Methods of incorporating amino acid analogs into proteins
a technology proteins, applied in the field of amino acid analogs in proteins, can solve the problems of limiting the chemical functionality of the method, unable to circumvent unable to achieve the specificity of the synthetase, so as to increase reduce the binding affinity, and enhance the half-life of the cytokine or growth factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0497]This invention is further illustrated by the following examples which should not be construed as limiting. The teachings of all references, patents and published patent applications cited throughout this application, as well as the Figures are hereby incorporated by reference.
example i
tRNA and Synthetase Construction
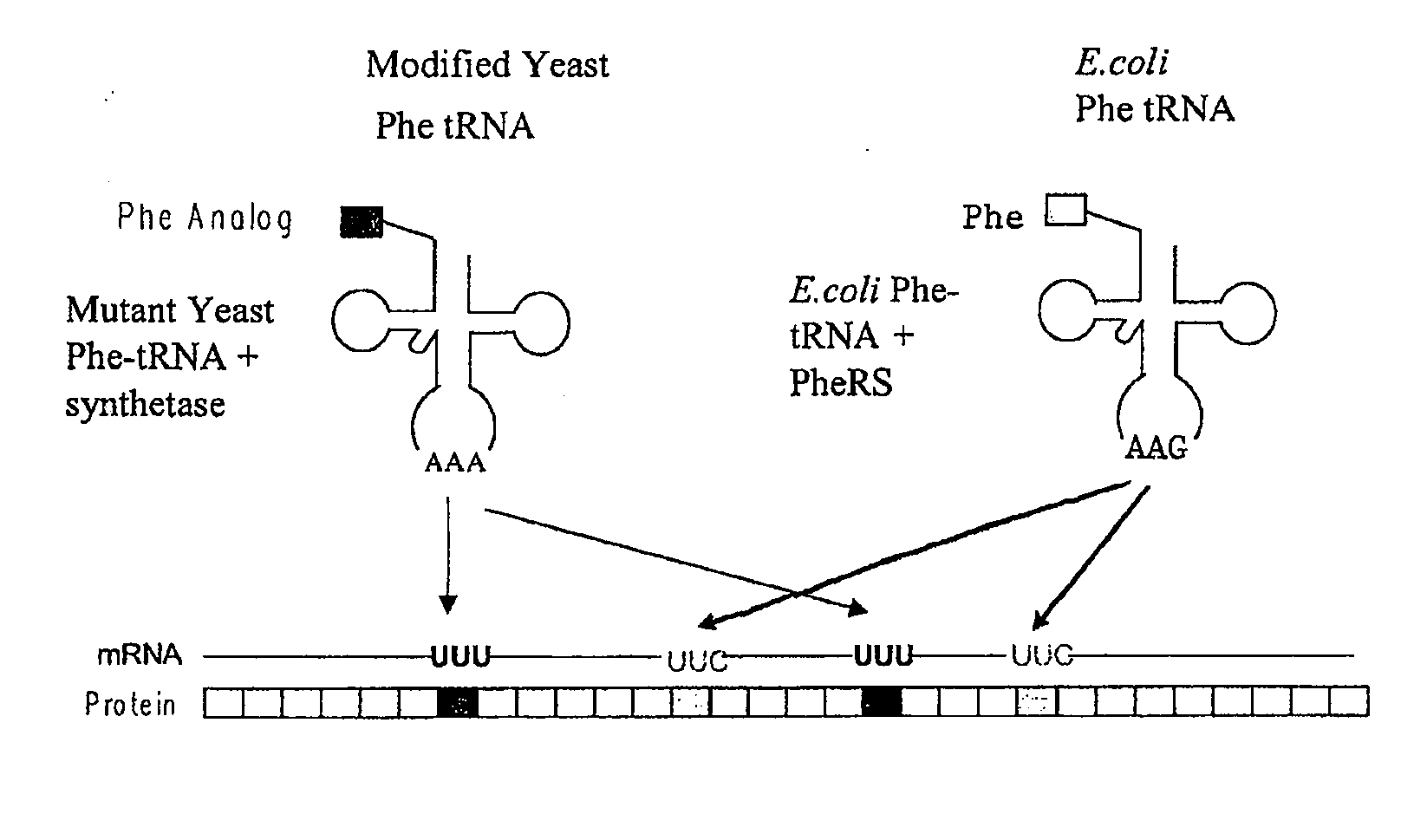
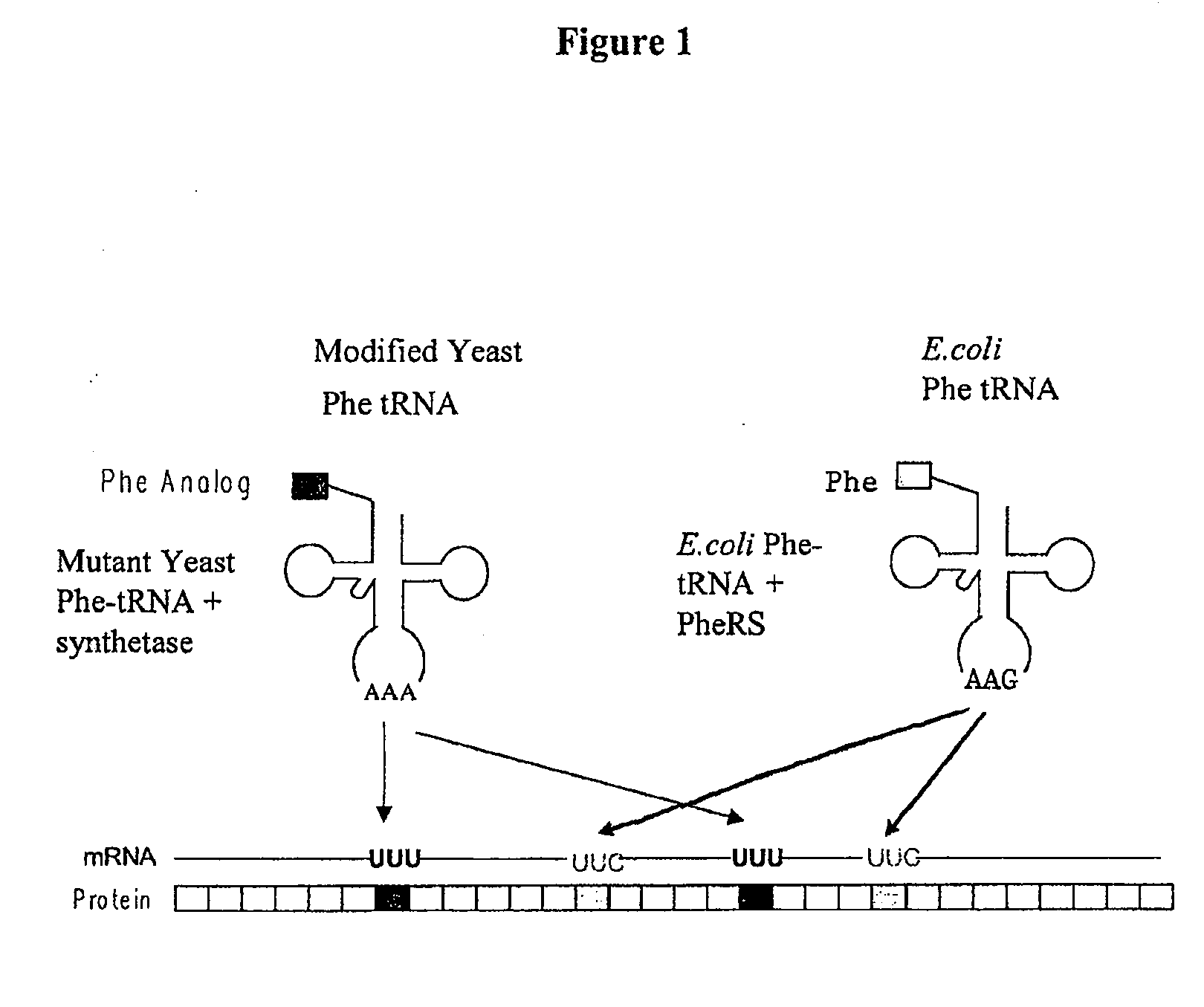
[0498]This example illustrates the incorporation of an amino acid analog in proteins at positions encoded by codons which normally encode phenylalanine (Phe). A schematic diagram is shown in FIG. 1. Similar approaches can be used for any other analogs.
[0499]Phe is encoded by two codons, UUC and UUU. Both codons are read by a single tRNA, which is equipped with the anticodon sequence GAA. The UUC codon is therefore recognized through standard Watson-Crick base-pairing between codon and anticodon; UUU is read through a G-U wobble base-pair at the first position of the anticodon (Crick, J. Mol. Biol. 19: 548, 1966; Soll and RajBhandary, J. Mol. Biol. 29: 113, 1967). Thermal denaturation of RNA duplexes has yielded estimates of the Gibbs free energies of melting of G-U, G-C, A-U, and A-C basepairs as 4.1, 6.5, 6.3, and 2.6 kcal / mol, respectively, at 37° C. Thus the wobble basepair, G-U, is less stable than the Watson-Crick basepair, A-U. A modified tRNAPh...
example ii
Generation of a Mutant Protein Containing Nal
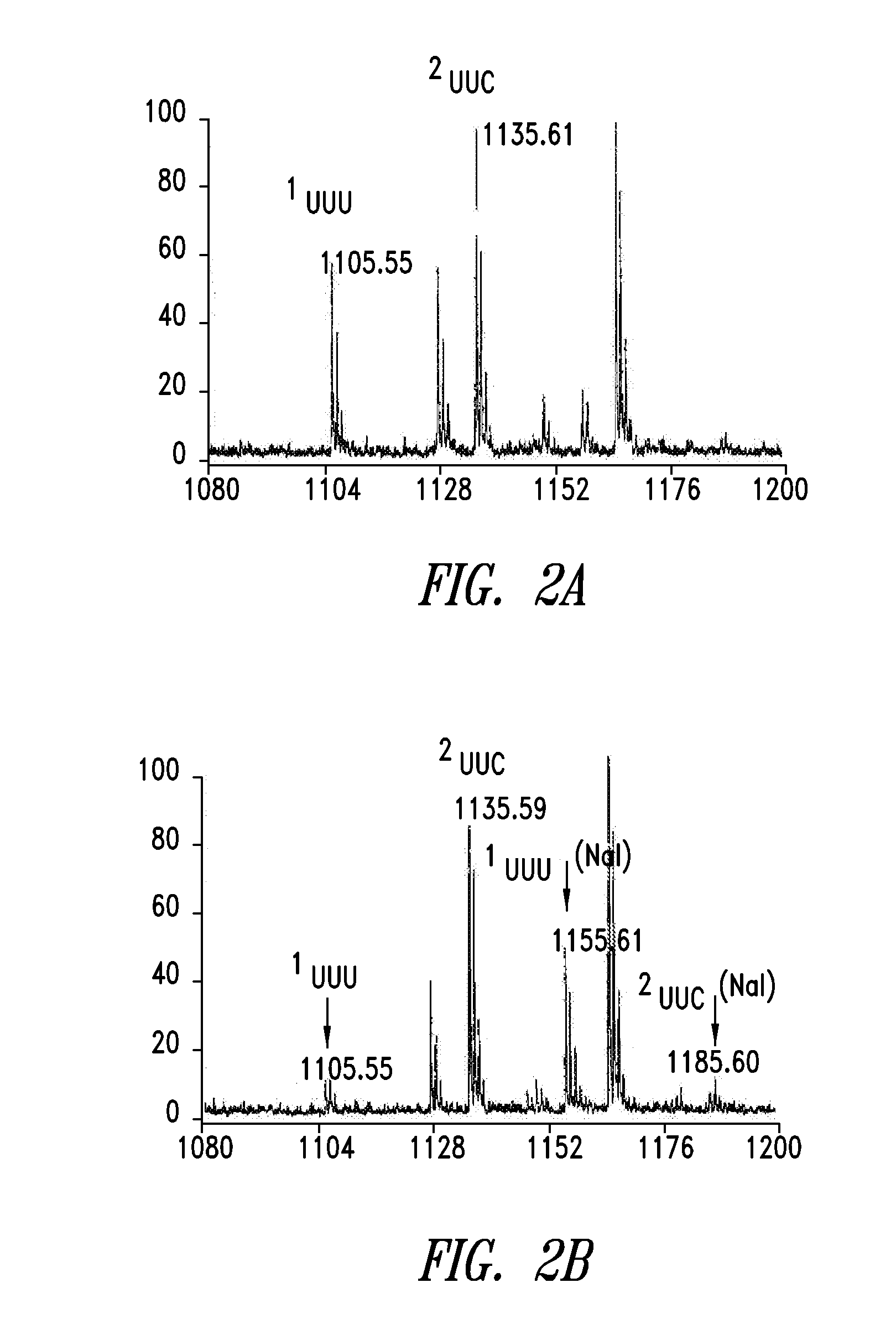
[0504]Murine dihydrofolate reductase (mDHFR), which contains nine Phe residues, was chosen as the test protein. The expression plasmid pQE16 encodes mDHFR under control of a bacteriophage T5 promoter; the protein is outfitted with a C-terminal hexahistidine (HIS6) tag to facilitate purification via immobilized metal affinity chromatography.
[0505]In this construct, four of the Phe residues of mDHFR are encoded by UUC codons, five by UUU. A full-length copy of the mu-yPheRS gene, under control of a constitutive tac promoter, was inserted into pQE16. The gene encoding ytRNAPheAAA was inserted into the repressor plasmid pREP4 (Qiagen) under control of the constitutive promoter 1 pp. E. coli transformants harboring these two plasmids were incubated in Phe-depleted minimal medium supplemented with 3 mM Nal and were then treated with 1 mM IPTG to induce expression of mDHFR. Although the E. coli strain (K10-F6) used in this study is a Phe auxotro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescent | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| thermal stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com