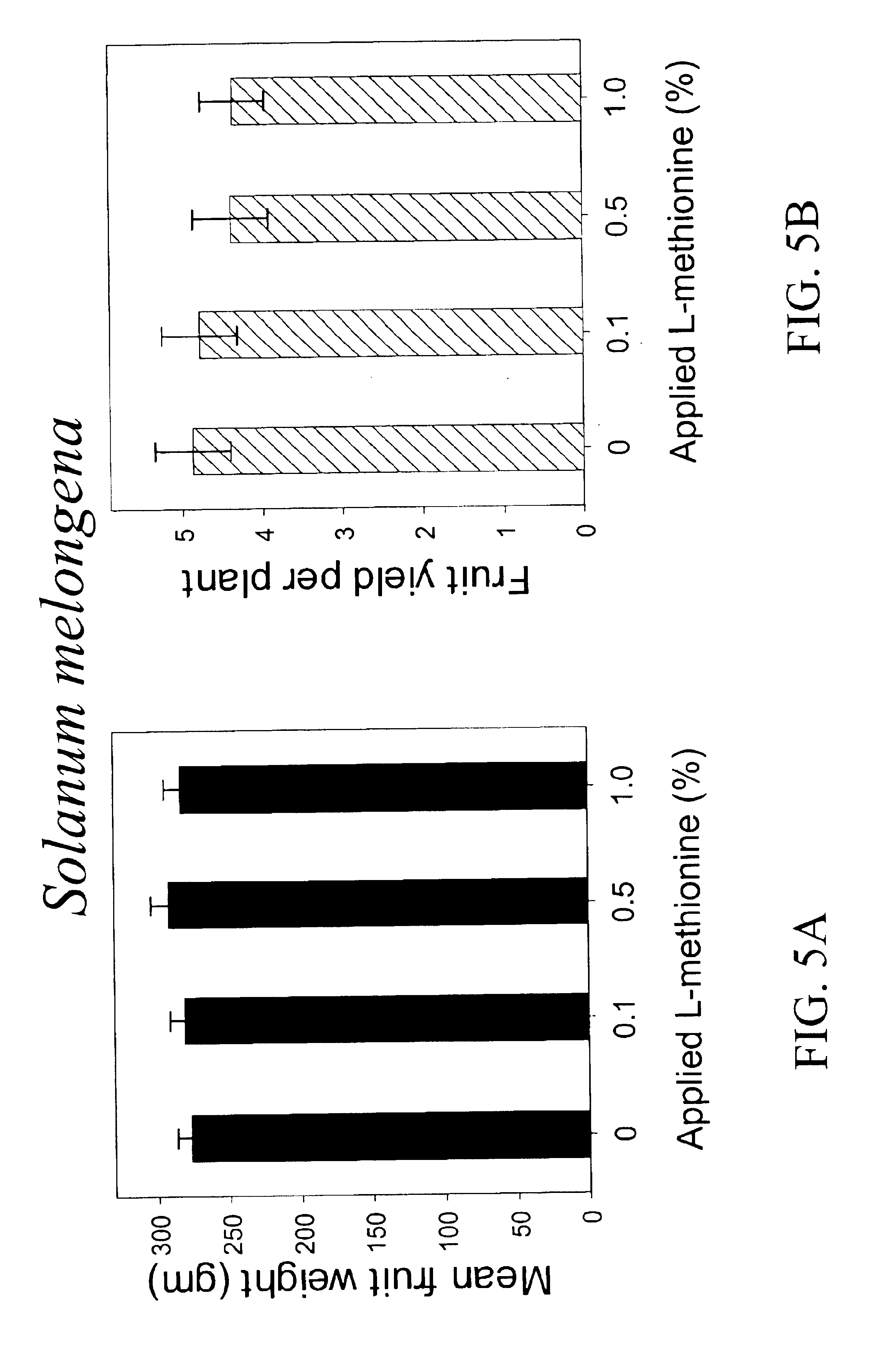
Materials and methods for controlling pests
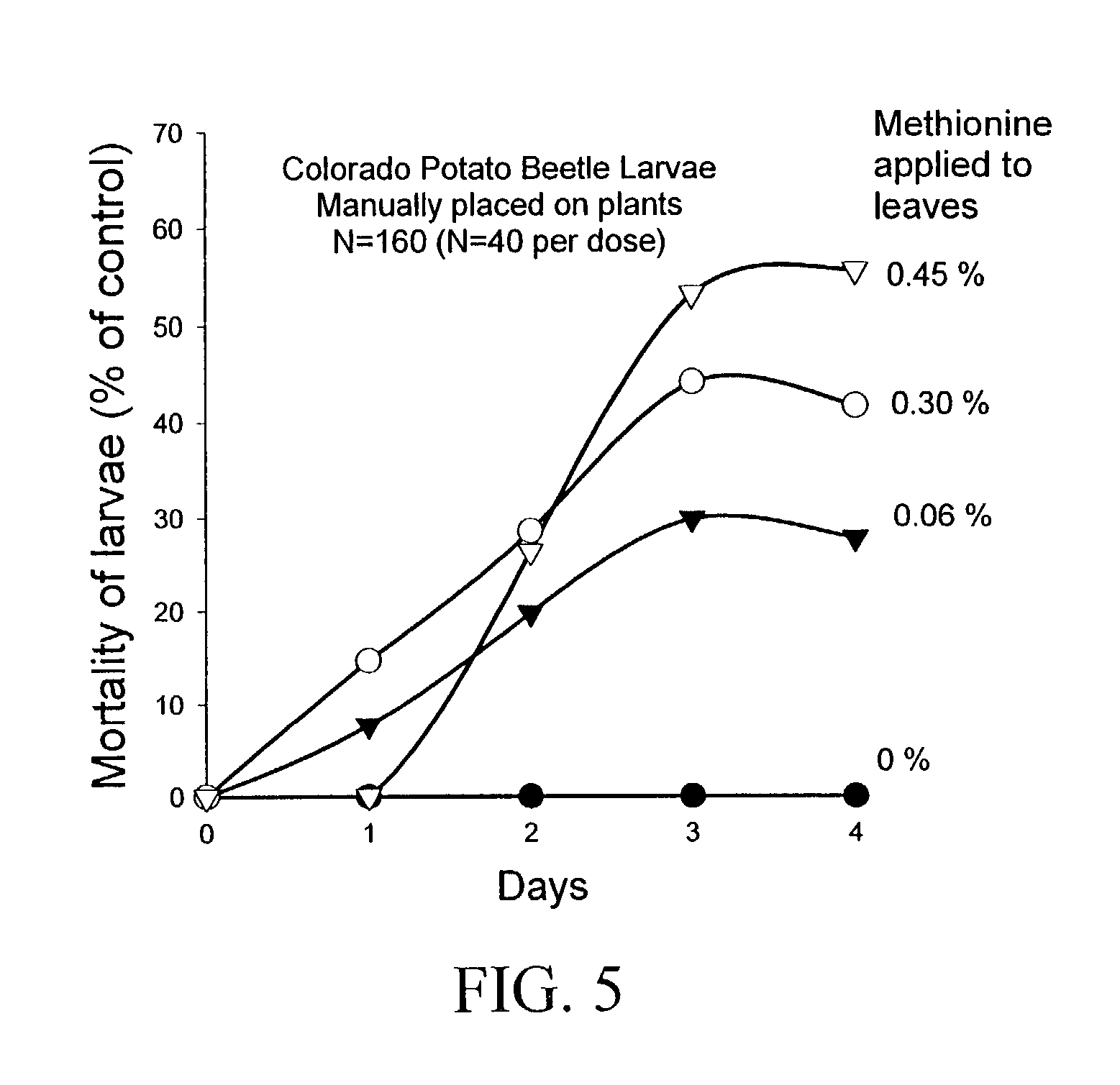
InactiveUS20030154508A1Low toxicitySustainable, pesticide-free food supplyOrganic active ingredientsBiocideSolute transportersMethionine biosynthesis
Owner:FLORIDA UNIV OF A FLORIDA +1
Methods of incorporating amino acid analogs into proteins
InactiveUS20050287639A1Extended half-lifeReduced binding affinityFungiSugar derivativesBase pairAmino acid
The invention provides a method of incorporating nonstandard amino acids into a protein by utilizing a modified aminoacyl-tRNA synthetase to charge the nonstandard amino acid to a modified tRNA, which forms strict Watson-Crick base-pairing with a codon that normally forms wobble base-pairing with natural tRNAs.
Owner:CALIFORNIA INST OF TECH
Melanocortin 1 receptor selective compounds
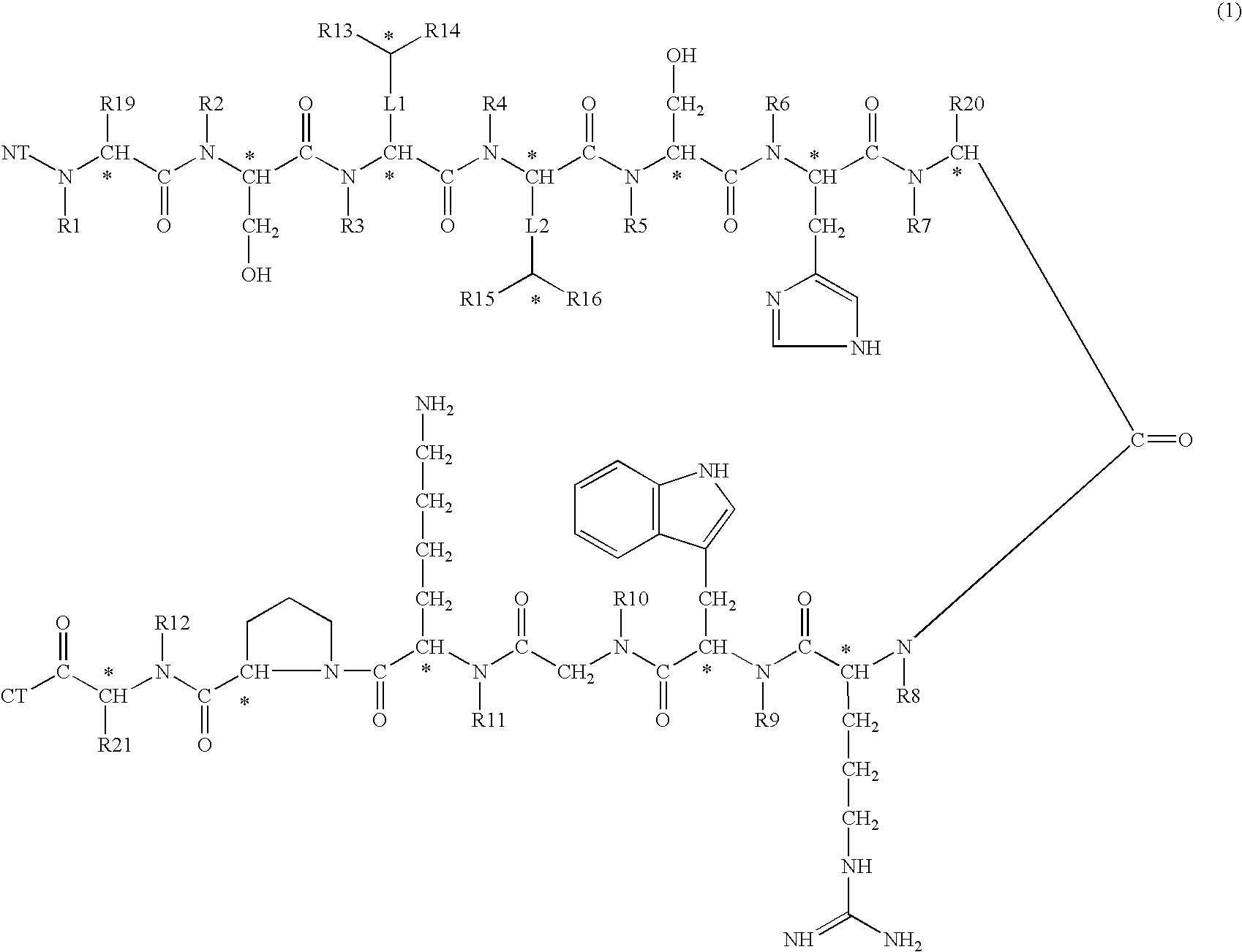
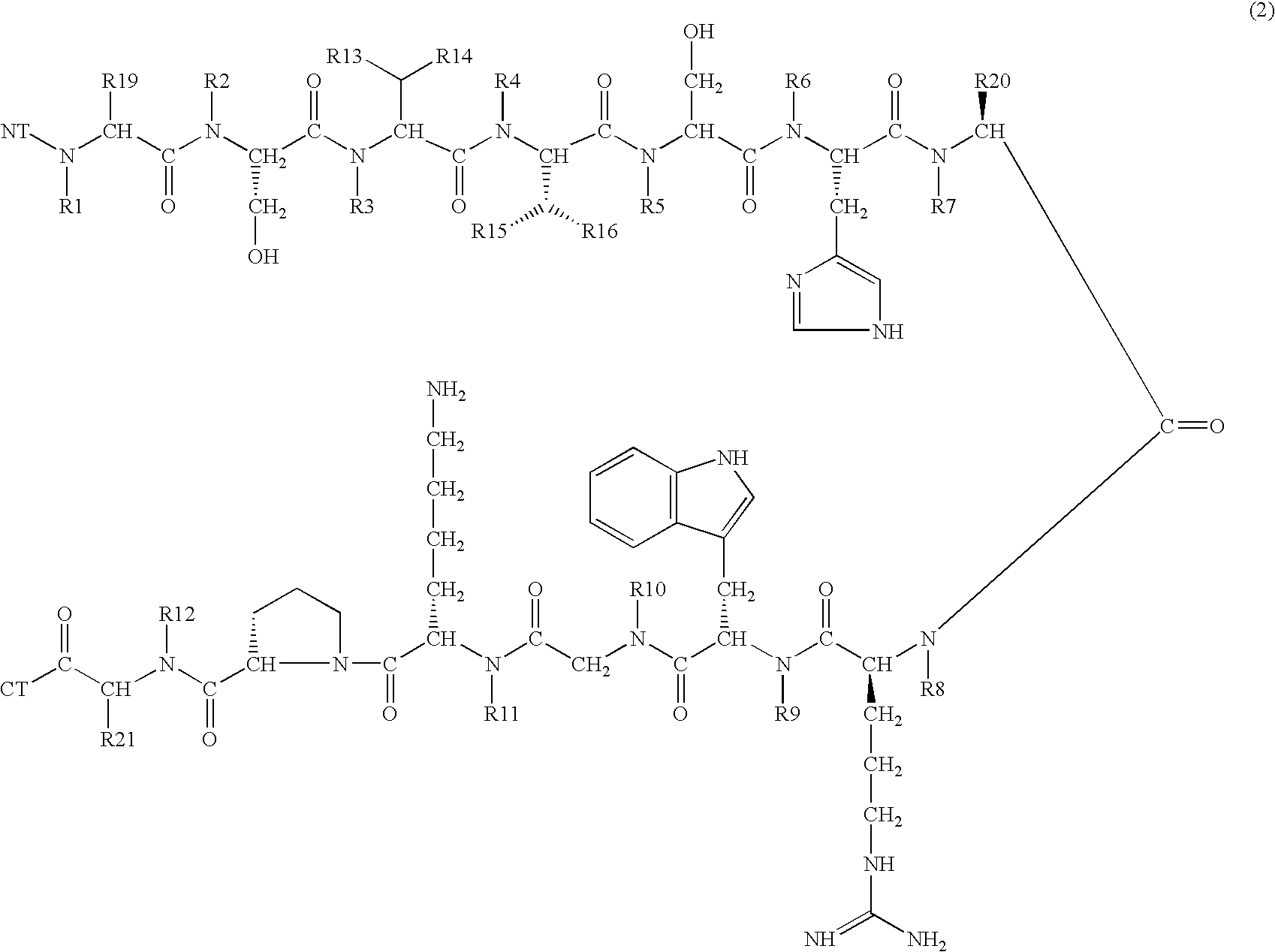
A compound of general formula (1) wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11 and R12 are H or methyl, R13, R14, R15 and R16 are H or alkyl, wherein L1 and L2 are linkers selected from single bond, methyl, ethyl, wherein R19, R20 and R21 are H or —CH2X, NT is selected from H, hydroxyl, alkyl, aminoacid, aminoacid analogue, polypeptide and functional group, CT is selected from hydrogen, hydroxyl, alkyl, aminoacid, aminoacid analogue, polypeptide and functional group shows high selectivity and high affinity for MC1-receptors in combination with effective stimulation or inhibiton of cAMP formation in MC1-receptor expressing cells but low affinity for other subtypes of MC-receptors and may be used to treat a wide range of inflammatory conditions. Also disclosed is a DNA molecule and a corresponding vector encoding the compound, a fusion protein comprising a copy of it, a vector comprising DNA encoding the fusion protein, and a pharmaceutical composition comprising the compound.
Owner:ACTION PHARM AS
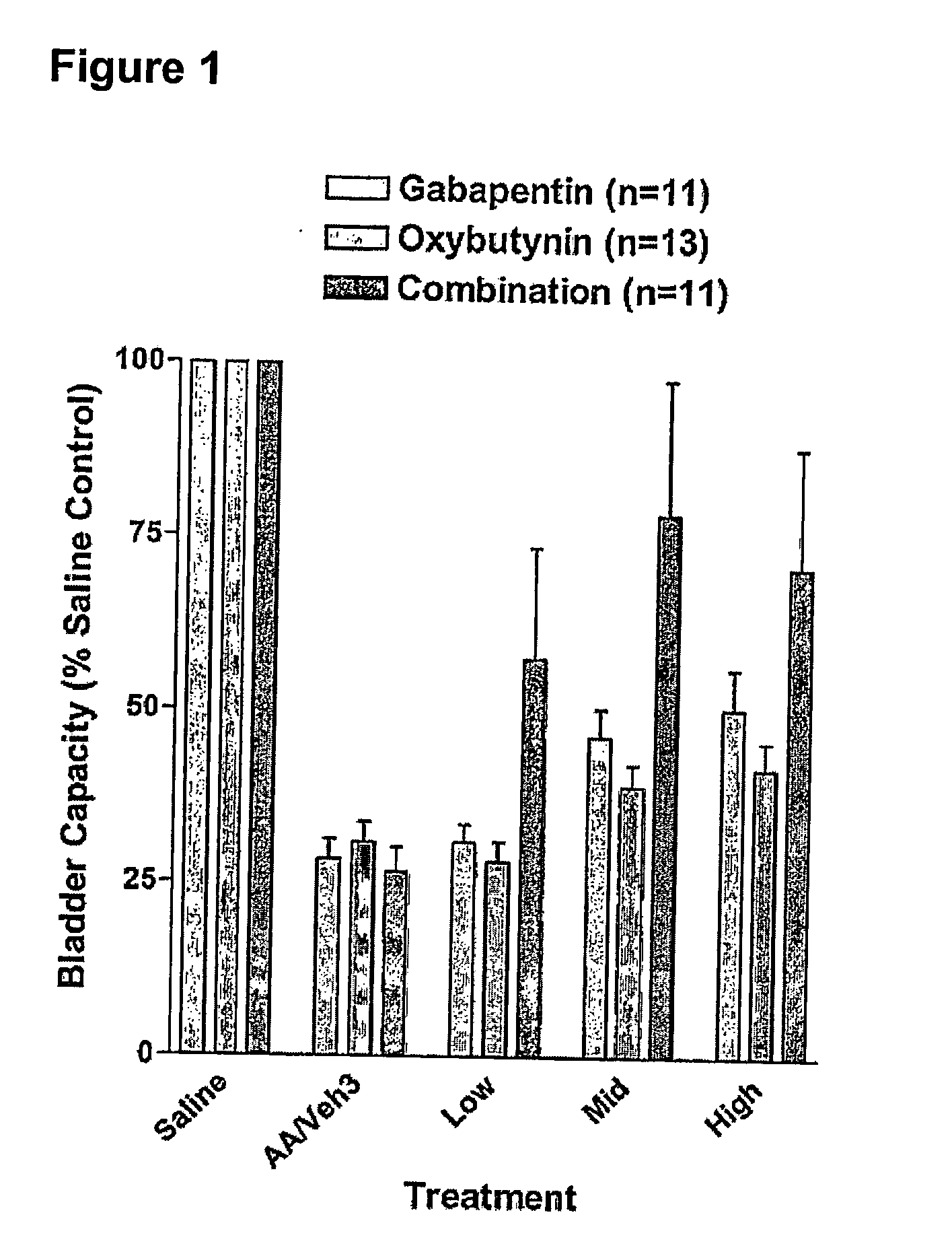
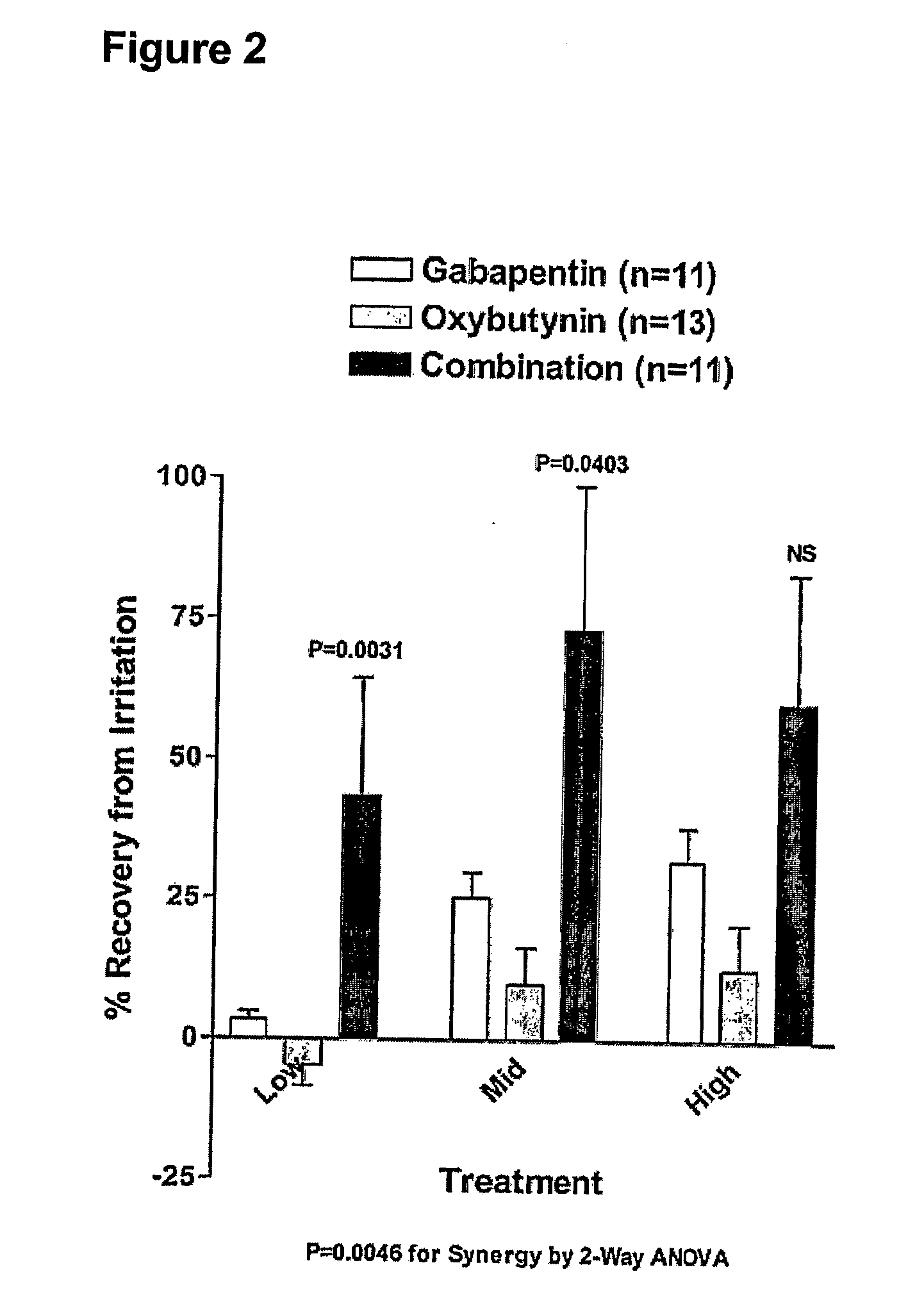
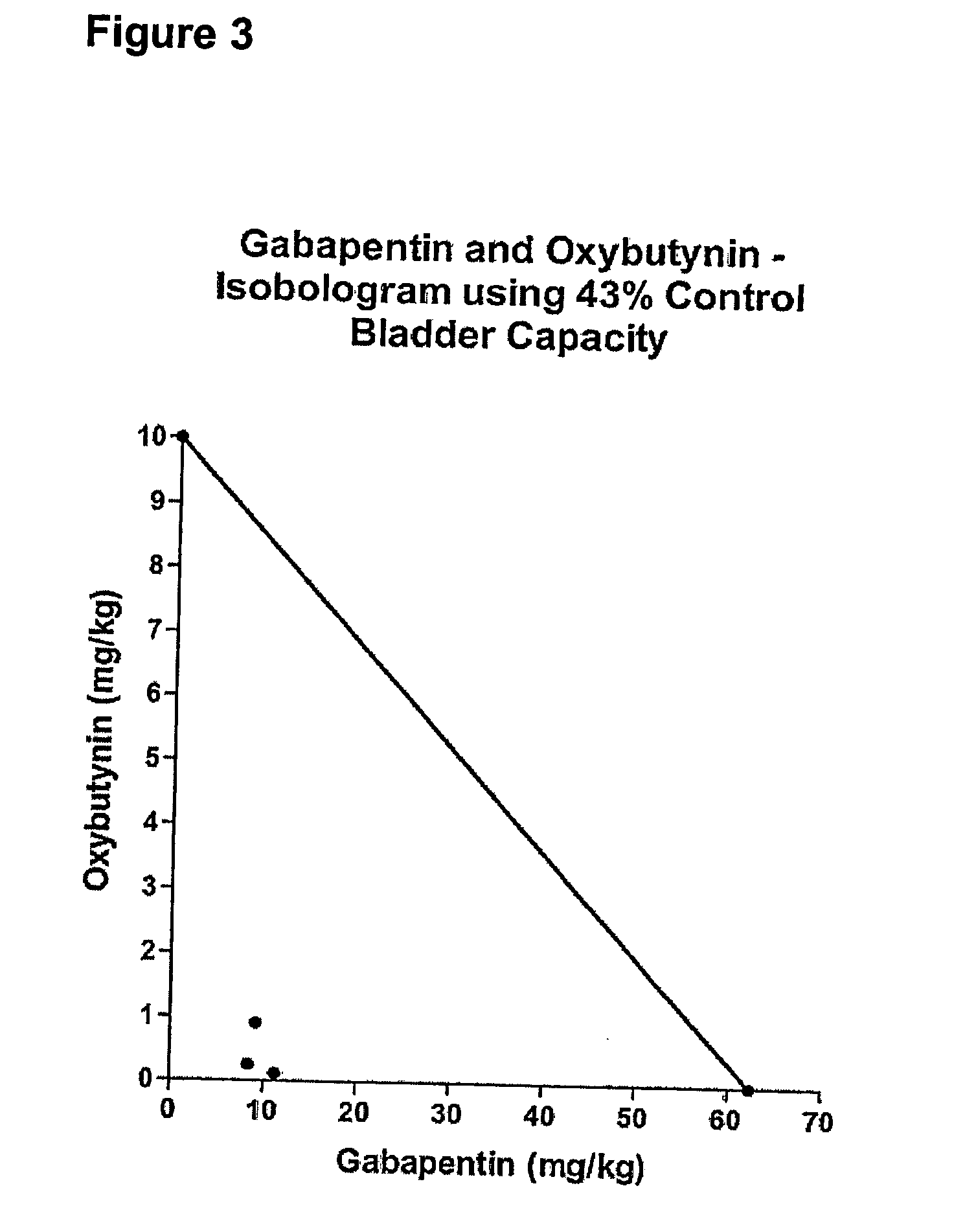
Methods for treating pain using smooth muscle modulators and a2 subunit calcium channel modulators
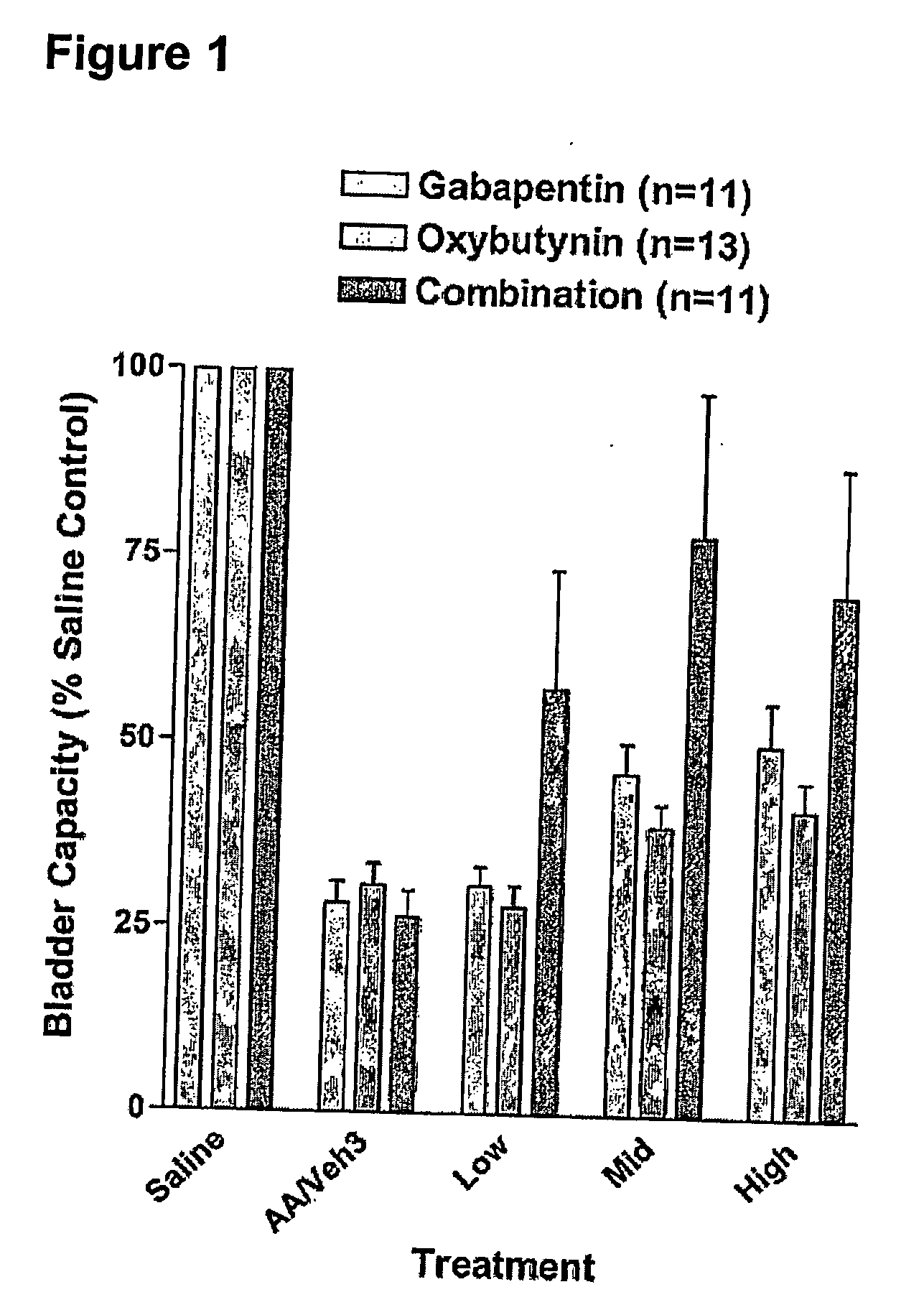
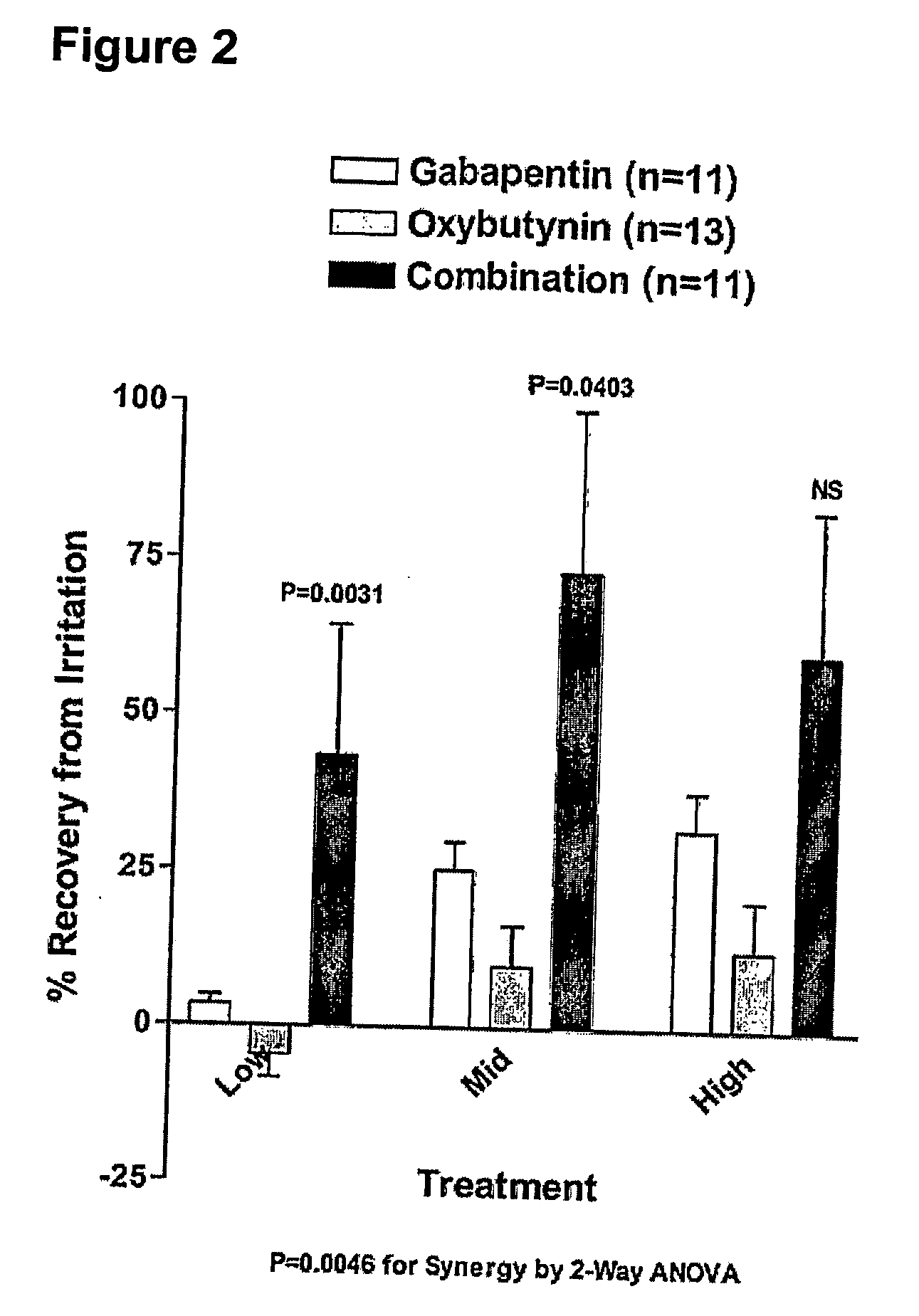
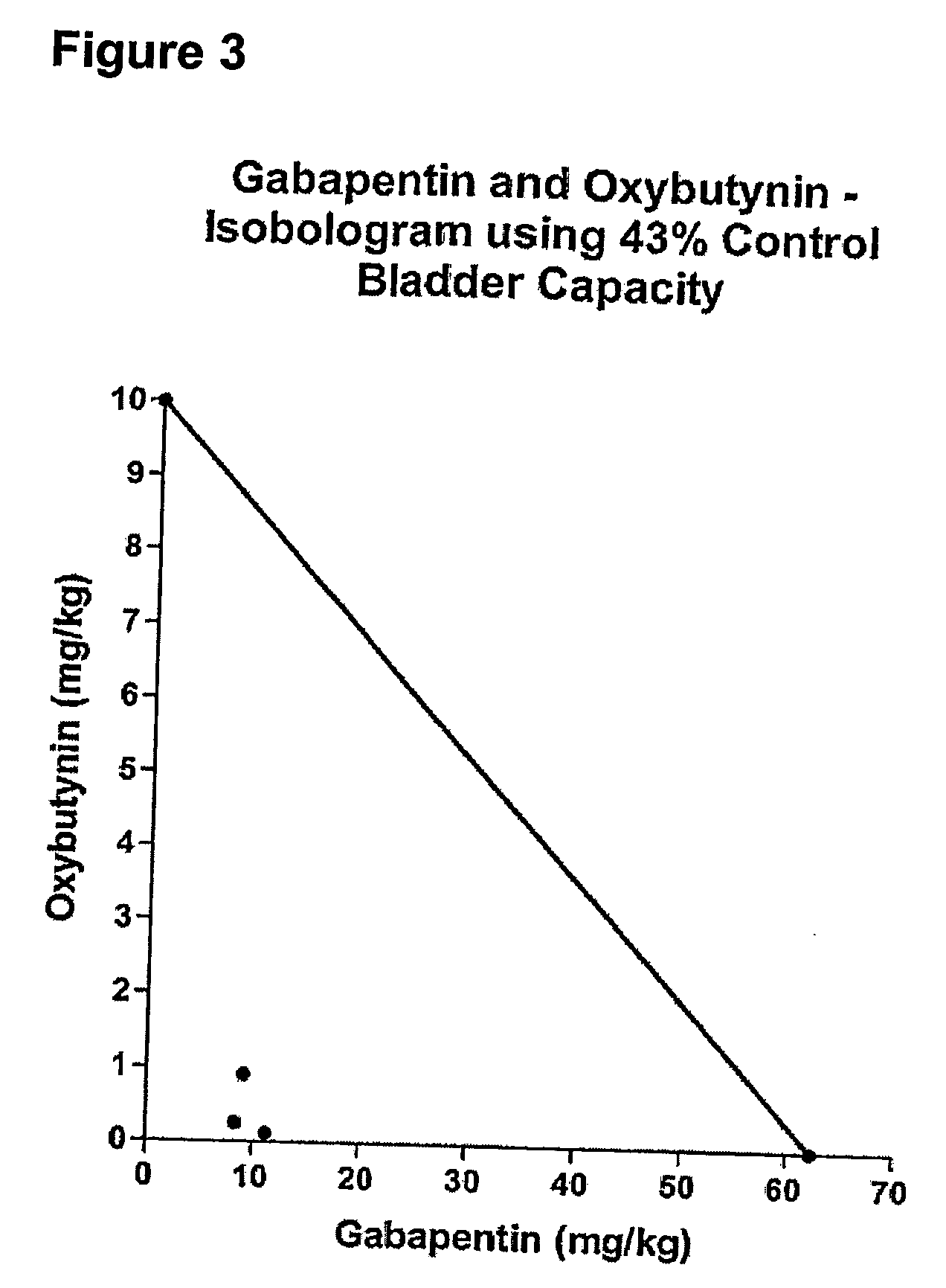
InactiveUS20060264509A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsGabapentinAdrenergic receptor agonists
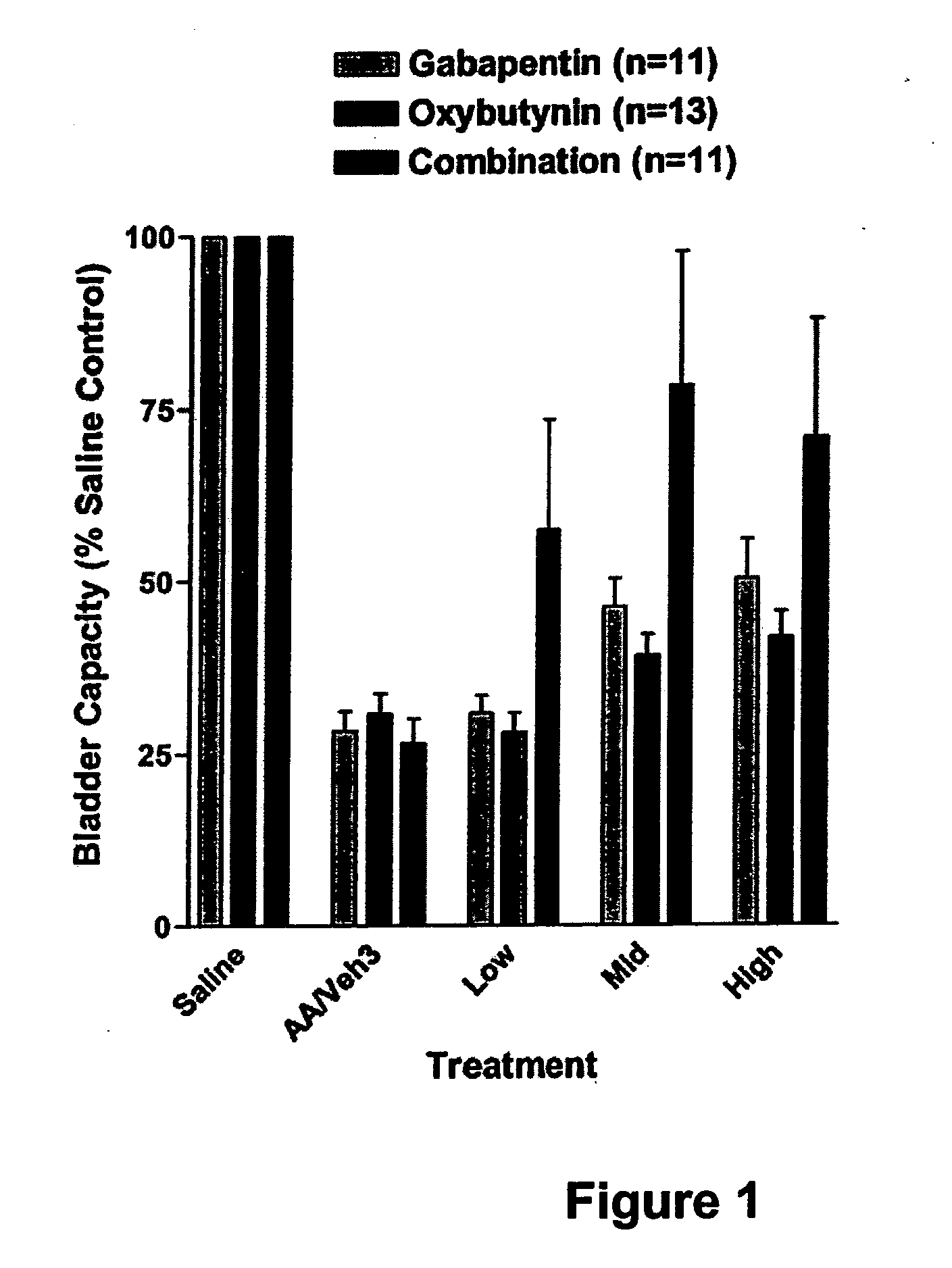
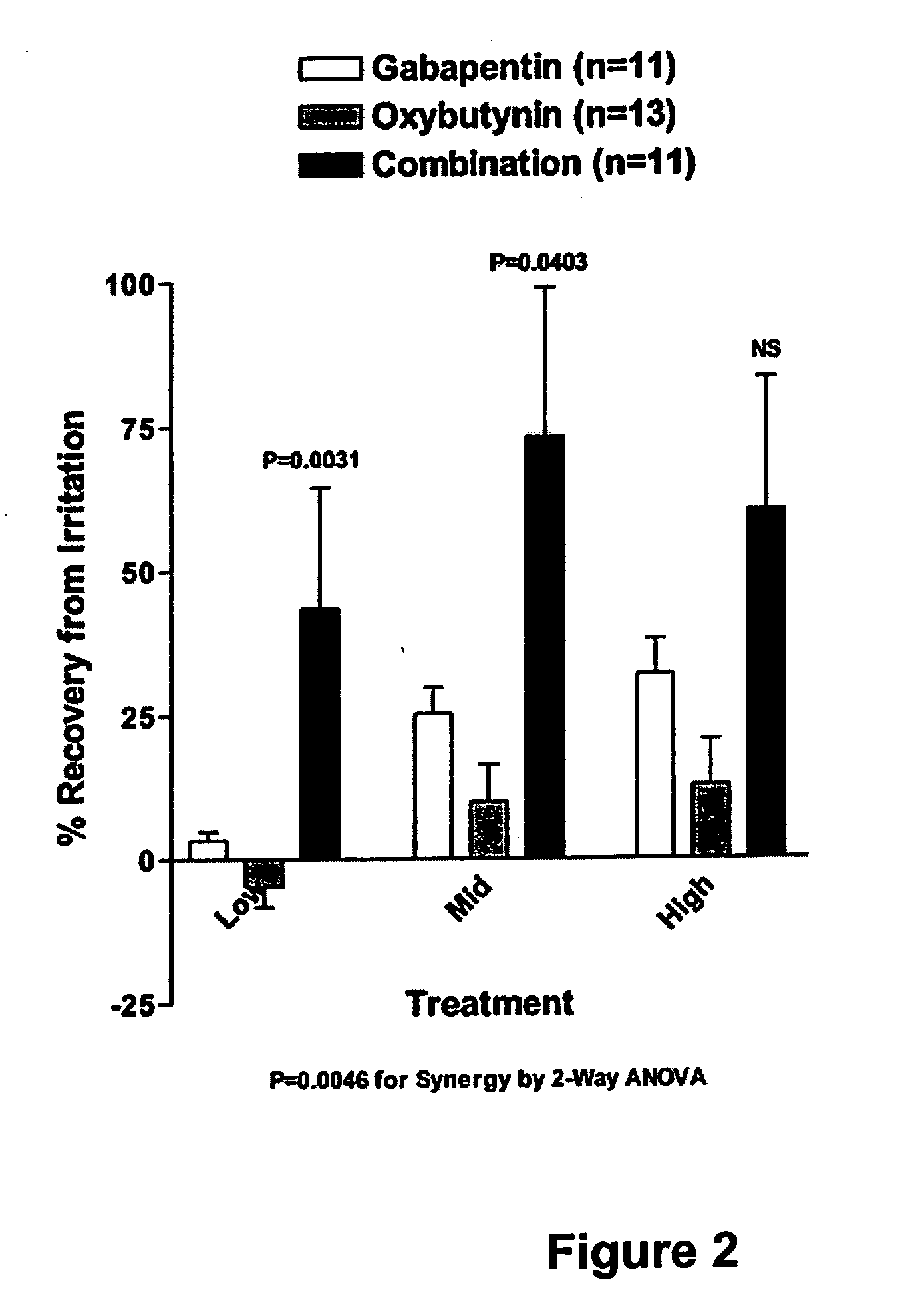
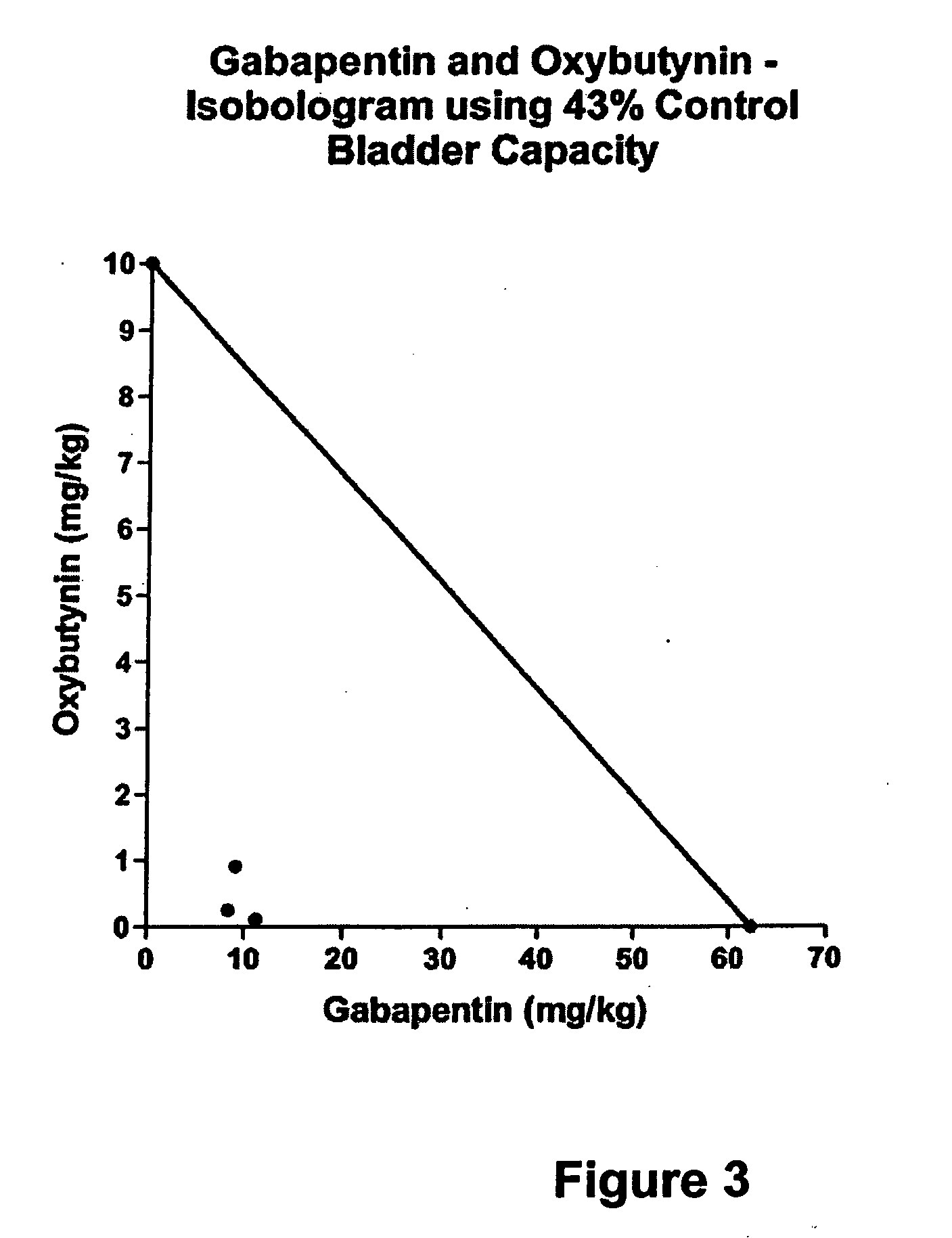
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat pain. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g., gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
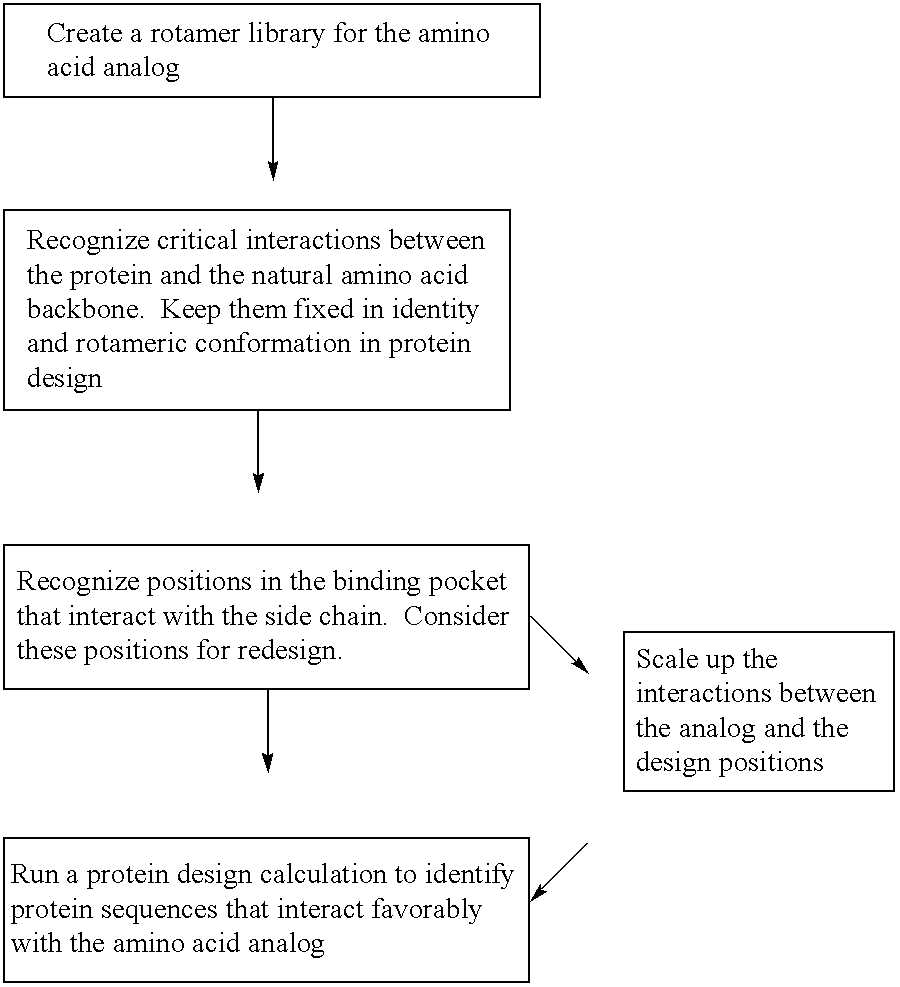
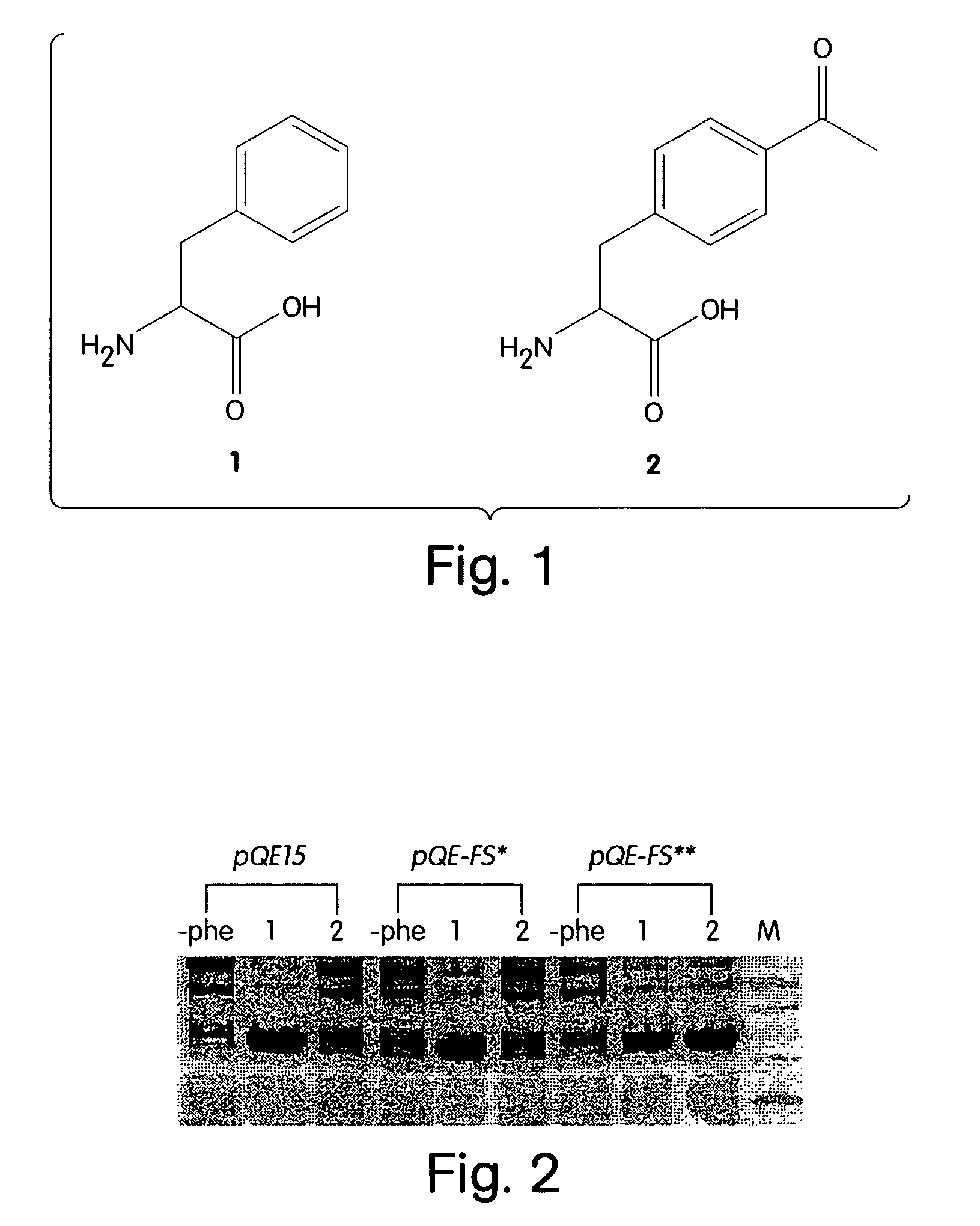
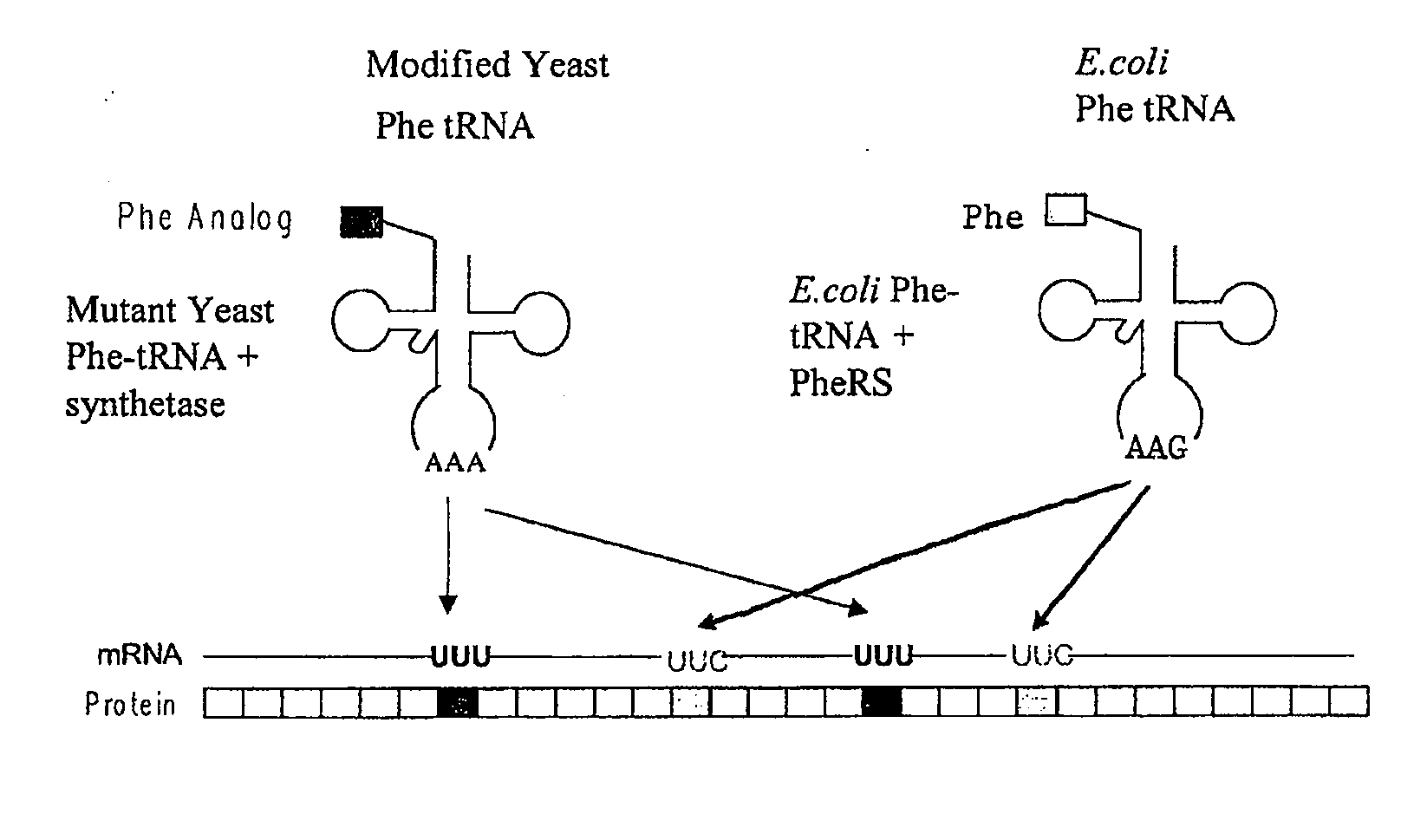
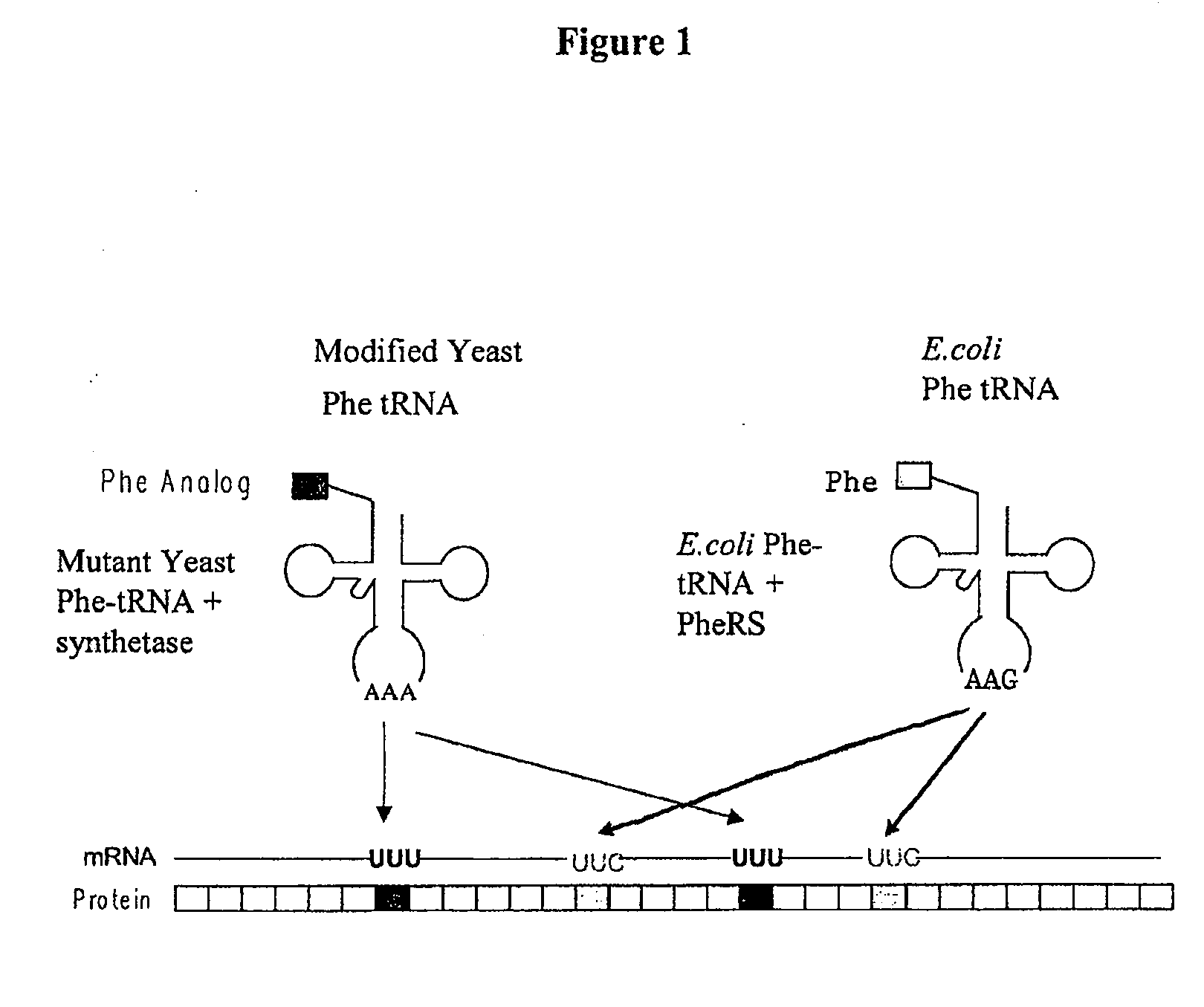
Computational method for designing enzymes for incorporation of non natural amino acids into proteins
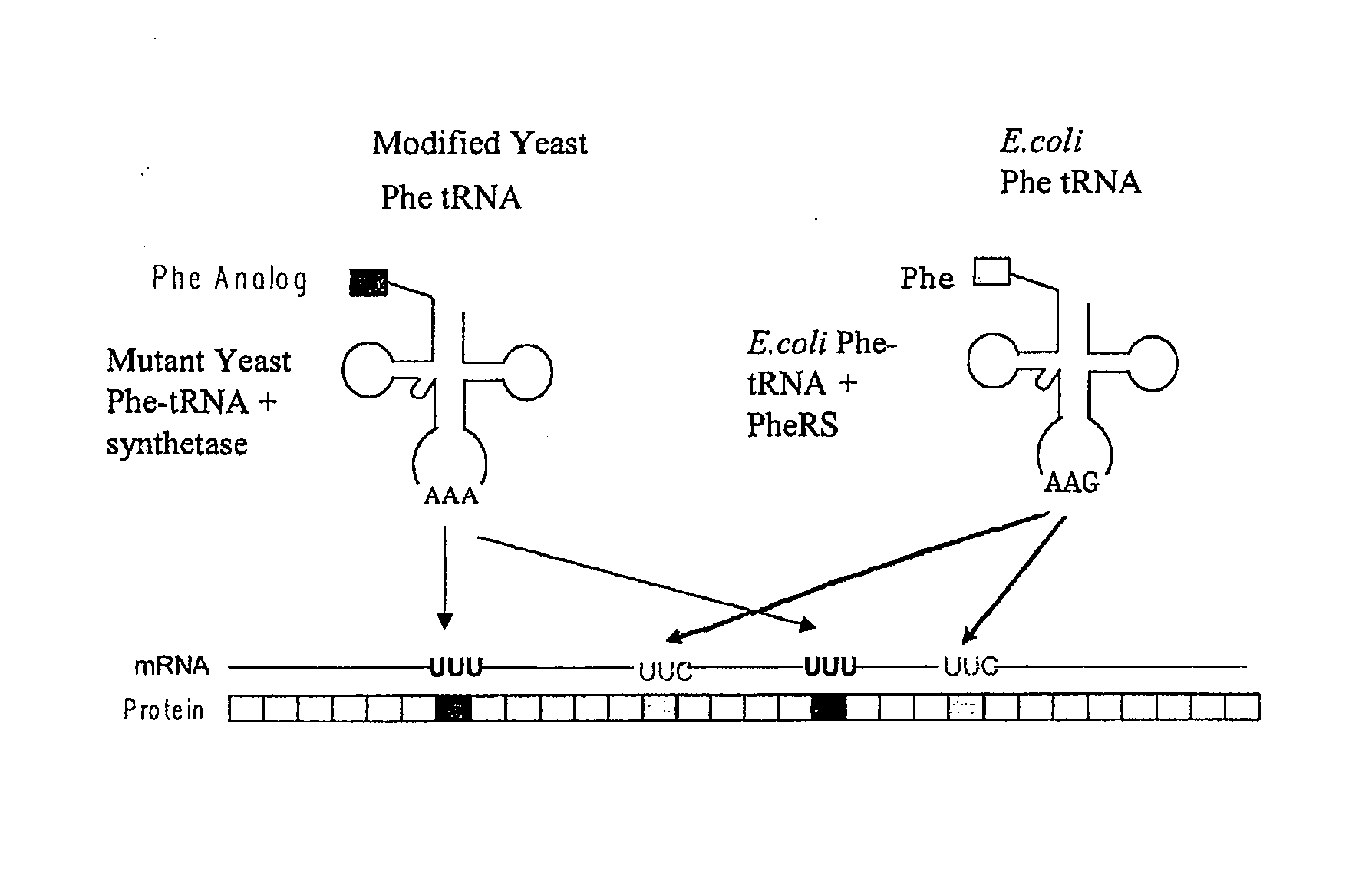
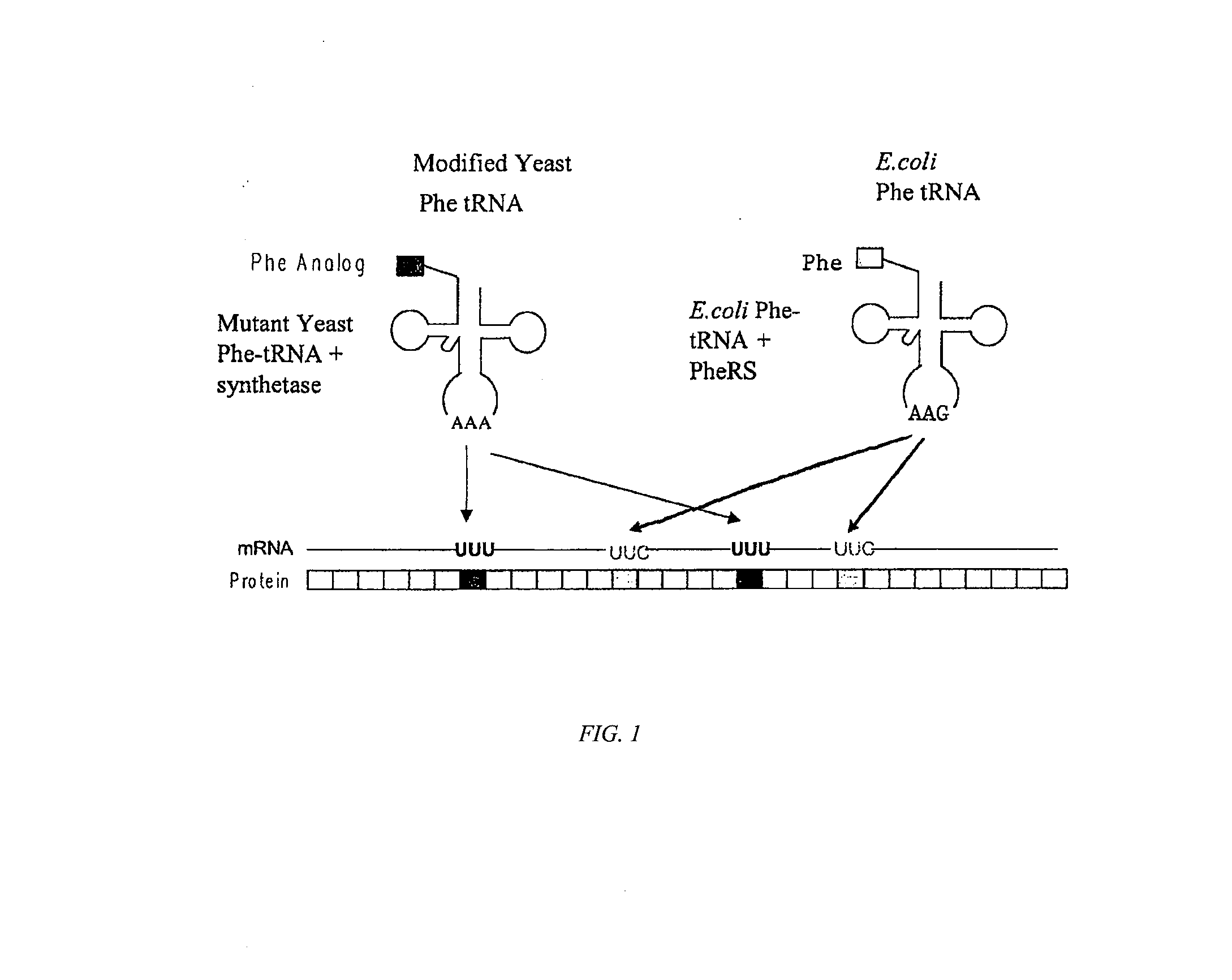
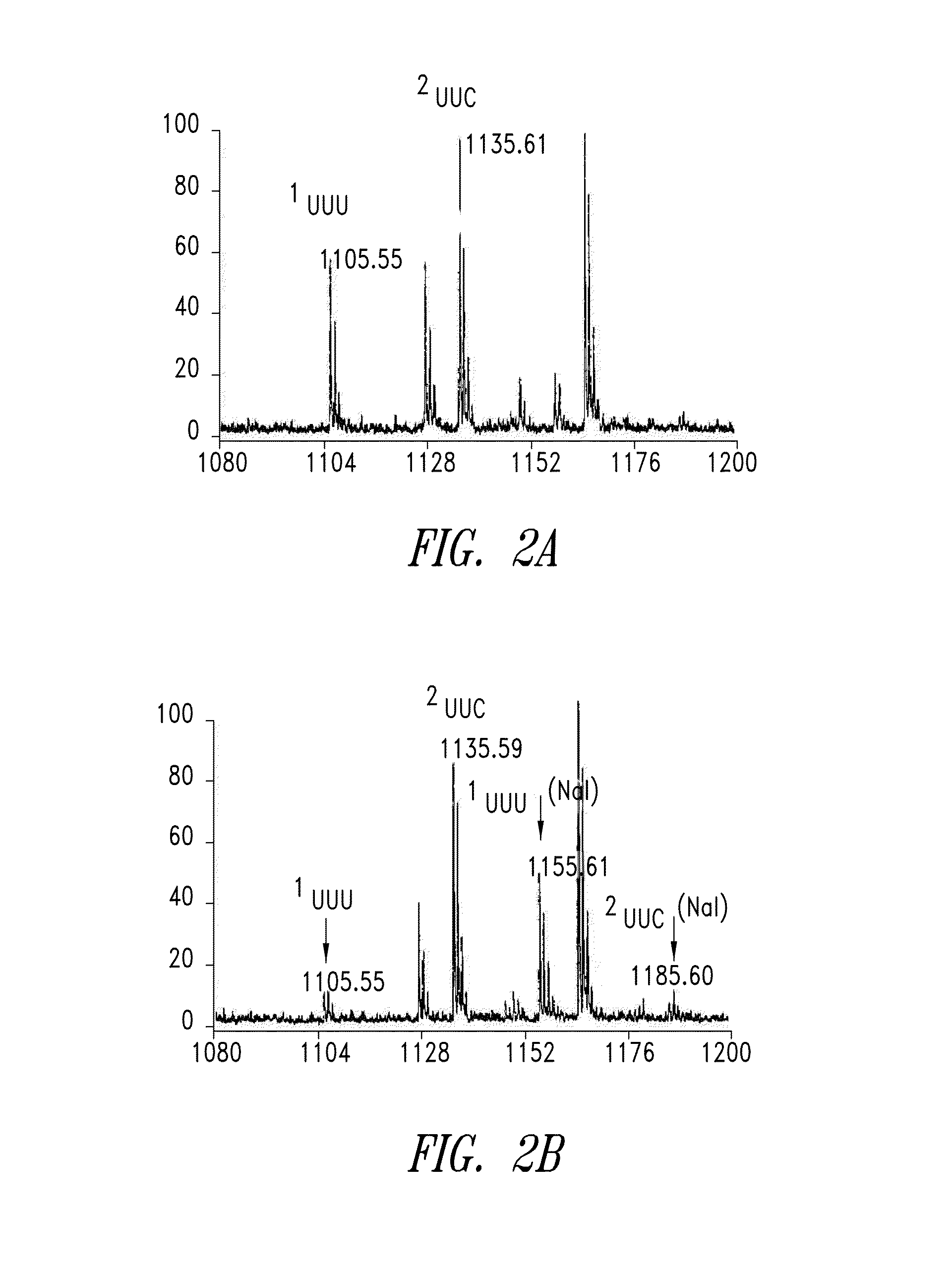
The instant invention provides methods, reagents, and computational tools for designing non-natural substrate analogs for enzymes, especially for designing unnatural amino acid analogs for aminoacyl tRNA Synthetases (AARSs), such as the Phe tRNA Synthetase. The instant invention also provides methods to incorporate unnatural amino acid analogs, especially those with interesting functional groups, into protein products to generate proteins of modified or novel functions.
Owner:CALIFORNIA INST OF TECH
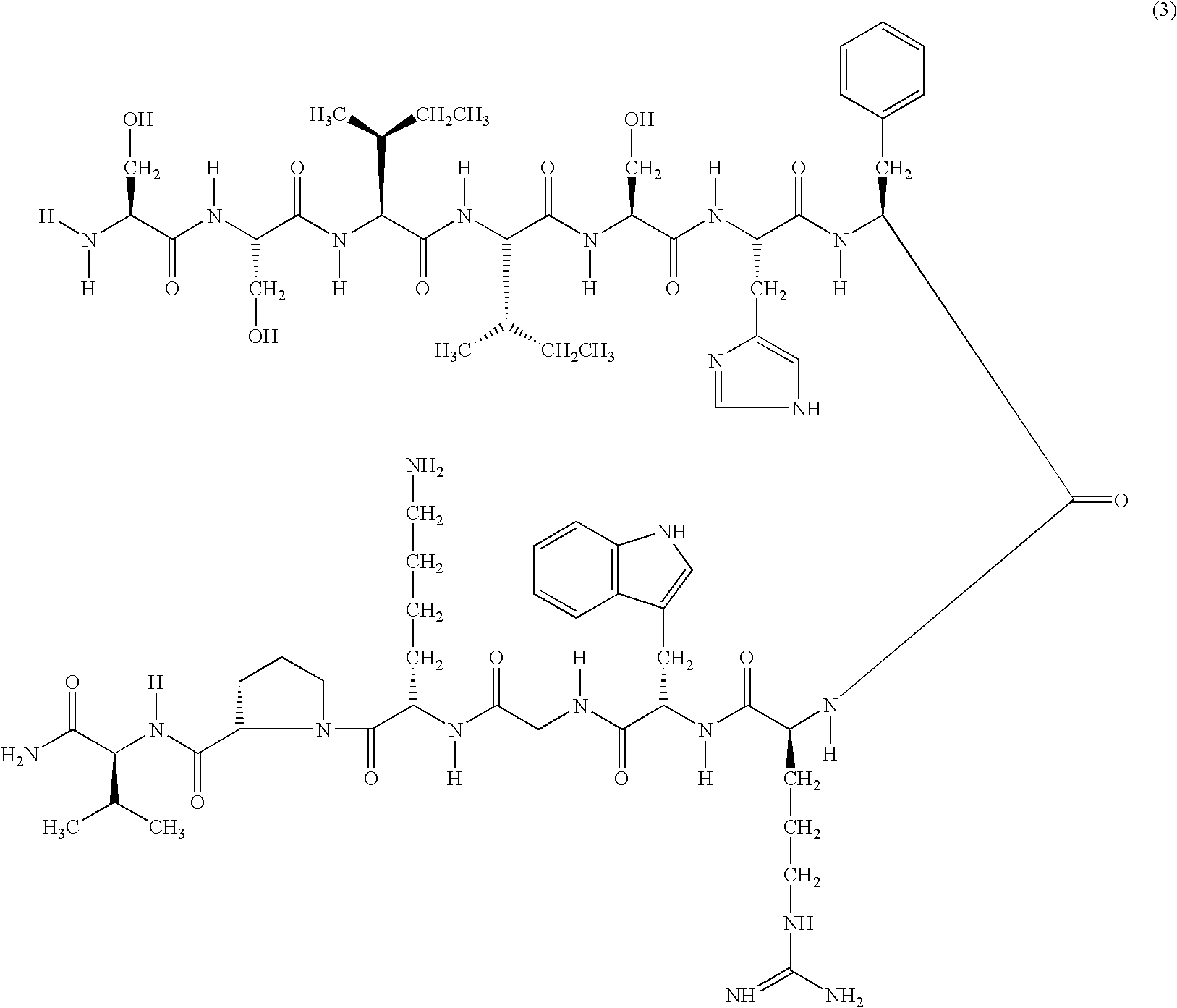
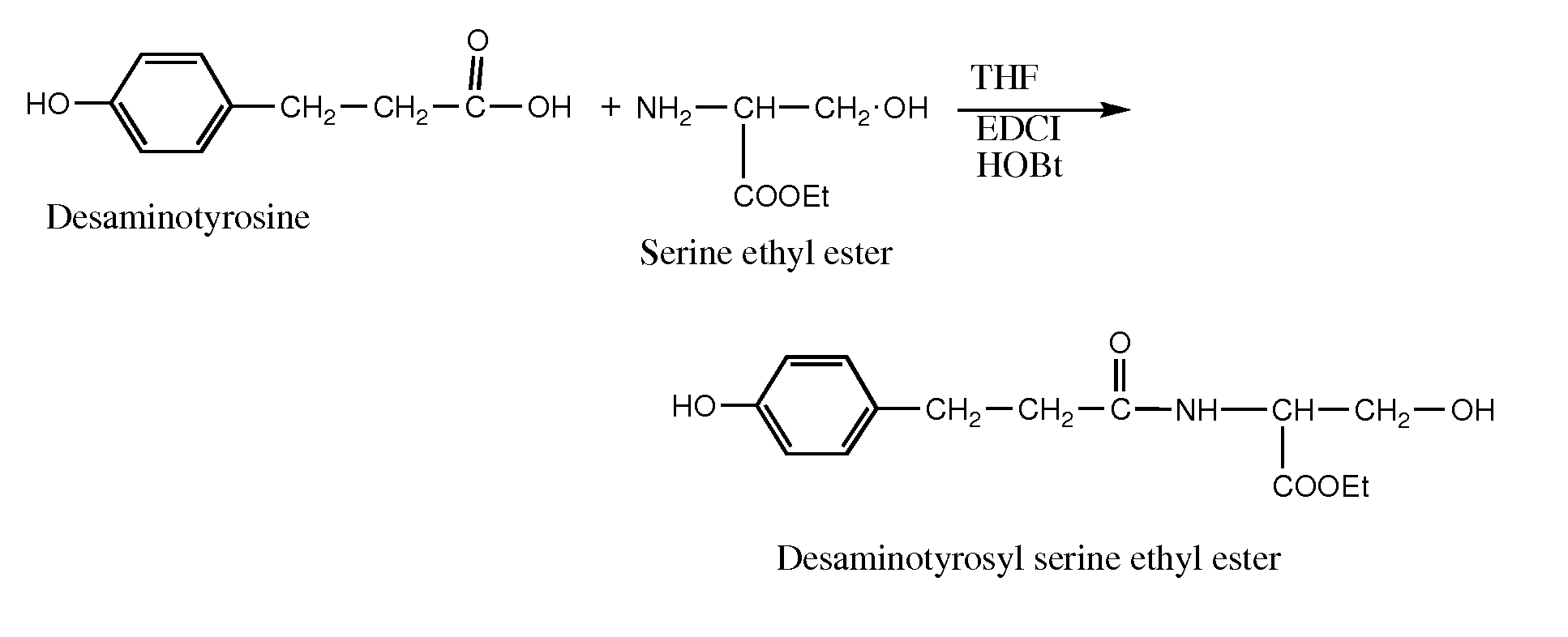
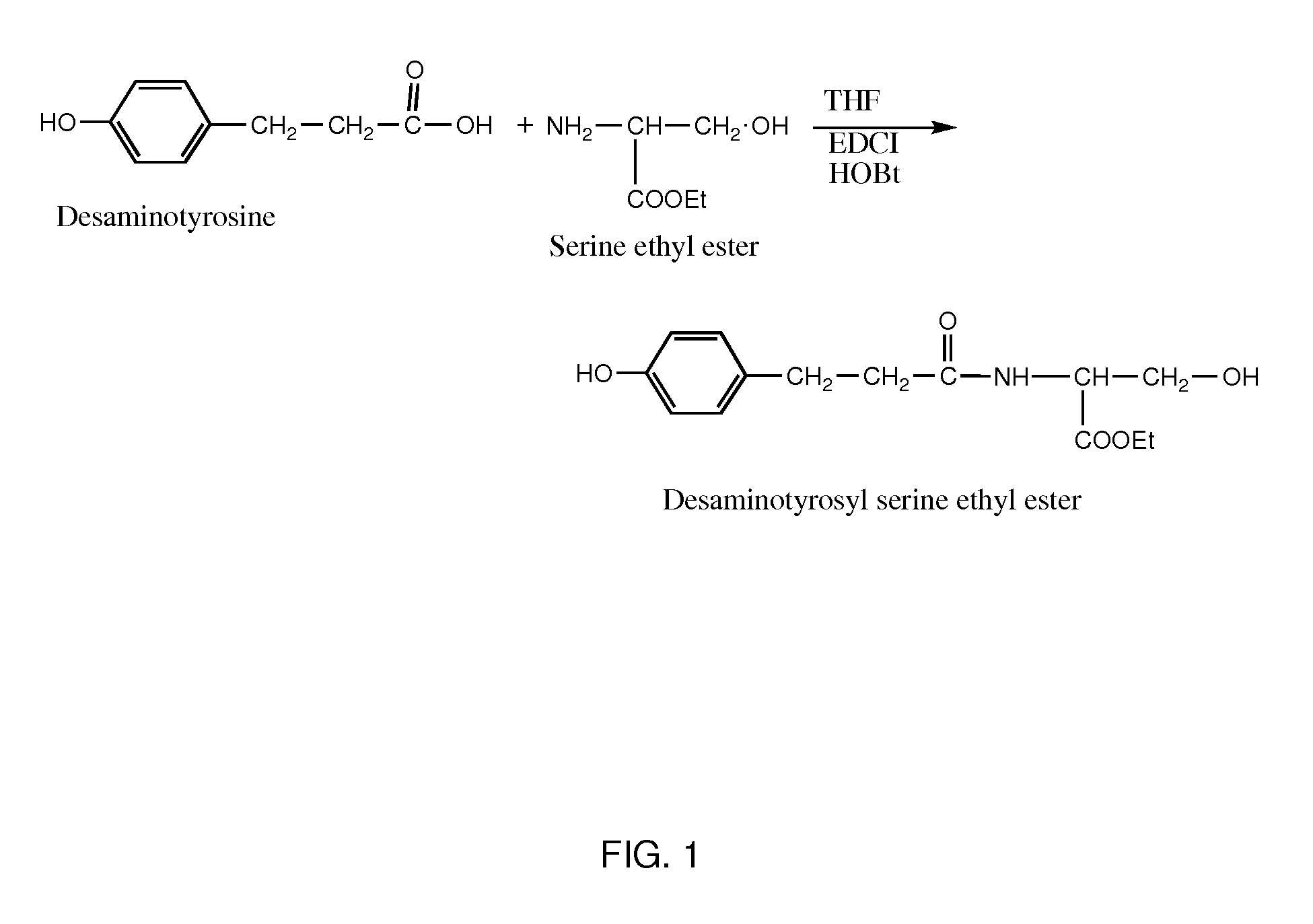
Conjugates of water soluble polymer-amino acid oligopeptide-drug, preparation method and use thereof
A conjugate of water soluble polymer-amino acid oligopeptide-drug of Formula (I) below and a pharmaceutical composition comprising the conjugate are provided. In the conjugate, P is a water soluble polymer; X is a linking group, wherein the linking group links P and A1; each of A1, A2 and A3 is independently same or different amino acid residue or amino acid analogue residue; each of D1 and D2 is independently same or different drug molecule residue; a is 0 or 1; b is an integer of 2-12; c is an integer of 0-7; d is 0 or 1. The conjugate could improve drug load capacity, water solubility, stability and activity of the drug.
Owner:JENKEM TECH CO LTD TIANJIN
Methods for decreasing detrusor muscle overactivity
InactiveUS20050239890A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsDiseaseGabapentin
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs, e.g., gabapentin and pregabalin, fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS20040058415A1High yieldRapid and predictable approachFungiBacteriaDihydrofolic acidAminoacid analog
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
Transgenic high tryptophan plants
The present invention provides resistance to amino acid analogues of tryptophan and / or modification of tryptophan content in plants by introducing and expressing an isolated DNA fragment encoding anthranilate synthase in plant cells Methods. Also provided are transgenic plants transformed with the isolated DNA fragment encoding an anthranilate synthase, as well as human or animal food, seeds and progeny derived from these plants.
Owner:RENESSEN +1
Materials and methods for controlling pests
InactiveUS20030140371A1Low toxicitySustainable, pesticide-free food supplyBiocideOrganic active ingredientsSolute transportersProviding material
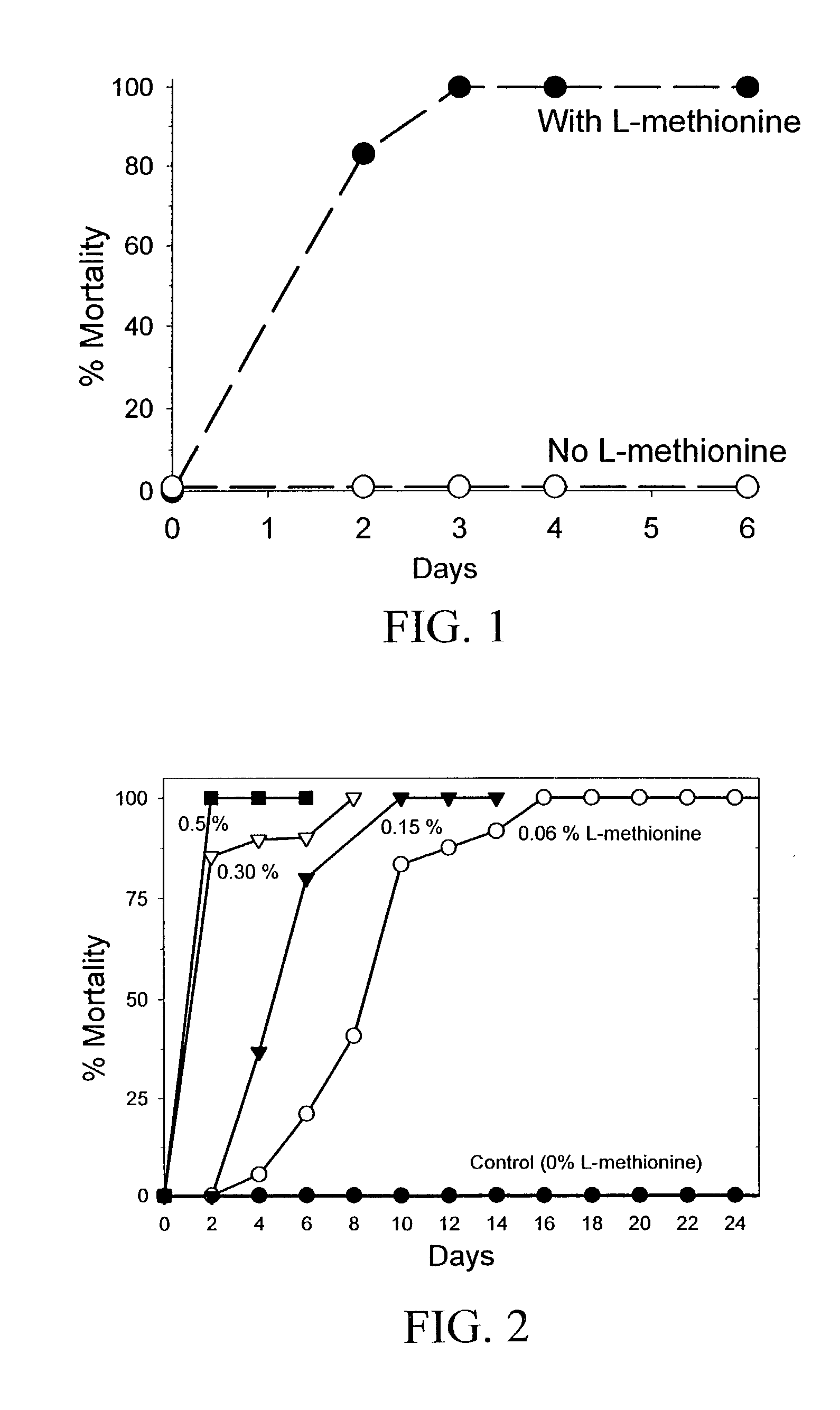
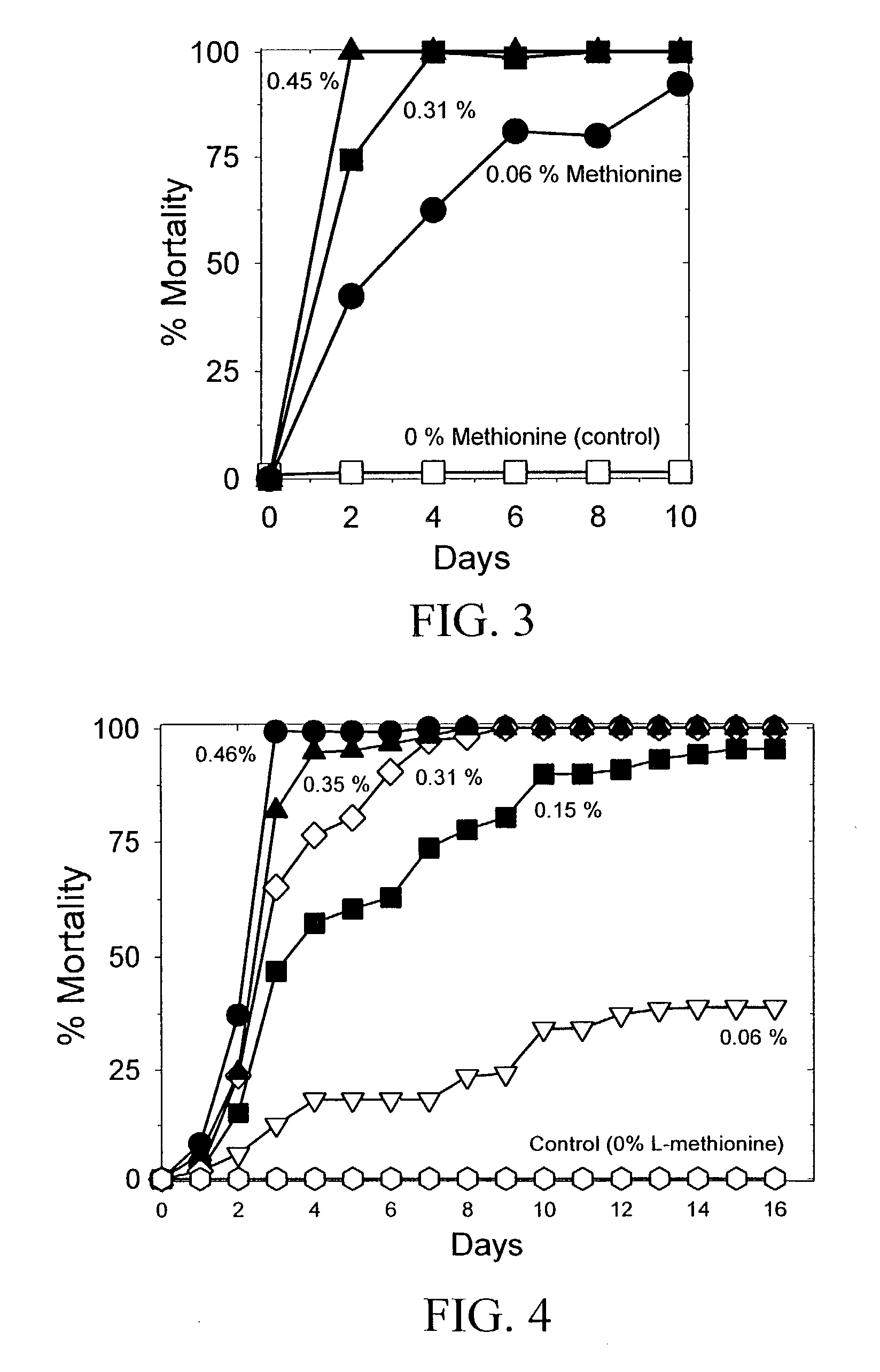
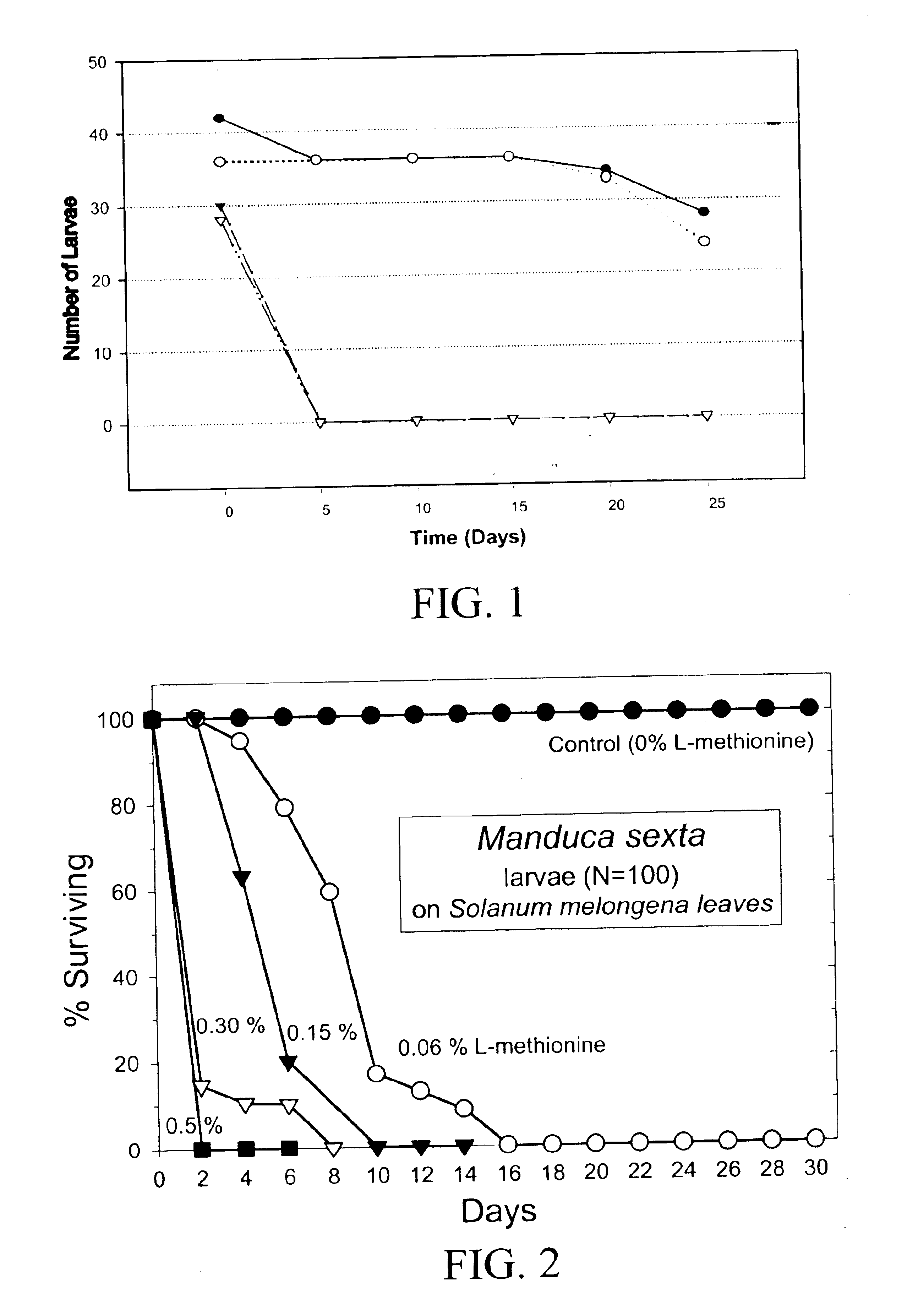
The present invention provides materials and methods for pest control. The subject invention provides pesticidal compositions that contain one or more compounds that interact with organic solute transporter / ligand-gated ion channel multifunction polypeptides in the pest, and / or alter amino acid metabolic pathways, and / or alter ionic homeostasis in the pest. Upon exposure to a target pest, these compositions either compromise pest growth and / or cause the death of the pest. In a preferred embodiment, the compositions of the subject invention contain one or more amino acids and / or amino acid analogs. In a particularly preferred embodiment, the methods of the subject invention involve exposing a pest to a composition that comprises methionine or leucine, or an analog thereof.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
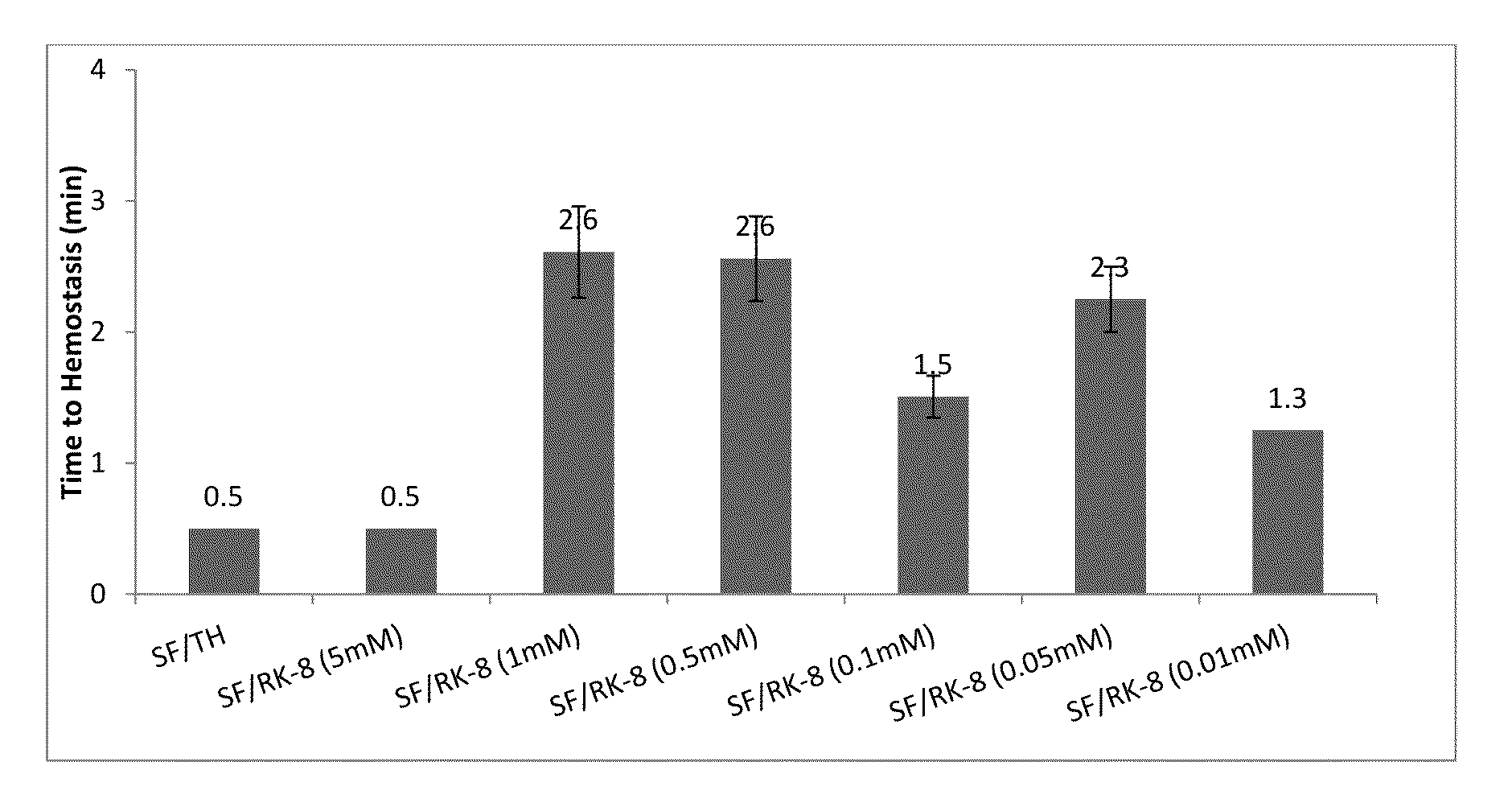
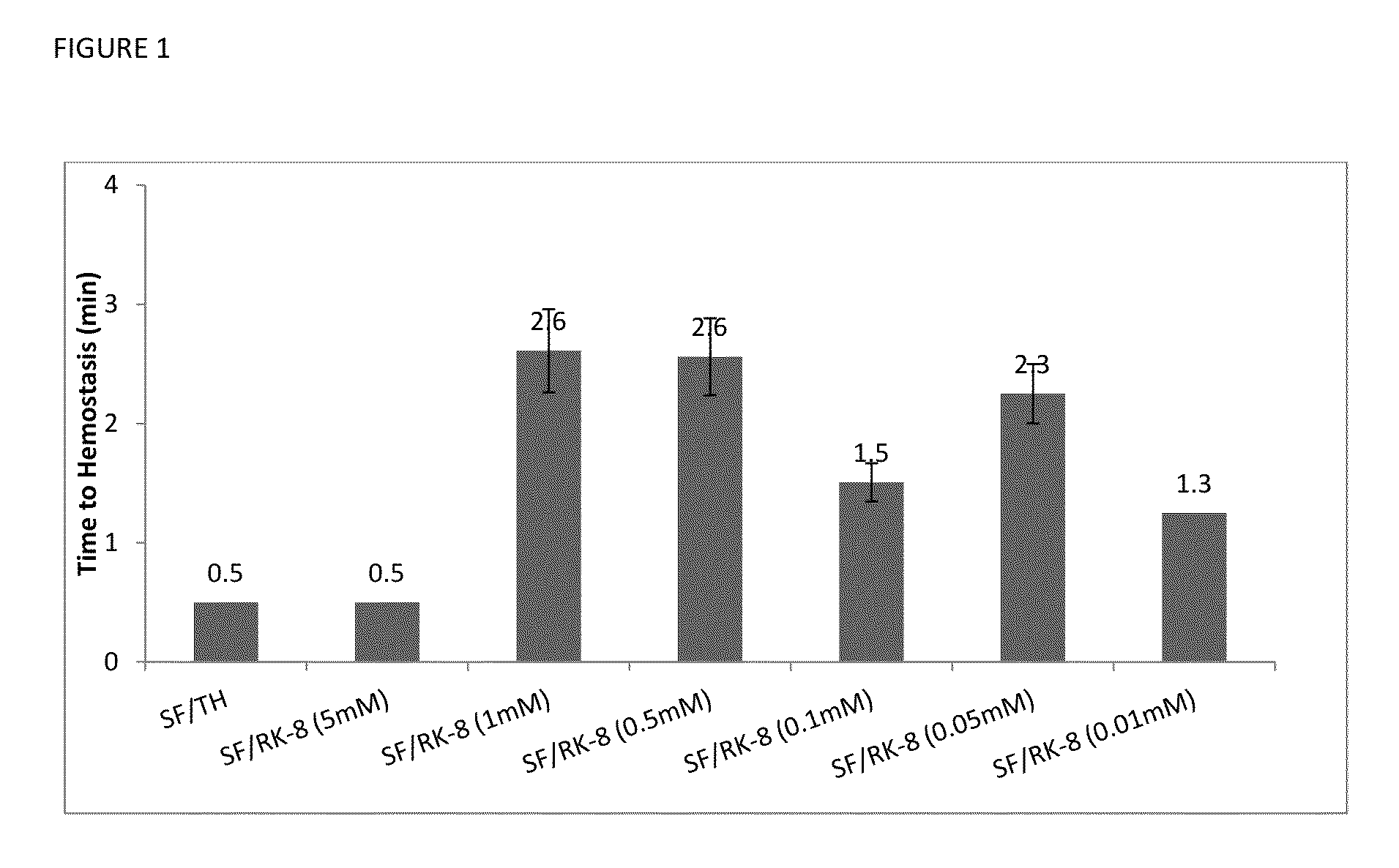
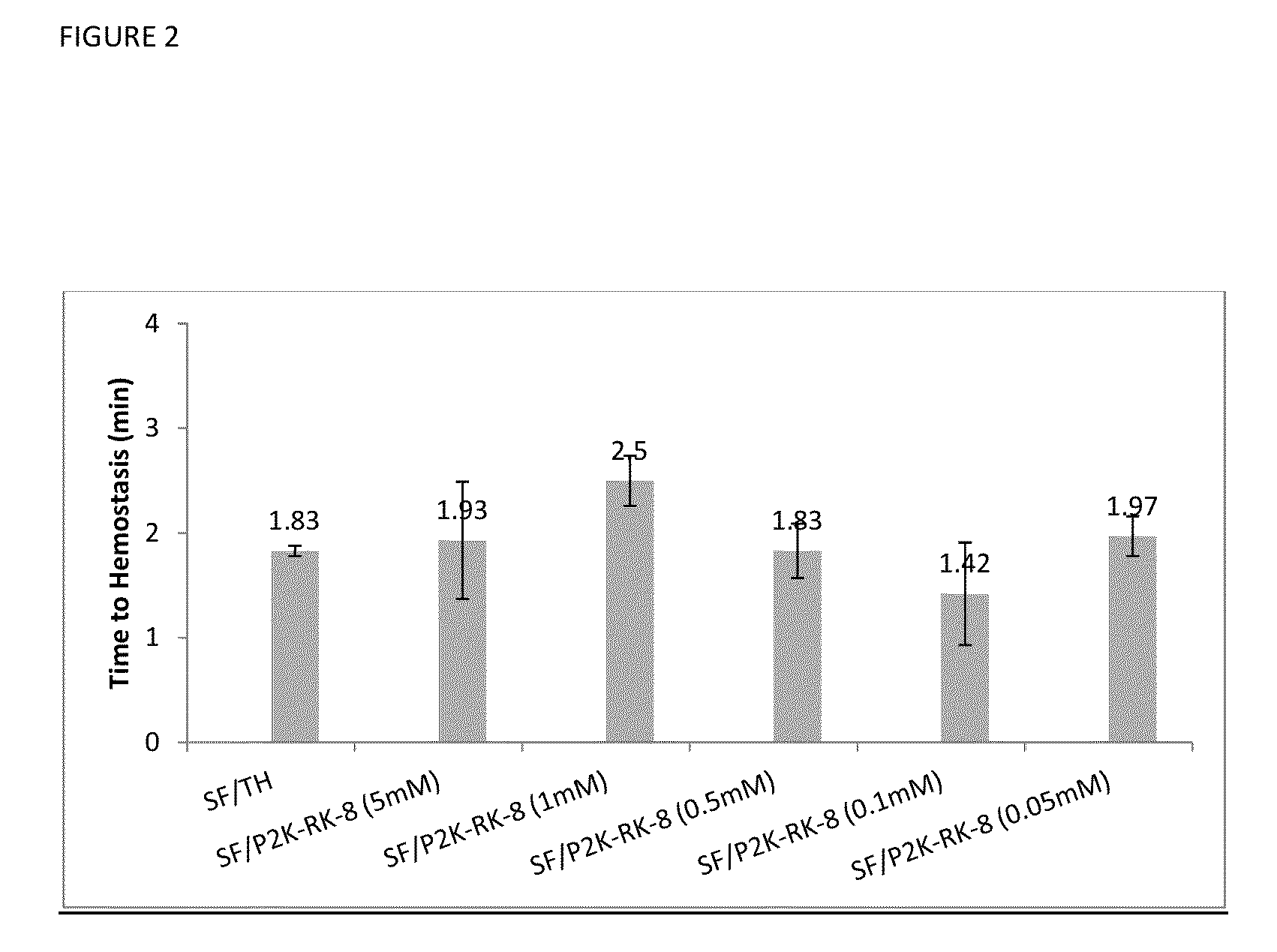
Procoagulant Peptides and Their Derivatives and Uses Therefor
ActiveUS20130004478A1Improve solubilityCost effectiveFibrinogenSurgical adhesivesAbsorbable polymersGenetically engineered
The present invention is directed to a hemostatic or tissue sealing material having (a) a peptide having a sequence SEQ ID NO: 1 or an amino acid analog sequence thereof, and (b) a scaffold for said peptide or amino acid analogue sequence. The scaffold is preferably hemostatic, such as a natural or genetically engineered absorbable polymer, a synthetic absorbable polymer, or combinations thereof. The natural or genetically engineered absorbable polymers can be selected from the group consisting of a protein, a polysaccharide, or combinations thereof.
Owner:ETHICON INC
Sulfation of wnt pathway proteins
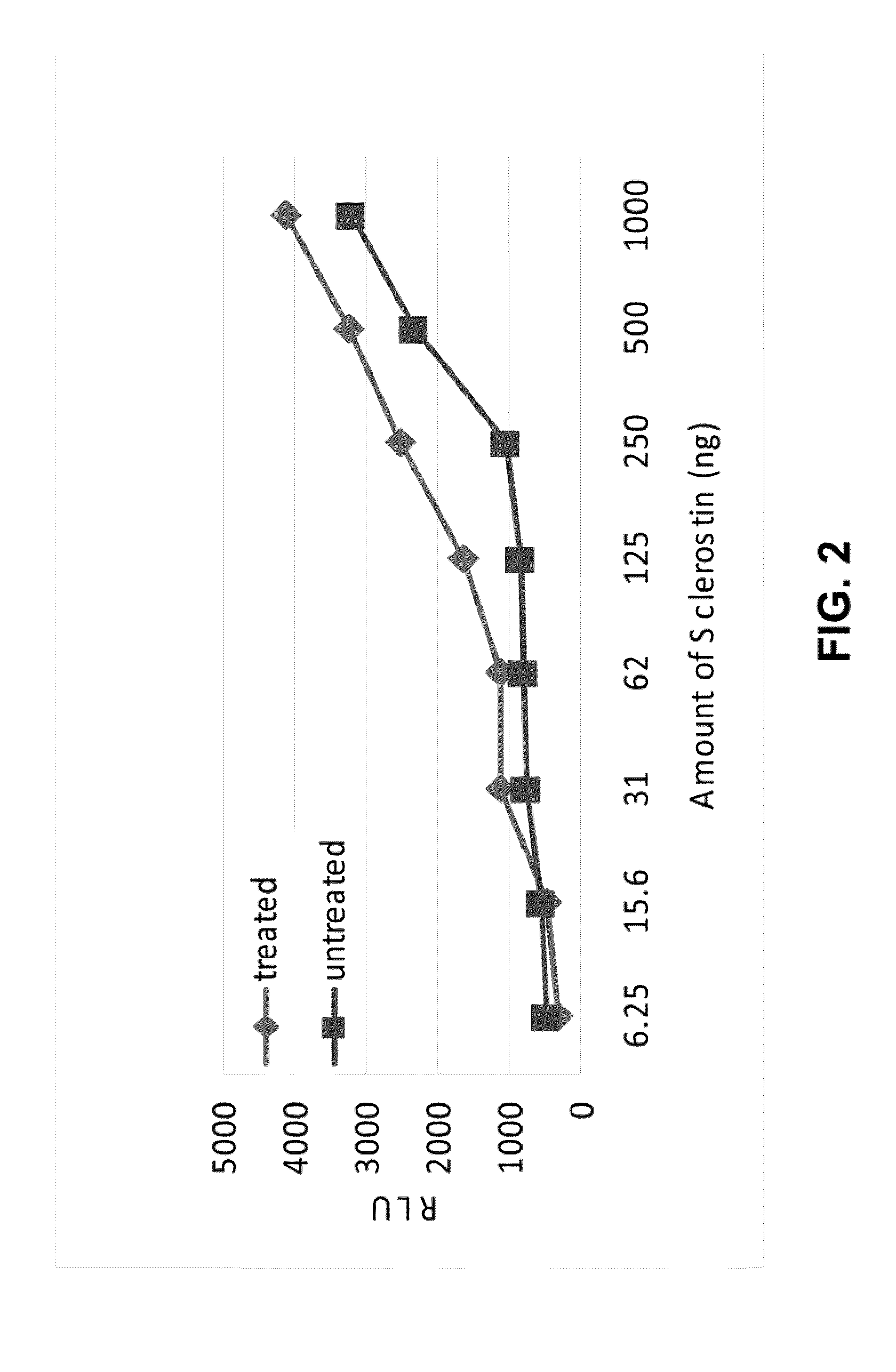
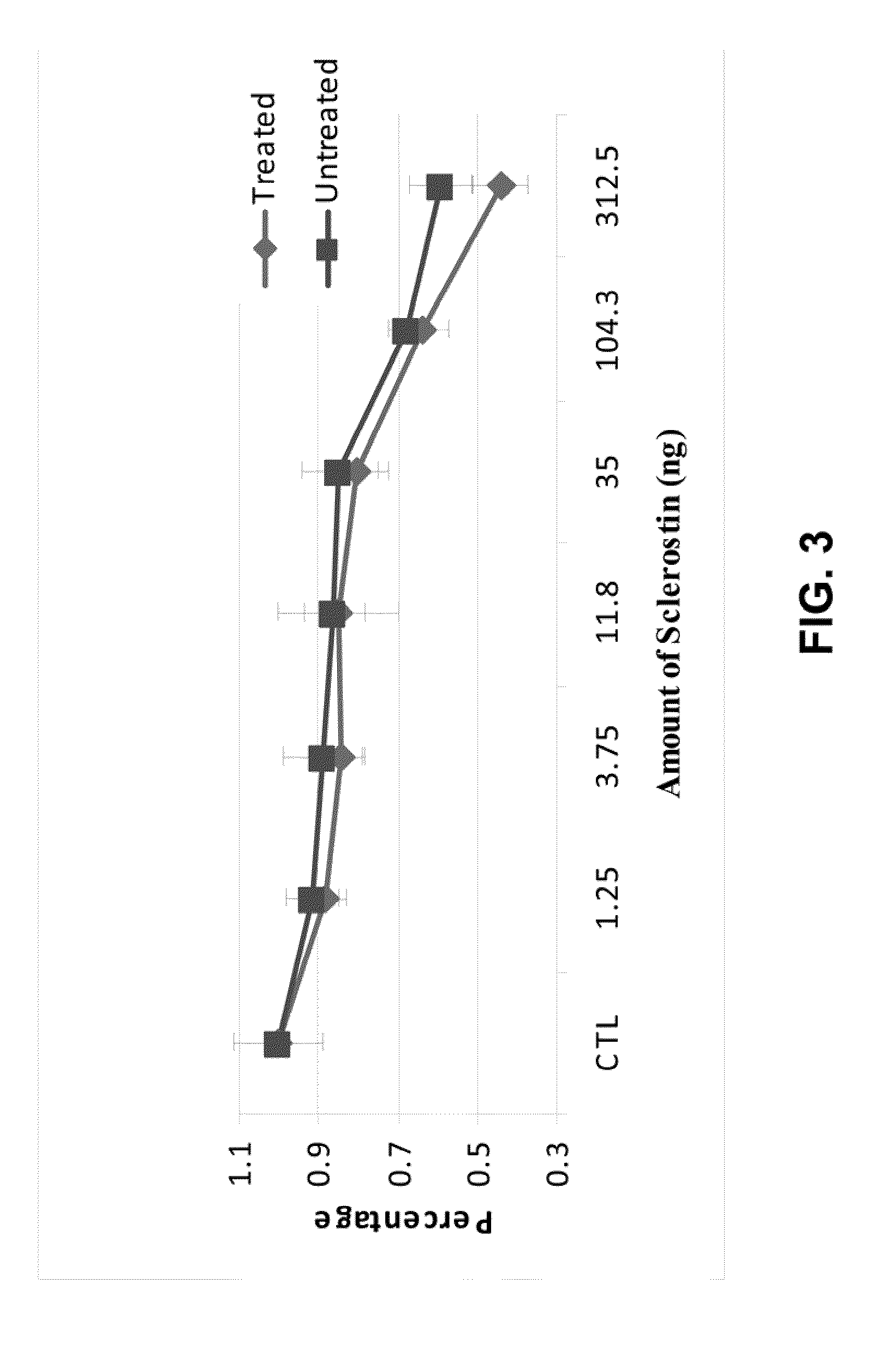
ActiveUS20120071399A1Reduce inhibitionPeptide/protein ingredientsSkeletal disorderCrystallographySulfation
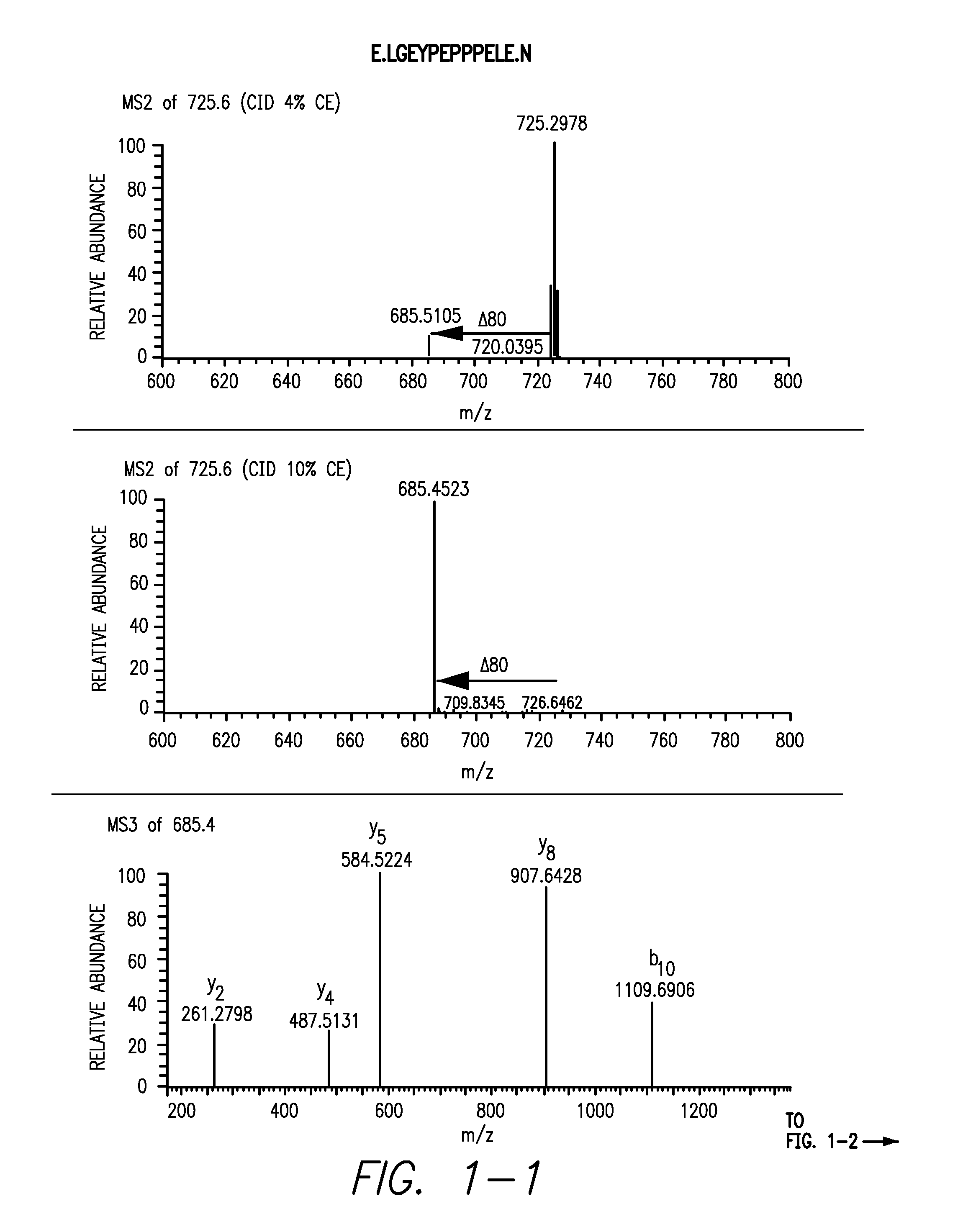
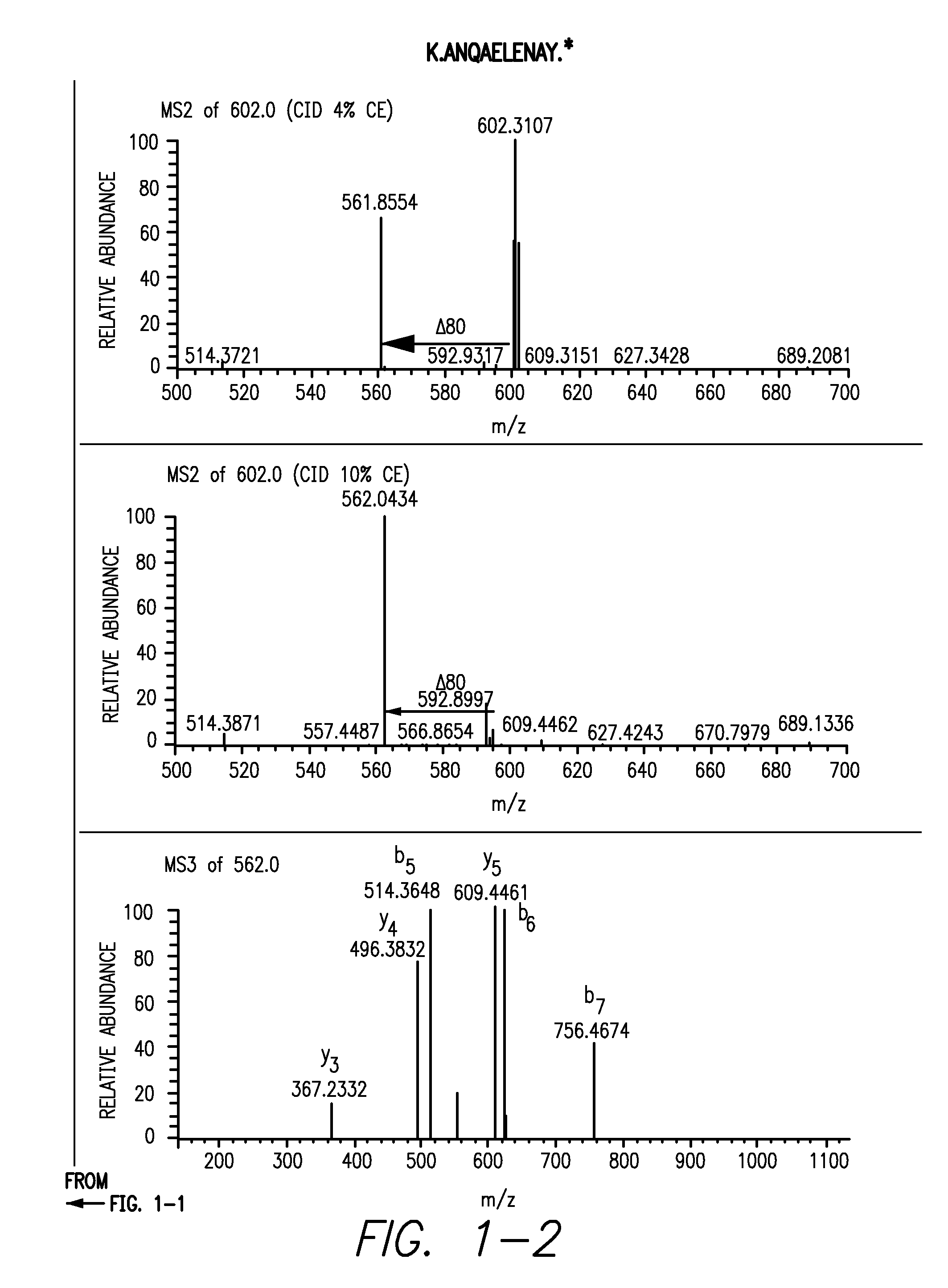
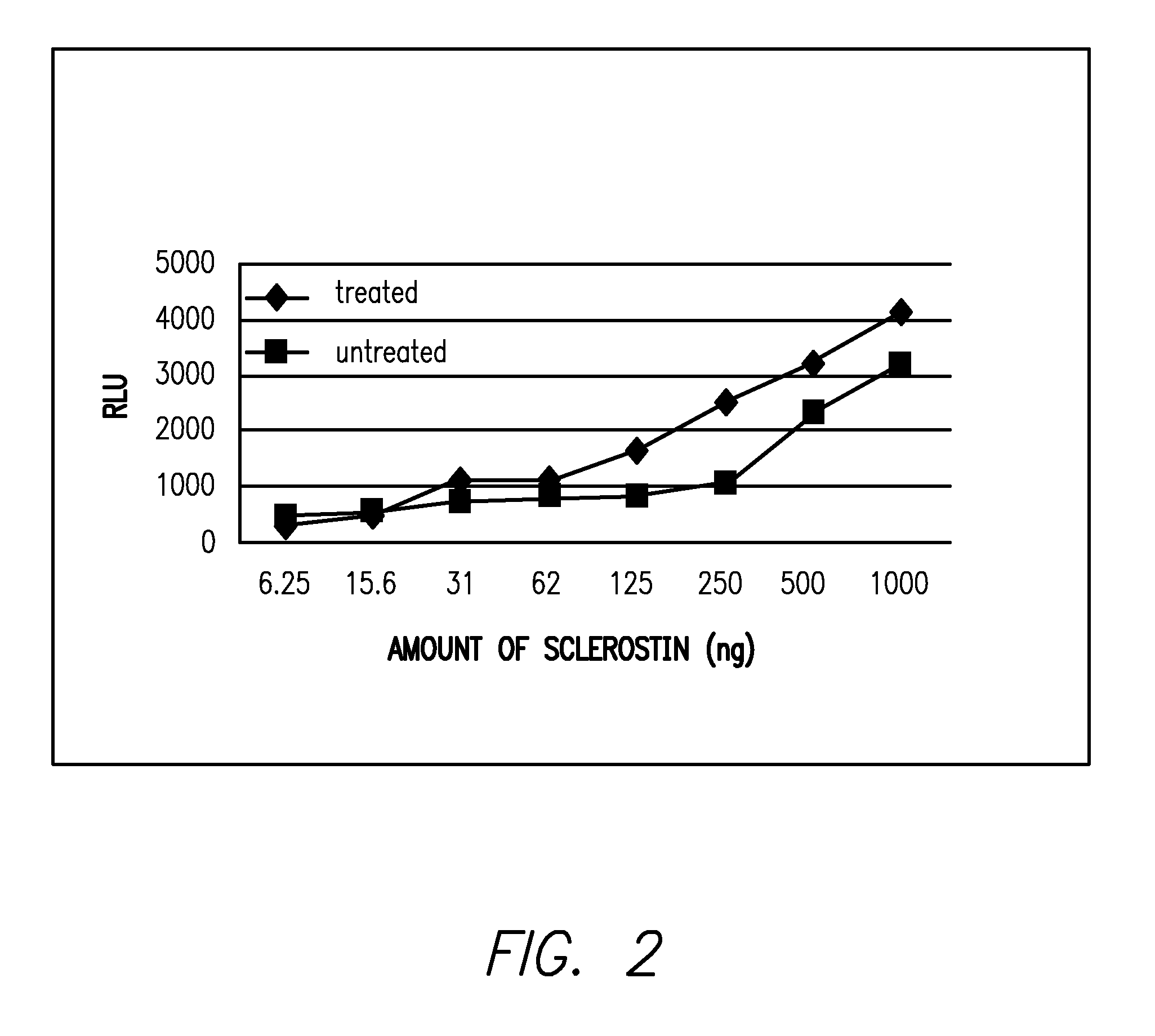
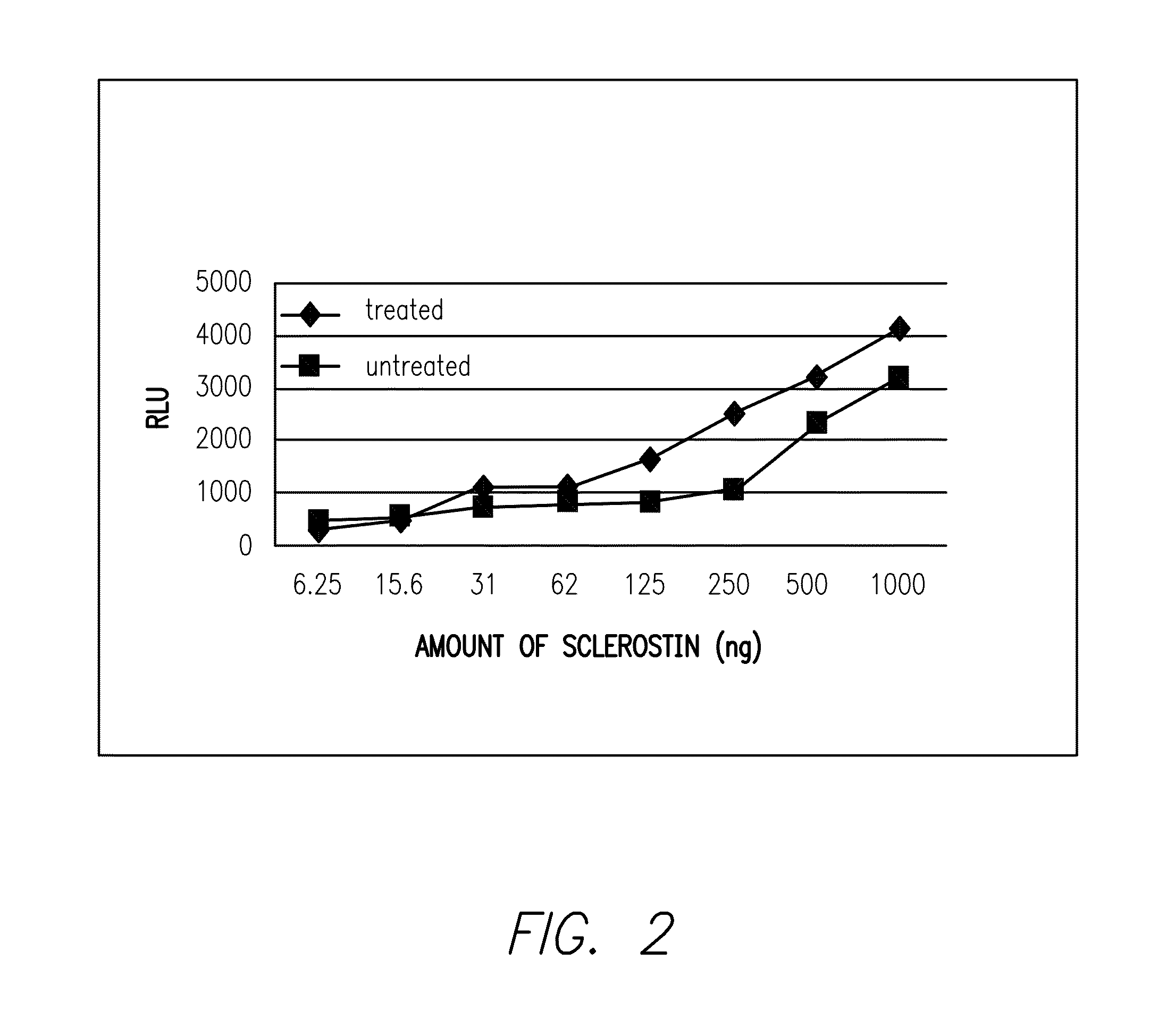
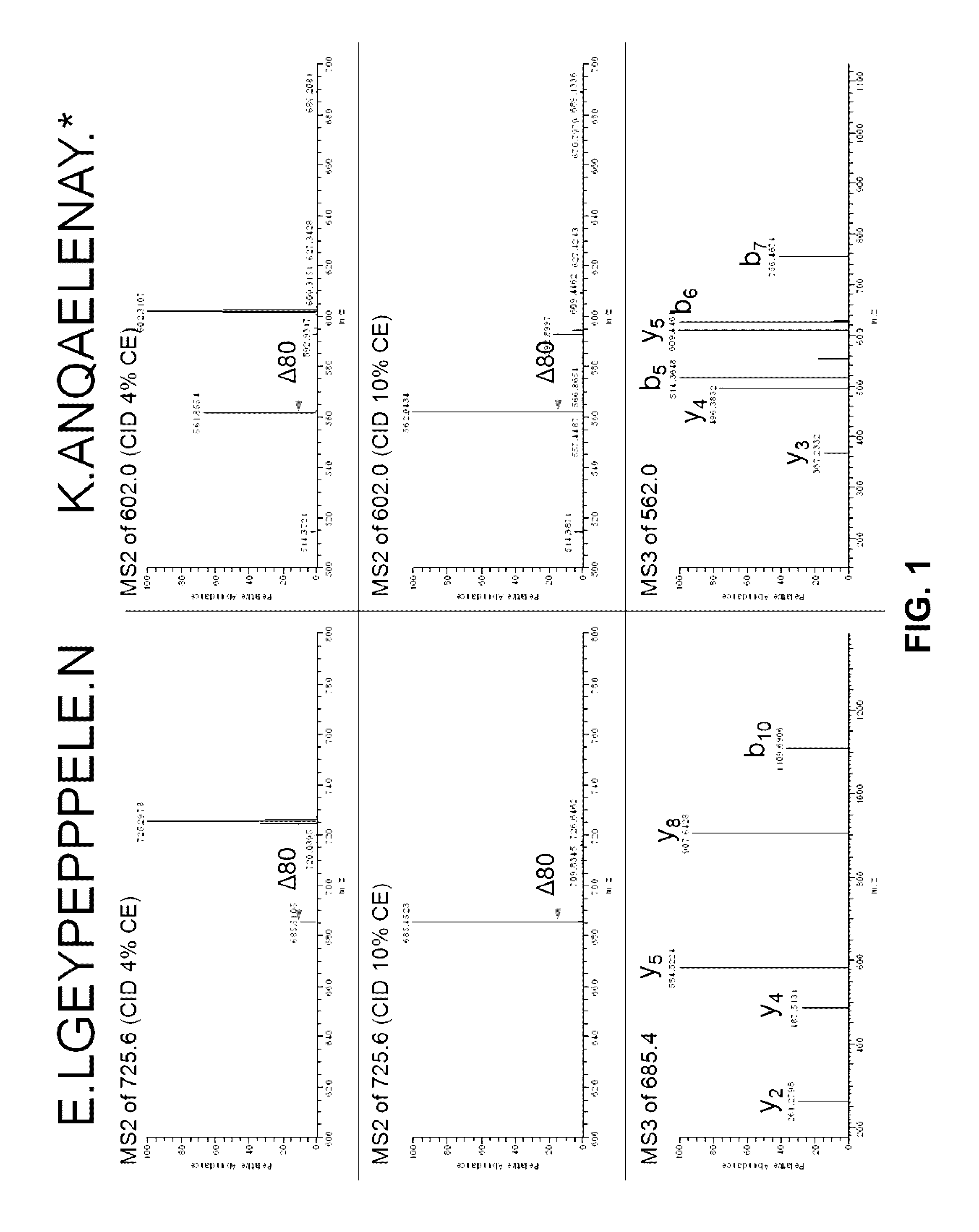
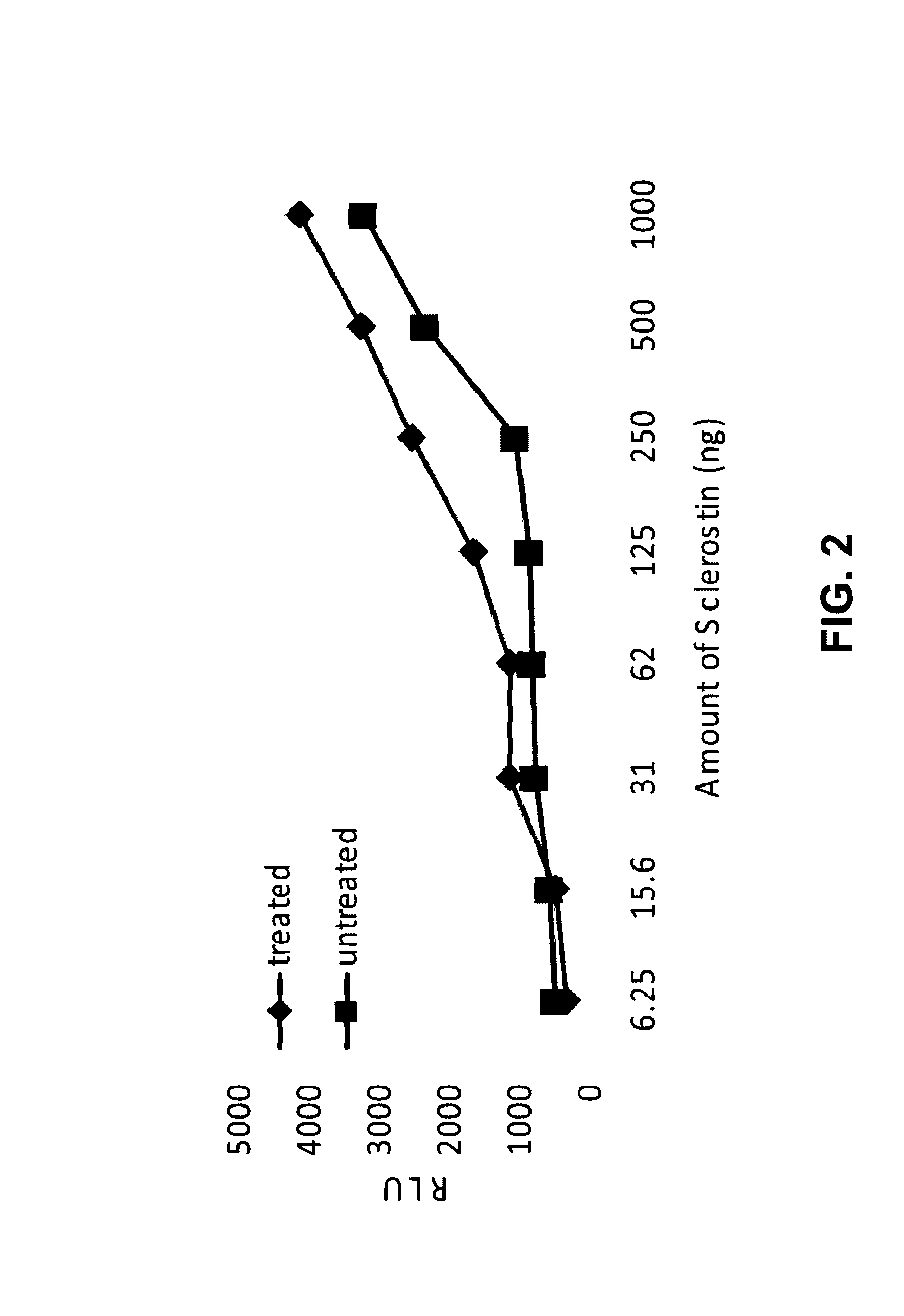
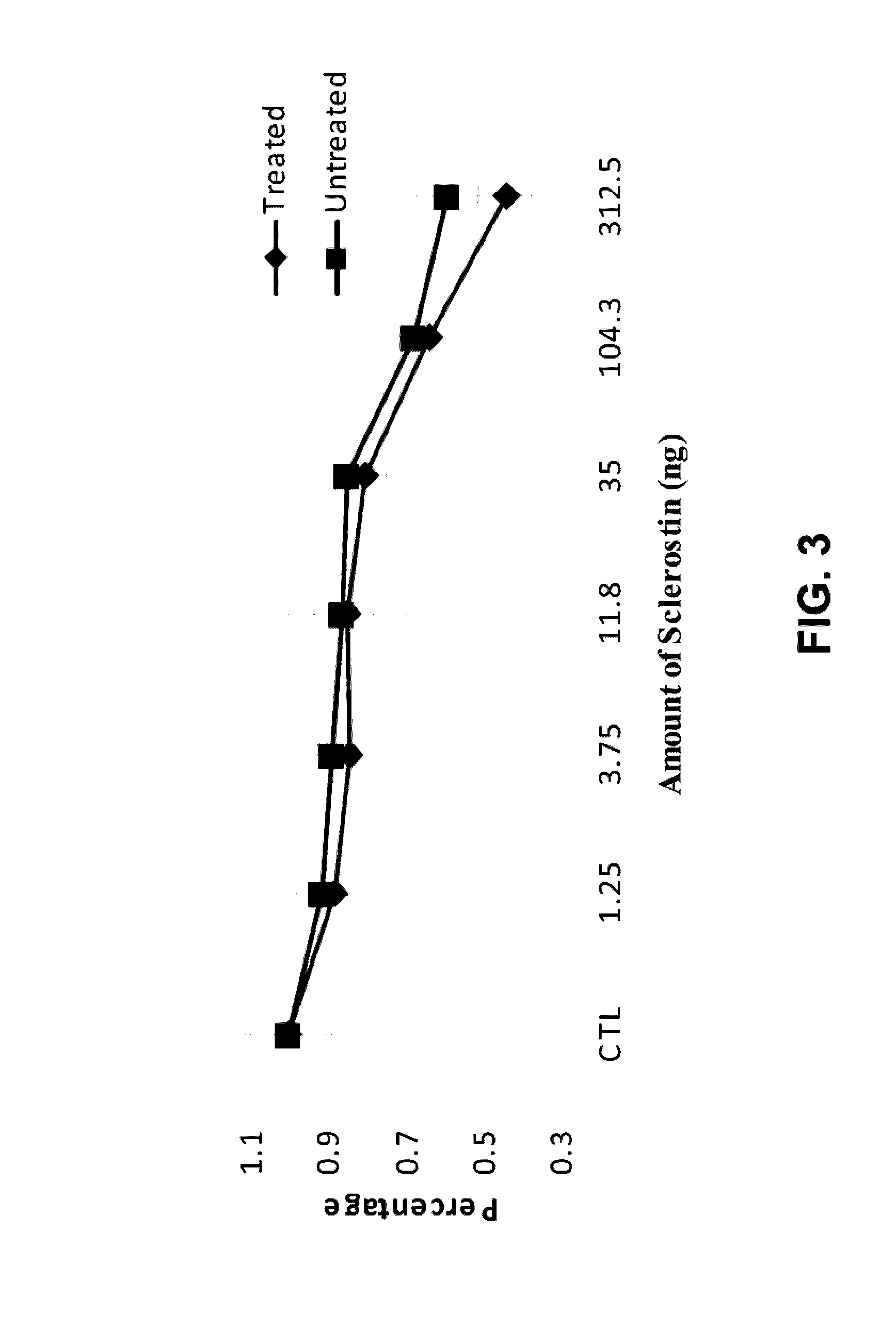
Provided is a composition comprising a peptide comprising amino acids and / or amino acid analogs comprising a continuous sequence of a sclerostin fragment comprising Tyr43 or Tyr213. Also provided is a composition comprising a peptide comprising less than about 75 amino acids and / or amino acid analogs including an amino acid or amino acid analog capable of being sulfated, where the composition is capable of inhibiting sclerostin binding to an LRP. Further provided is a composition comprising a peptide comprising less than about 75 amino acids and / or amino acid analogs including an amino acid or amino acid analog capable of being post-translationally sulfated, where the composition is capable of inhibiting binding of a protein ligand comprising a sulfation site to its binding partner. Additionally provided is a method of enhancing a Wnt signaling pathway comprising contacting an LRP5 / 6 receptor in the Wnt signaling pathway with either of the above-described compositions that comprise a sequence of a sclerostin fragment or is capable of inhibiting sclerostin binding to an LRP, where the tyrosine or tyrosine analog is not sulfated, in a manner sufficient to enhance the Wnt signaling pathway. Further provided is a method of treating a subject having a disease exacerbated by inhibition of a Wnt signaling pathway comprising administering either of the above-described compositions that comprise a sequence of a sclerostin fragment or is capable of inhibiting sclerostin binding to an LRP, where the tyrosine or tyrosine analog is not sulfated, to the subject in a manner sufficient to reduce the inhibition of the Wnt signaling pathway. Also, a method of inhibiting binding of a protein ligand comprising a sulfation site to its binding partner is provided. The method comprises adding the above-described composition that is capable of inhibiting binding of a protein ligand to its binding partner to the protein ligand and its binding partner in a manner sufficient to inhibit binding of the protein ligand to its binding partner.
Owner:ENZO THERAPEUTICS +1
Materials and methods for controlling pests
InactiveUS6766613B2Low toxicitySustainable, pesticide-free food supplyBiocideOrganic active ingredientsSolute transportersMethionine biosynthesis
Owner:FLORIDA UNIV OF A FLORIDA +1
Methods of incorporating amino acid analogs into proteins
InactiveUS20110008828A1Improved and enhanced enzymatic propertyEfficient chargingFungiSugar derivativesBase pairAmino acid
The invention provides a method of incorporating nonstandard amino acids into a protein by utilizing a modified aminoacyl-tRNA synthetase to charge the nonstandard amino acid to a modified tRNA, which forms strict Watson-Crick base-pairing with a codon that normally forms wobble base-pairing with natural tRNAs.
Owner:CALIFORNIA INST OF TECH
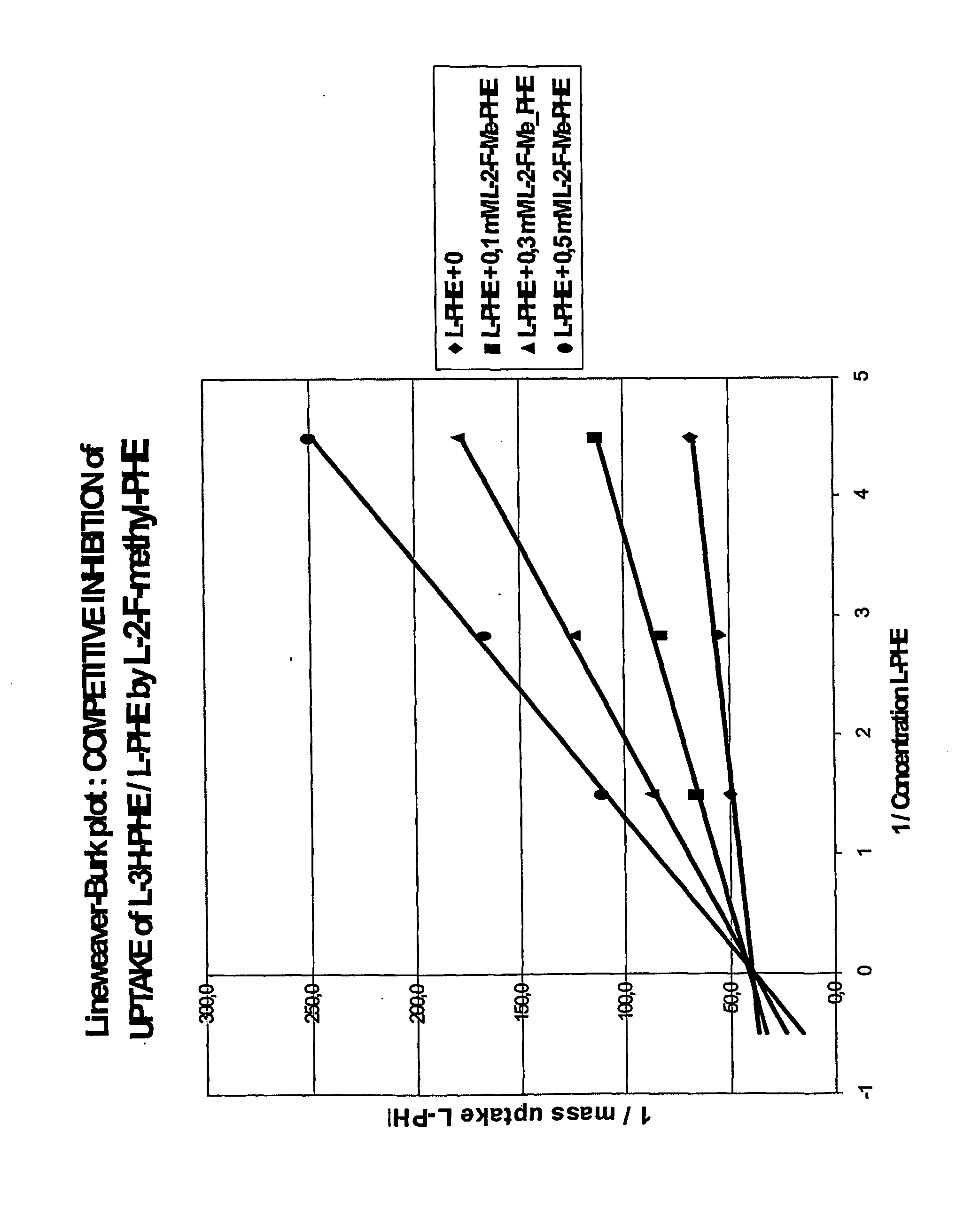
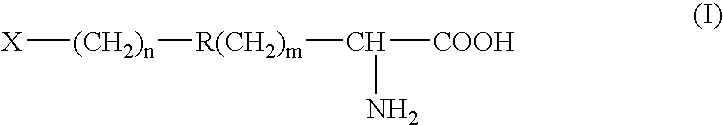
Radioactively labelled amino acid analogues, their preparation and use
InactiveUS20060127306A1Synthesis is simple and fastHigh labeling yieldBiocidePeptide/protein ingredientsHalogenCompound (substance)
The present invention relates to Halogenated amino acid analogues for use in diagnosis, which compounds have the genera formula (I) wherein: R is (C1-C6) alkyl optionally substituted with thioether or ether oxygen atom when n=0, or a substituted aromatic or heteraromatic ring when n=1-6; and m=0 or 1; and X is a halogen atom. The invention further relates to precursor compounds for these analogues, to a method of preparing these analogues, to a pharmaceutical composition comprising these analogues and to the use of these analogues and compositions in the diagnosis of cancer.
Owner:VRIJE UNIV BRUSSEL
Methods of incorporating amino acid analogs into proteins
ActiveUS20130210073A1Improved and enhanced enzymatic propertyEfficient chargingFungiBacteriaOrganic chemistryAmino acid
The invention provides a method of incorporating nonstandard amino acids into a protein by utilizing a modified aminoacyl-tRNA synthetase to charge the nonstandard amino acid to a modified tRNA, which forms strict Watson-Crick base-pairing with a codon that normally forms wobble base-pairing with natural tRNAs.
Owner:CALIFORNIA INST OF TECH
Staphylococcus peptides for bacterial interference
InactiveUS6953833B2Effective treatmentTripeptide ingredientsCyclic peptide ingredientsThioester synthesisBacteroides
The present invention provides a cyclic peptide comprising the structure: wherein X is selected from the group consisting of an amino acid, an amino acid analog, a peptidomimetic and a non-amide isostere, Z is selected from the group consisting of a synthetic amino acid and a biosynthetic amino acid, R is selected from the group consisting of oxygen, nitrogen, sulfur and carbon, n is 0 to 10 and y is 1 to 10. The present invention also provides a cyclic peptide comprising the amino acid sequence of NH2—X(n)-Z-X(y)—COOH and a cyclic bond between the Z residue and COOH other than a thioester bond, wherein X is selected from the group consisting of an amino acid, an amino acid analog, a peptidomimetic and a non-amide isostere, Z is selected from the group consisting of a synthetic amino acid and a biosynthetic amino acid, n is 0 to 10 and y is 1 to 10. Methods of preparation including a cyclization protocol, and methods of use of the cyclic peptides of the invention are also disclosed.
Owner:THE ROCKEFELLER UNIV +1
Sulfation of Wnt pathway proteins
Provided is a composition comprising a peptide comprising amino acids and / or amino acid analogs comprising a continuous sequence of a sclerostin fragment comprising Tyr43 or Tyr213. Also provided is a composition comprising a peptide comprising less than about 75 amino acids and / or amino acid analogs including an amino acid or amino acid analog capable of being sulfated, where the composition is capable of inhibiting sclerostin binding to an LRP. Further provided is a composition comprising a peptide comprising less than about 75 amino acids and / or amino acid analogs including an amino acid or amino acid analog capable of being post-translationally sulfated, where the composition is capable of inhibiting binding of a protein ligand comprising a sulfation site to its binding partner. Additionally provided is a method of enhancing a Wnt signaling pathway comprising contacting an LRP5 / 6 receptor in the Wnt signaling pathway with either of the above-described compositions that comprise a sequence of a sclerostin fragment or is capable of inhibiting sclerostin binding to an LRP, where the tyrosine or tyrosine analog is not sulfated, in a manner sufficient to enhance the Wnt signaling pathway. Further provided is a method of treating a subject having a disease exacerbated by inhibition of a Wnt signaling pathway comprising administering either of the above-described compositions that comprise a sequence of a sclerostin fragment or is capable of inhibiting sclerostin binding to an LRP, where the tyrosine or tyrosine analog is not sulfated, to the subject in a manner sufficient to reduce the inhibition of the Wnt signaling pathway. Also, a method of inhibiting binding of a protein ligand comprising a sulfation site to its binding partner is provided. The method comprises adding the above-described composition that is capable of inhibiting binding of a protein ligand to its binding partner to the protein ligand and its binding partner in a manner sufficient to inhibit binding of the protein ligand to its binding partner.
Owner:ENZO THERAPEUTICS +1
Process for the synthesis of peptides amides by side-chain attachment to a solid phase
A process is provided for the solid-phase synthesis of a peptide amide which comprises attaching an α-nitrogen protected Cα-carboxamide amino acid to a solid support by its side chain, removing the α-nitrogen protecting group, assembling a peptide chain on said α-nitrogen and then cleaving the assembled peptide amide from the solid support. Novel amino acid analogues, peptide amides and solid-phase supports are also provided.
Owner:FUJIFILM DIOSYNTH BIOTECH UK
Antibodies specific for sulfated sclerostin
Provided is a protein comprising an antibody binding site that binds to a sulfated epitope of a Wnt pathway protein that is not Wnt5A, Wnt11, or Wnt3a. Also provided is a composition comprising an isolated and purified Wnt pathway protein, where the protein is sulfated but not glycosylated. Additionally provided is a preparation of a Wnt pathway protein comprising at least one sulfation site and at least one glycosylation site, where all of the Wnt pathway protein in the preparation is glycosylated but not sulfated. Further provided is a composition comprising a peptide less than 75 amino acids or amino acid analogs, the peptide consisting of a fragment of a Wnt pathway protein, wherein the fragment is sulfated. A modified Wnt pathway protein comprising a sulfation site that is not present in the native Wnt pathway protein is also provided. Also provided is a method of detecting or quantifying a sulfated Wnt pathway protein in a preparation. Additionally, a modified Wnt pathway protein lacking a sulfation site that is present in the native Wnt pathway protein is provided. Also provided is methods of treating a subject having a disease exacerbated by Wnt activation. Additionally, a method of treating a subject having a disease exacerbated by Wnt inhibition is provided.
Owner:ENZO BIOCHEM
Procoagulant peptides and their derivatives and uses therefor
ActiveUS9149511B2Improve solubilityCost effectiveFibrinogenSurgical adhesivesAbsorbable polymersGenetically engineered
The present invention is directed to a hemostatic or tissue sealing material having (a) a peptide having a sequence SEQ ID NO: 1 or an amino acid analog sequence thereof, and (b) a scaffold for said peptide or amino acid analog sequence. The scaffold is preferably hemostatic, such as a natural or genetically engineered absorbable polymer, a synthetic absorbable polymer, or combinations thereof. The natural or genetically engineered absorbable polymers can be selected from the group consisting of a protein, a polysaccharide, or combinations thereof.
Owner:ETHICON INC
Sulfation of wnt pathway proteins
Provided is a protein comprising an antibody binding site that binds to a sulfated epitope of a Wnt pathway protein that is not Wnt5A, Wnt11, or Wnt3a. Also provided is a composition comprising an isolated and purified Wnt pathway protein, where the protein is sulfated but not glycosylated. Additionally provided is a preparation of a Wnt pathway protein comprising at least one sulfation site and at least one glycosylation site, where all of the Wnt pathway protein in the preparation is glycosylated but not sulfated. Further provided is a composition comprising a peptide less than 75 amino acids or amino acid analogs, the peptide consisting of a fragment of a Wnt pathway protein, wherein the fragment is sulfated. A modified Wnt pathway protein comprising a sulfation site that is not present in the native Wnt pathway protein is also provided. Also provided is a method of detecting or quantifying a sulfated Wnt pathway protein in a preparation. Additionally, a modified Wnt pathway protein lacking a sulfation site that is present in the native Wnt pathway protein is provided. Also provided is methods of treating a subject having a disease exacerbated by Wnt activation. Additionally, a method of treating a subject having a disease exacerbated by Wnt inhibition is provided.
Owner:ENZO BIOCHEM
Methods for treating functional bowel disorders using alpha2 subunit calcium channel modulators with smooth muscle modulators
InactiveUS20060276542A1Limited efficacyReduce patient complianceBiocidePeptide/protein ingredientsGabapentinoidAdrenergic receptor agonists
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or, more compounds with smooth muscle modulatory effects to treat functional bowel disorders in patients in need of treatment. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs including gabapentin and pregabalin, fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Bioresorbable polymers synthesized from monomer analogs of natural metabolites
InactiveUS20110275782A1Improve the level ofReduce rateOrganic chemistryProsthesisMetabolitePolymer science
New bioresorbable polymers are synthesized from monomer analogs of natural metabolites In particular, polymers are polymerized from analogs of amino acids that contribute advantageous synthesis, processing and material properties to the polymers prepared therefrom, including particularly advantageous degradation profiles
Owner:RUTGERS THE STATE UNIV
Non-natural amino acids and neurotensin analogues
InactiveUS20080234202A1Selective and long-lasting biological activityNervous disorderPeptide/protein ingredientsArgininePeptide sequence
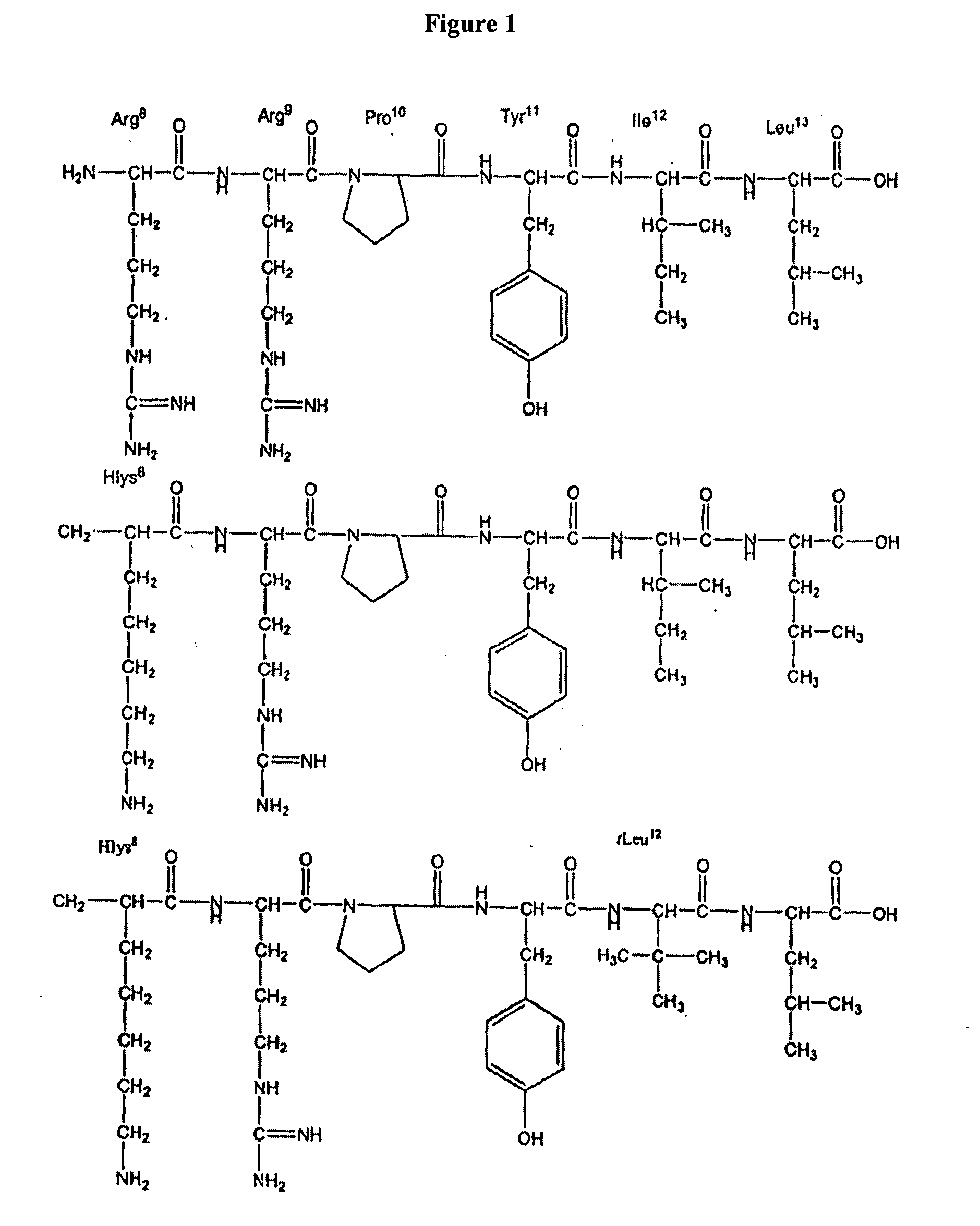
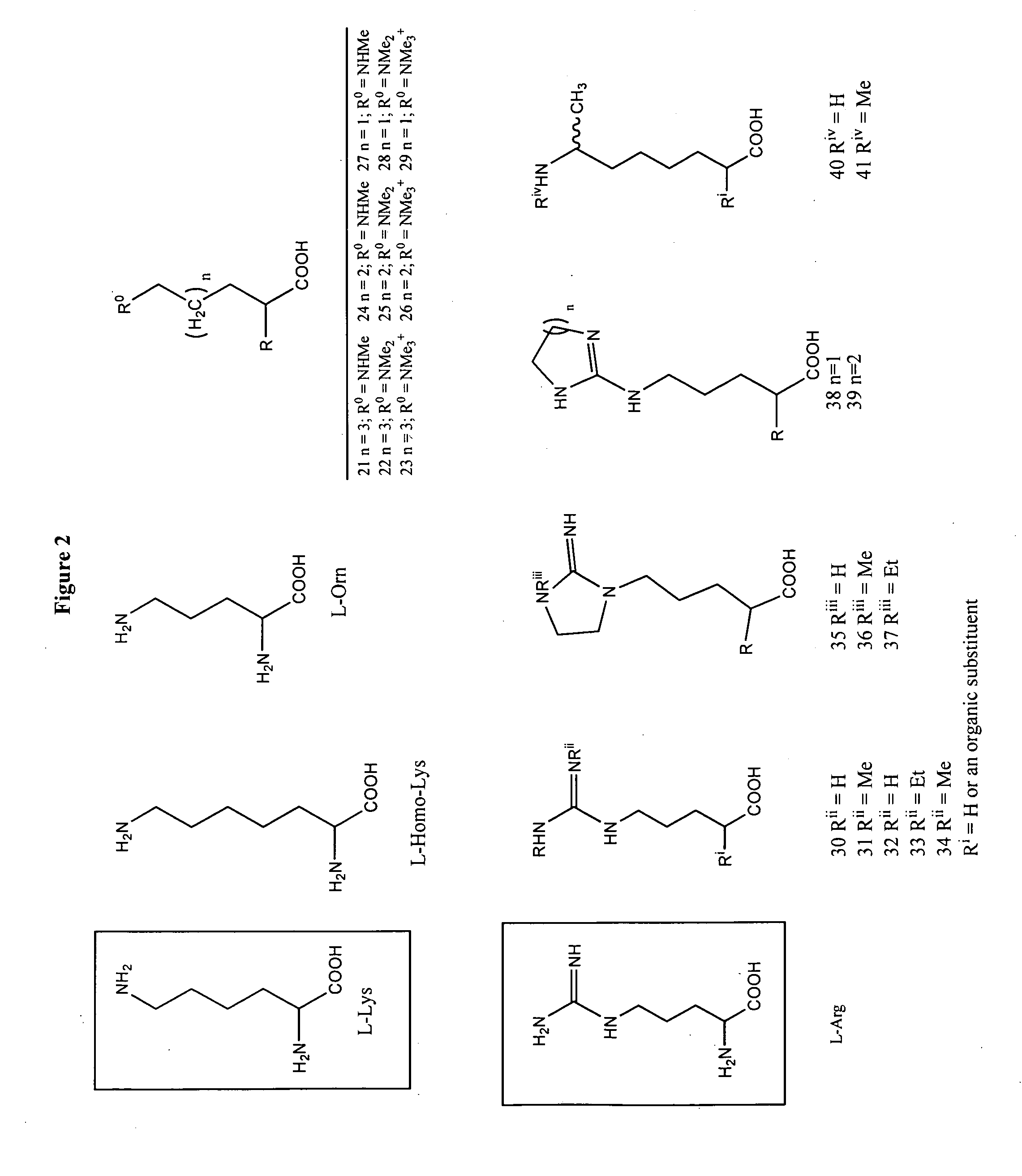
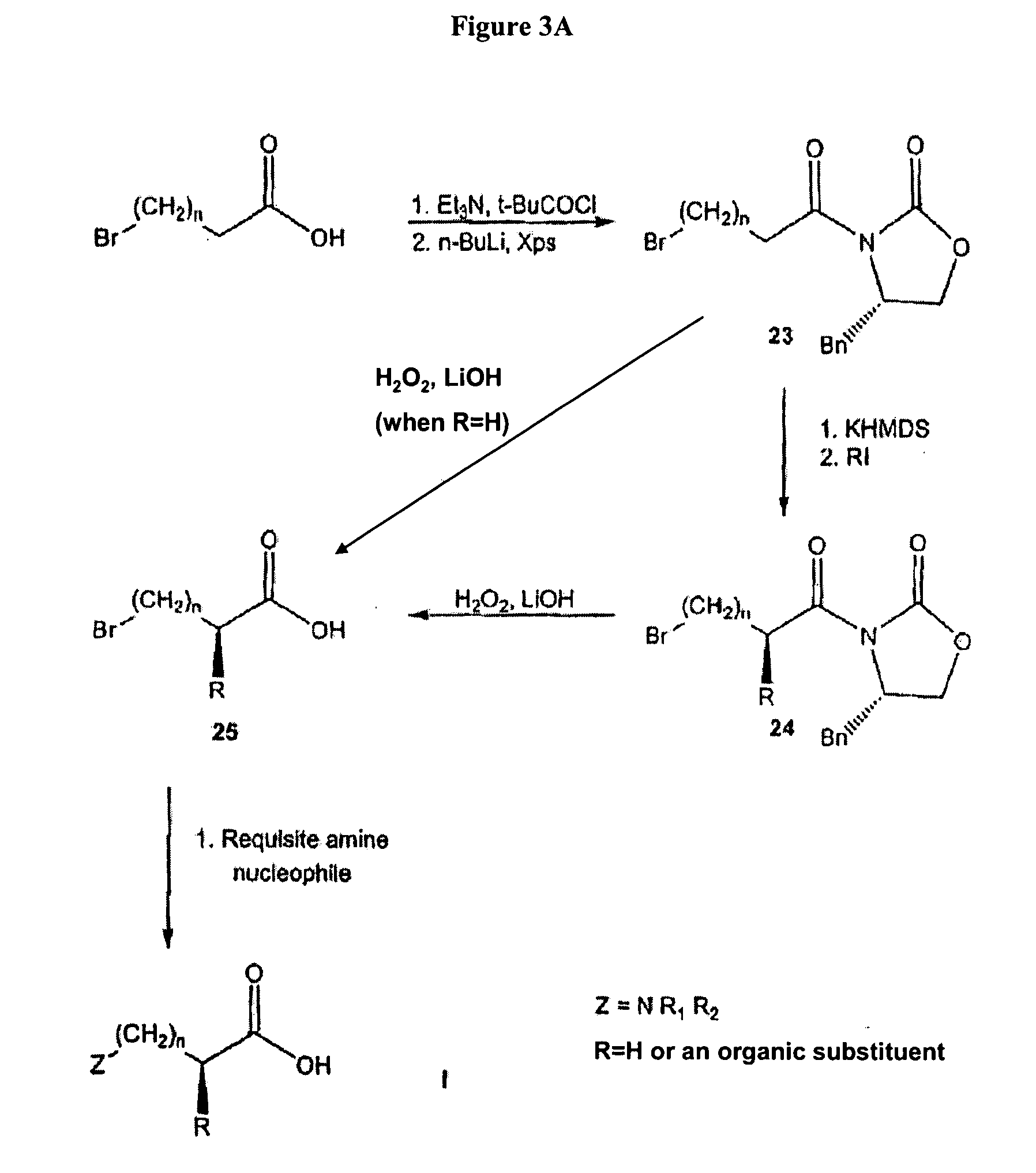
This invention relates to methods of synthesis of amino acid analogs contained within peptide sequences that is adapted for preparation of peptides containing analogs of basic amino acids lysine and arginine.
Owner:THE MEDICAL UNIV OF SOUTH CAROLINA +1
Beta-substituted beta-amino acids and analogs as chemotherapeutic agents and uses thereof
ActiveCN108026026AHigh uptake selectivityLow nonspecific uptakeNervous disorderOrganic chemistry methodsCytotoxicityIn vivo
Beta-Substituted Beta-amino acids, Beta-substituted Beta-amino acid derivatives, and Beta-substituted Beta-amino acid analogs and (bio)isosteres and their use as chemotherapeutic agents are disclosed.The Beta-substituted Beta-amino acid derivatives and Beta -substituted Beta-amino acid analogs and (bio)isosteres are selective LAT1 / 4F2hc substrates and exhibit rapid uptake and retention in tumorsexpressing the LAT1 / 4F2hc transporter. Methods of synthesizing the Beta-substituted Beta-amino acid derivatives and Beta-substituted Beta-amino acid analogs and methods of using the compounds for treating cancer are also disclosed. The Beta-substituted Beta-amino acid derivatives and Beta-substituted Beta-amino acid analogs exhibit selective uptake in tumor cells expressing the LAT1 / 4F2hc transporter and accumulate in cancerous cells when administered to a subject in vivo. The Beta-substituted Beta-amino acid derivatives and Beta-substituted Beta-amino acid analogs and (bio)isosteres exhibit cytotoxicity toward several tumor types.
Owner:QUADRIGA BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com