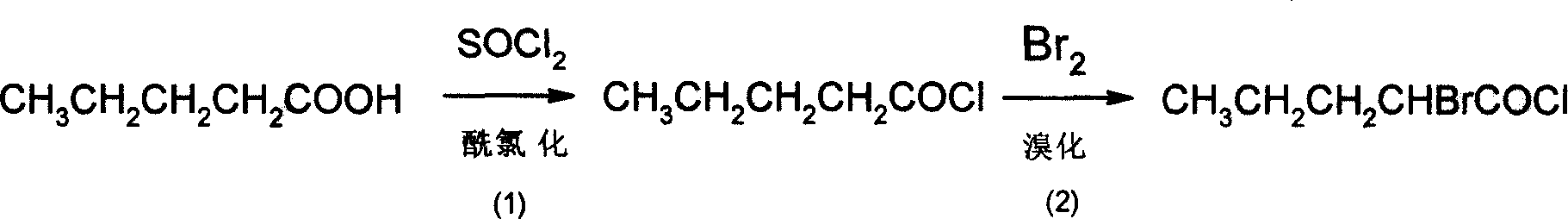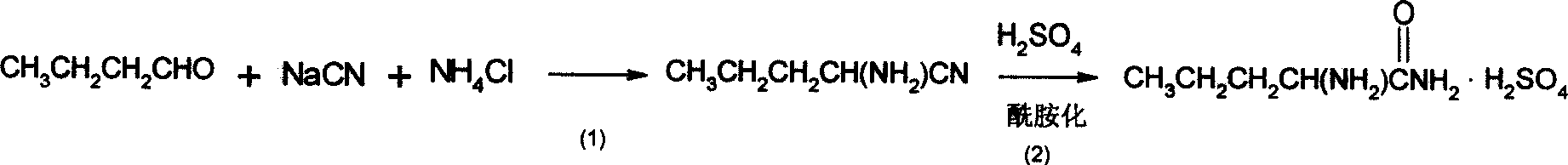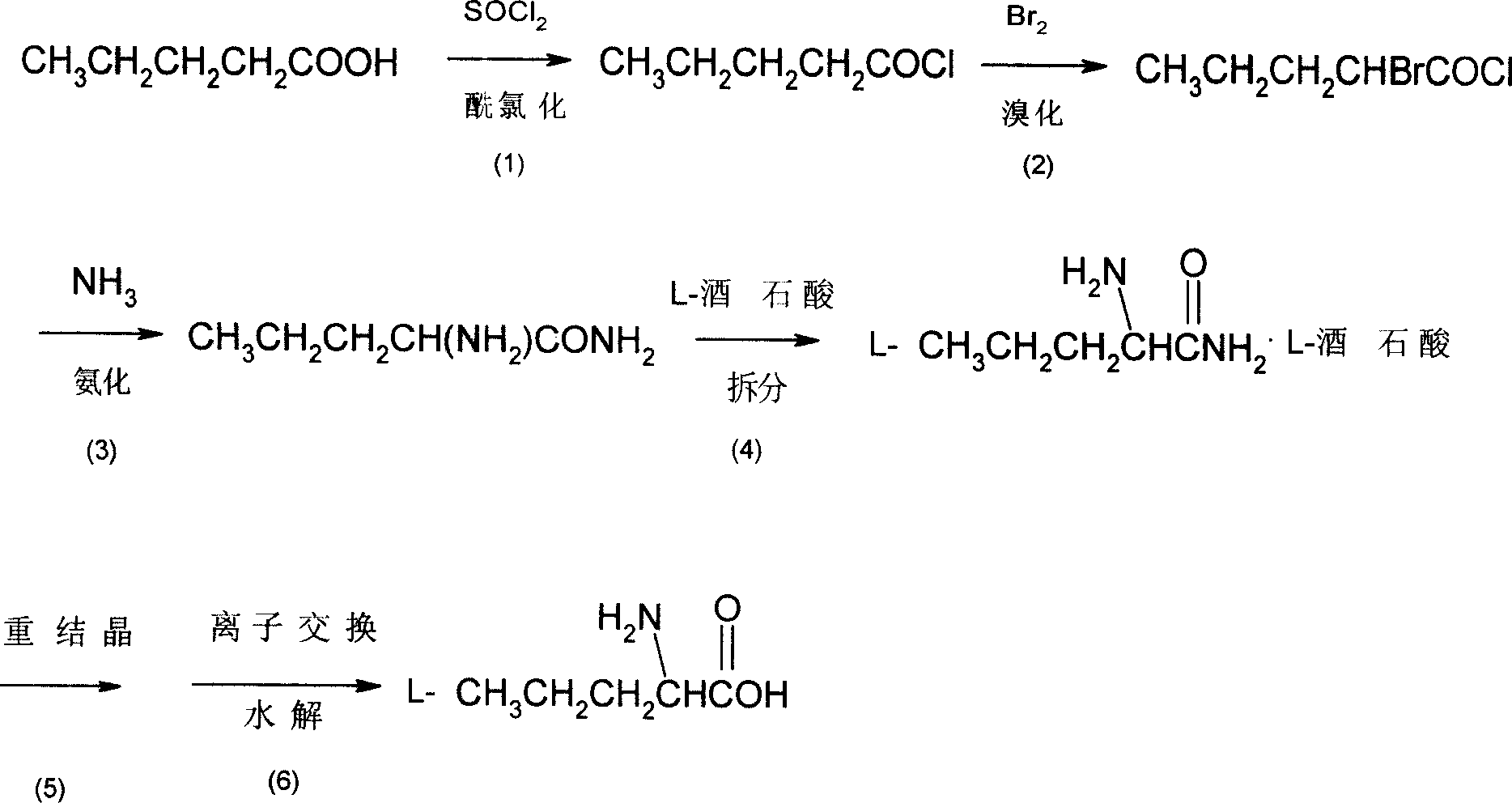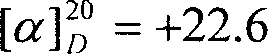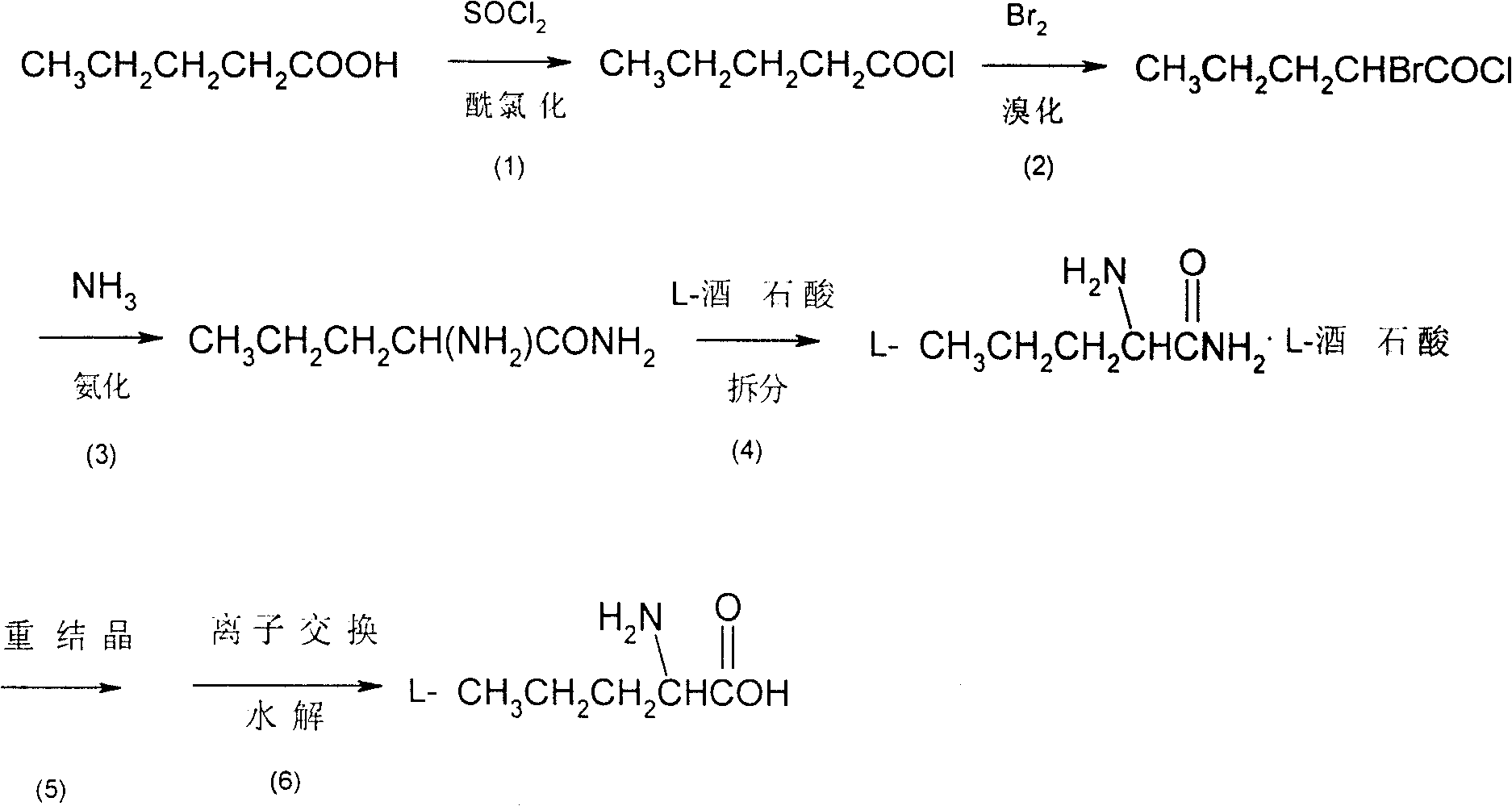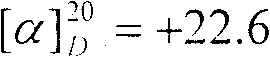Patents
Literature
45 results about "Norvaline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
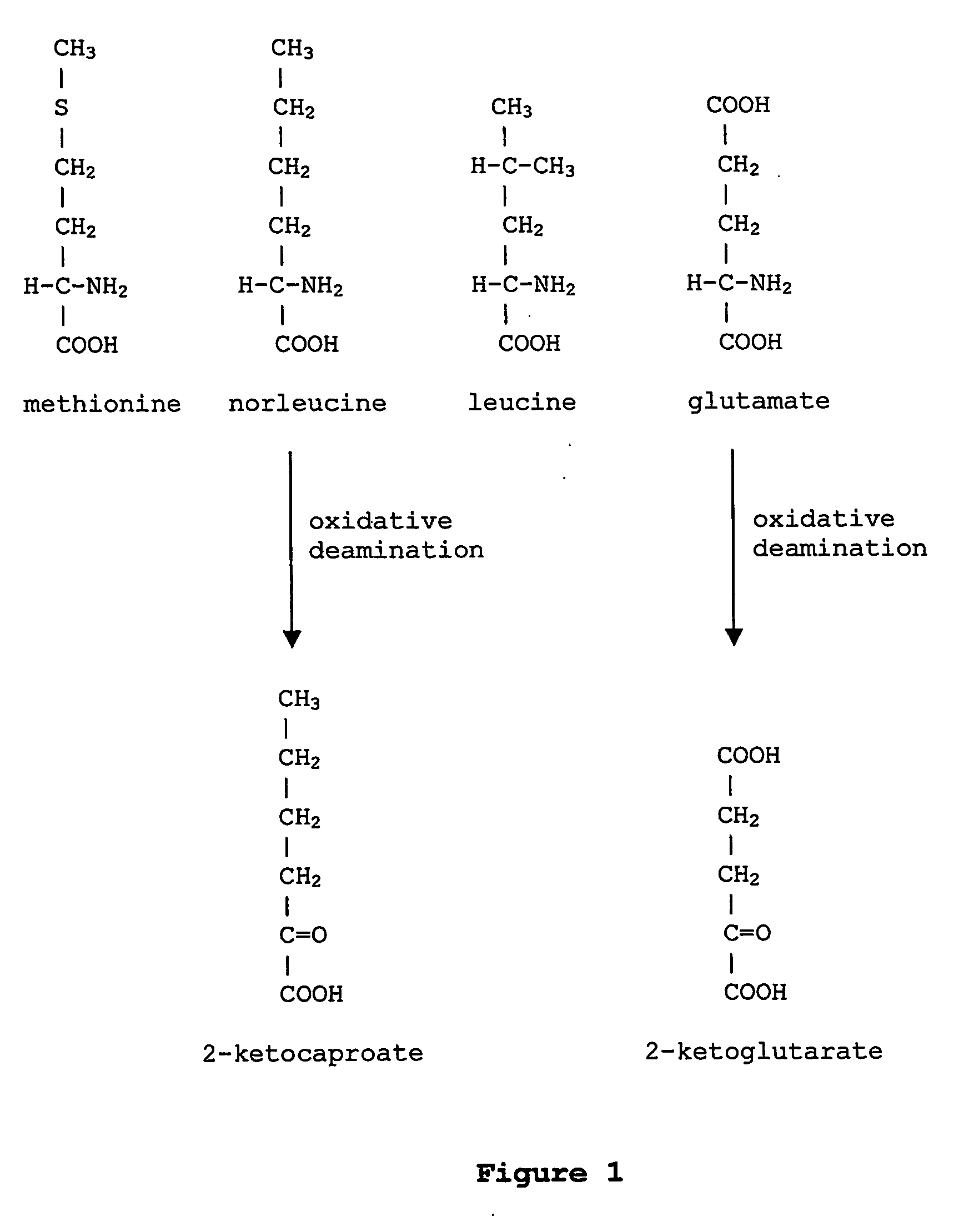
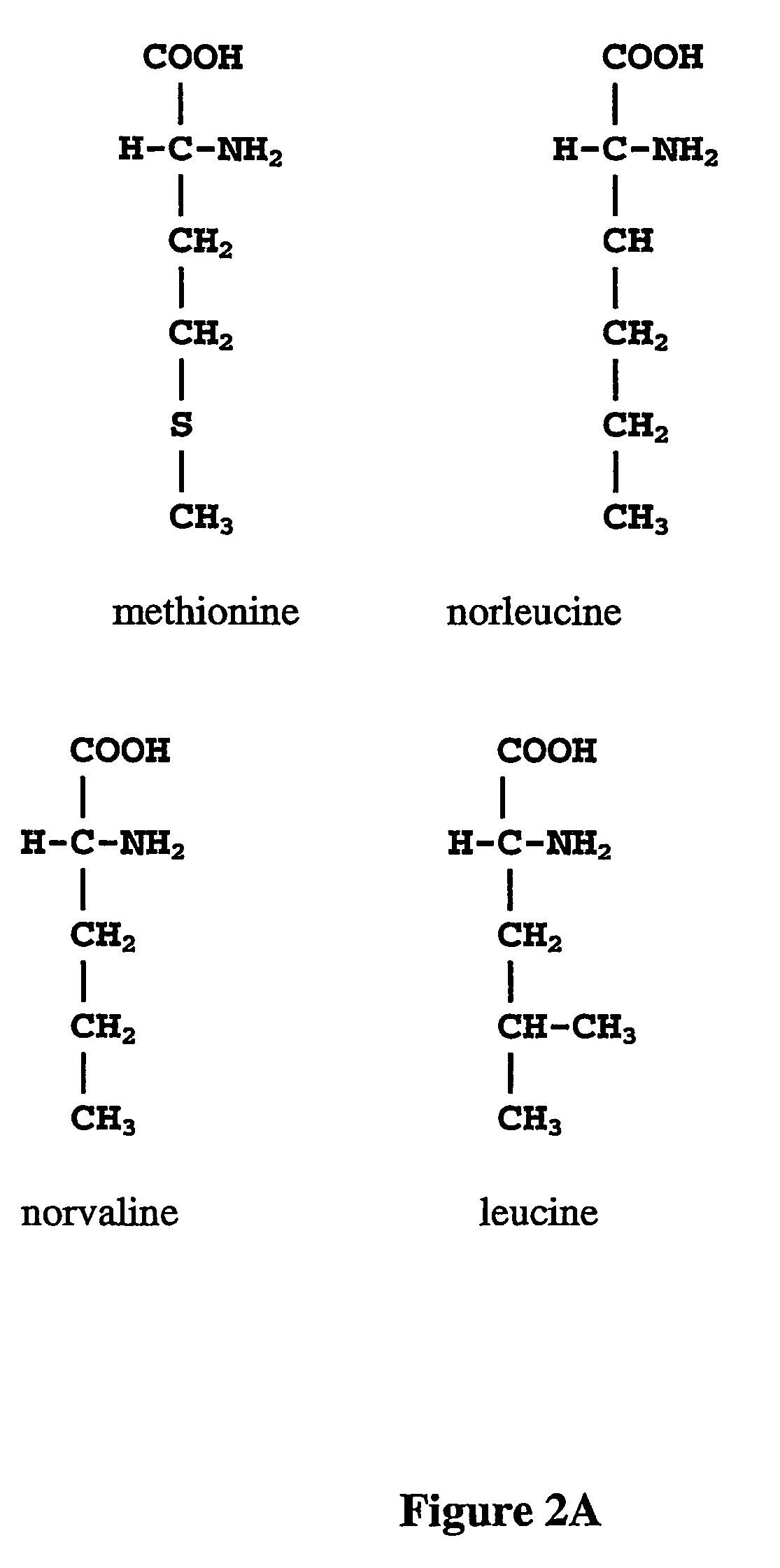
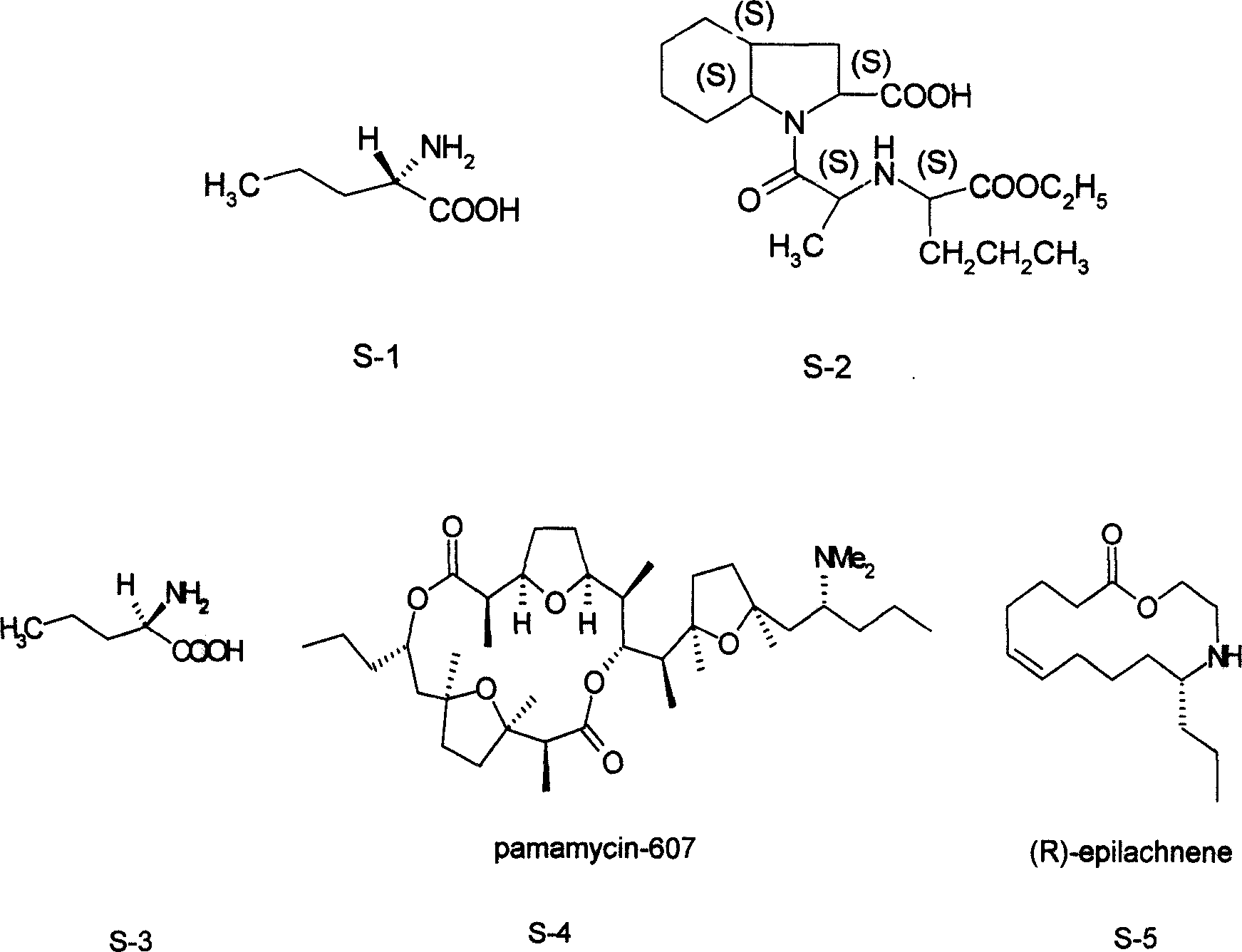
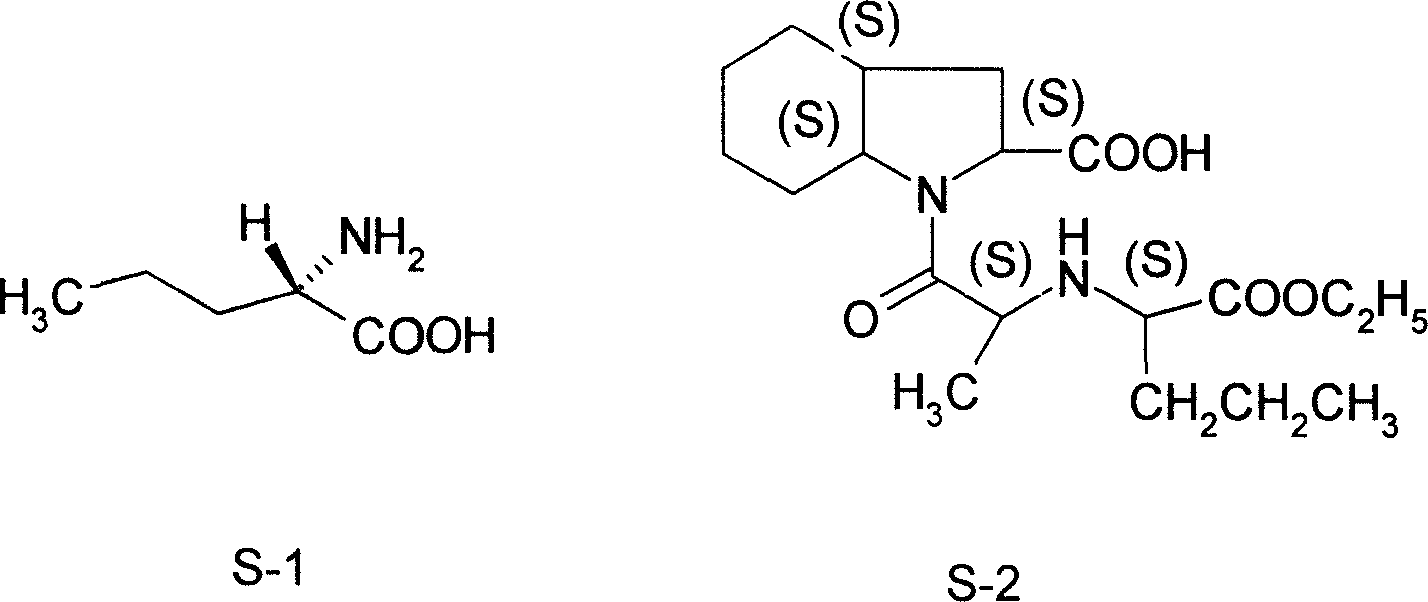
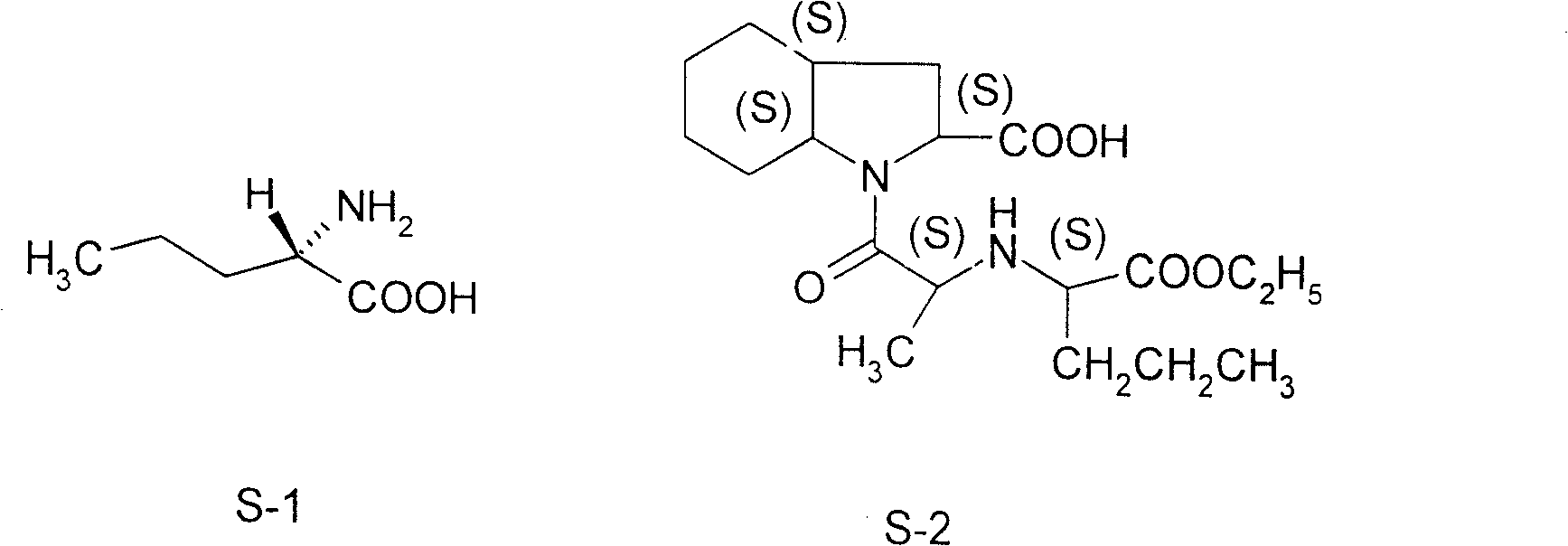
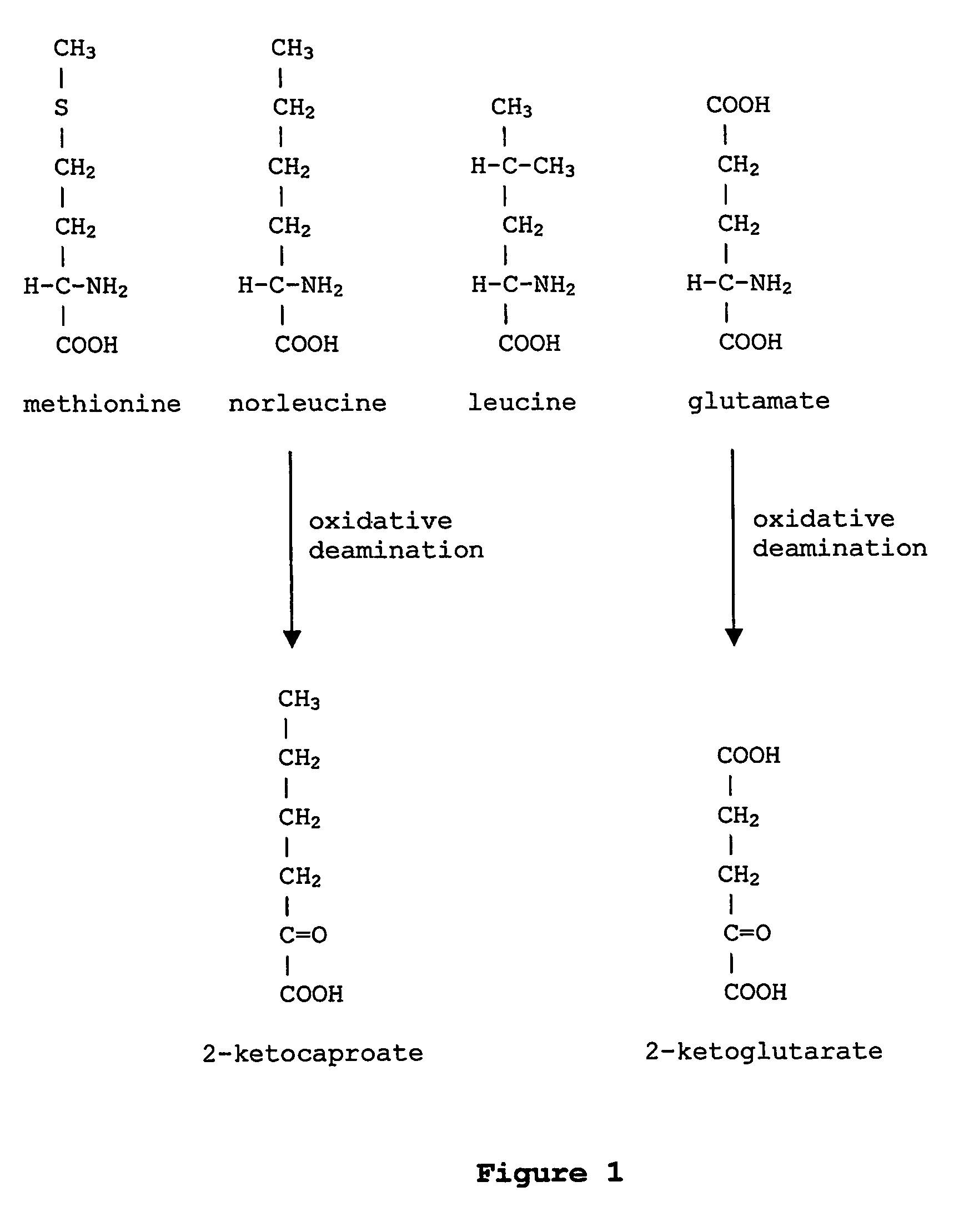
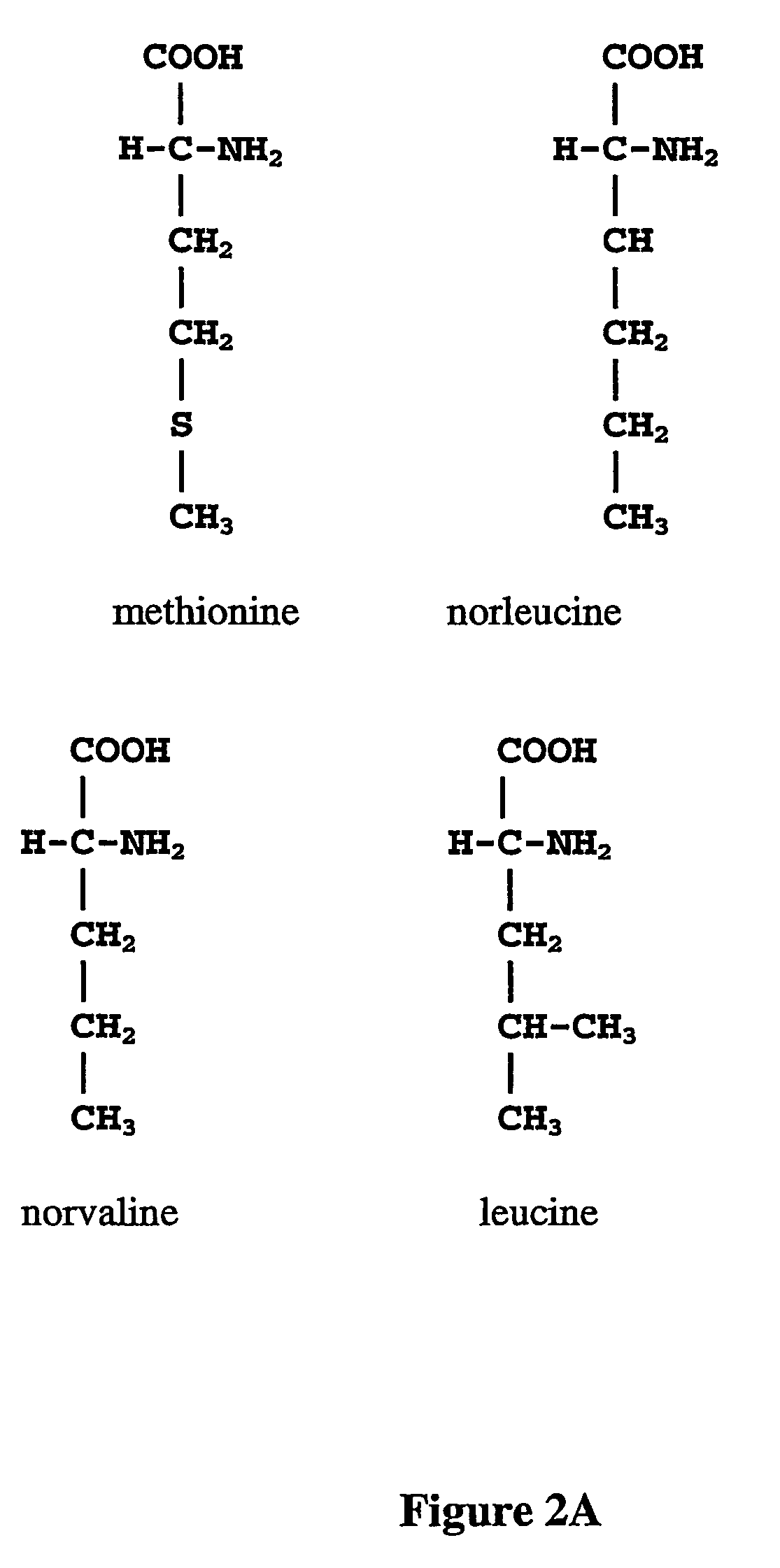
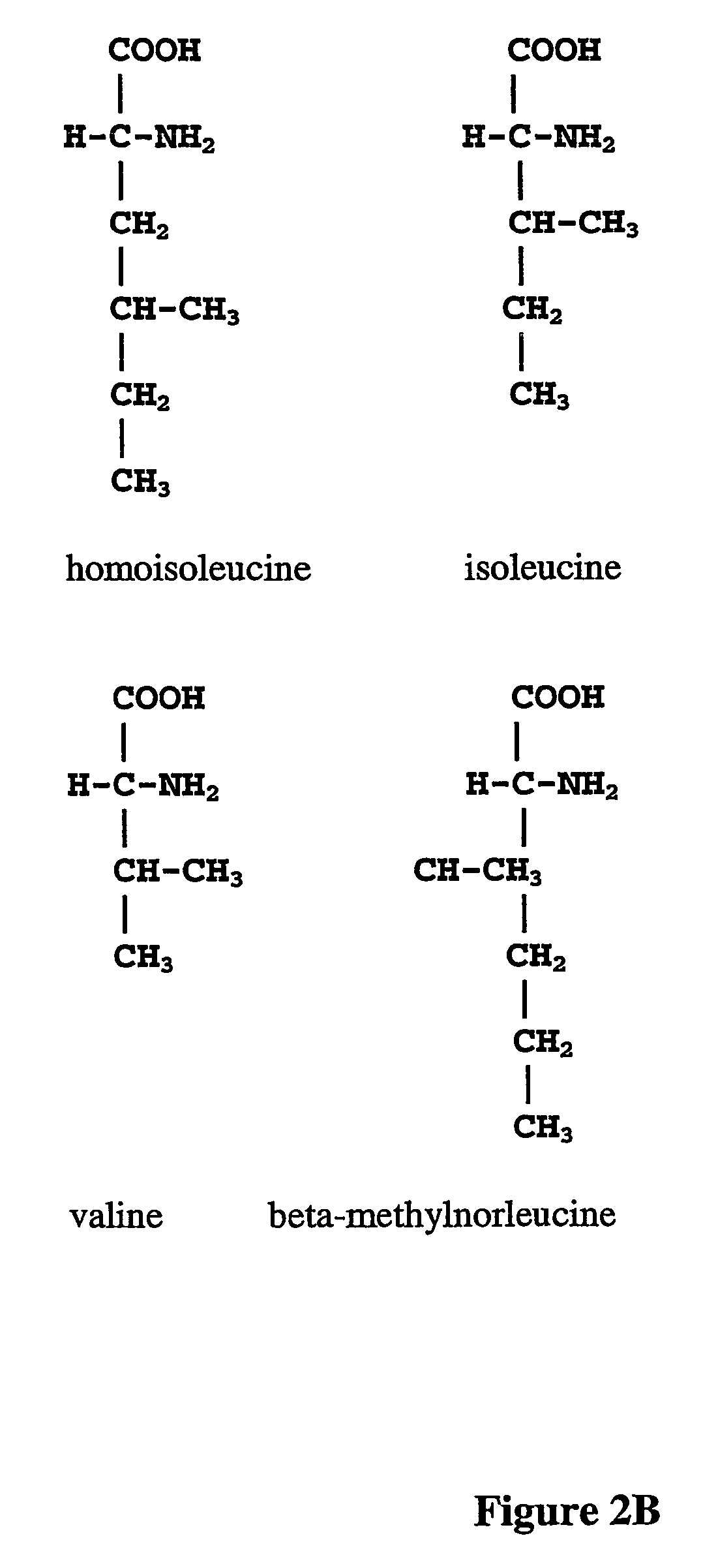
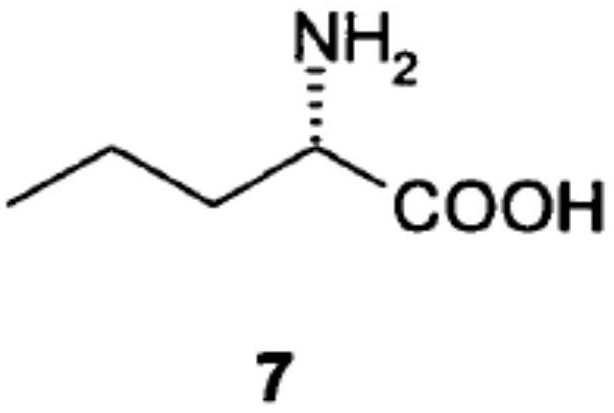
Norvaline (abbreviated as Nva) is an amino acid with the formula CH₃(CH₂)₂CH(NH₂)CO₂H. The compound is an isomer of the more common amino acid valine. Like most other α-amino acids, norvaline is chiral. It is a white, water-soluble solid.
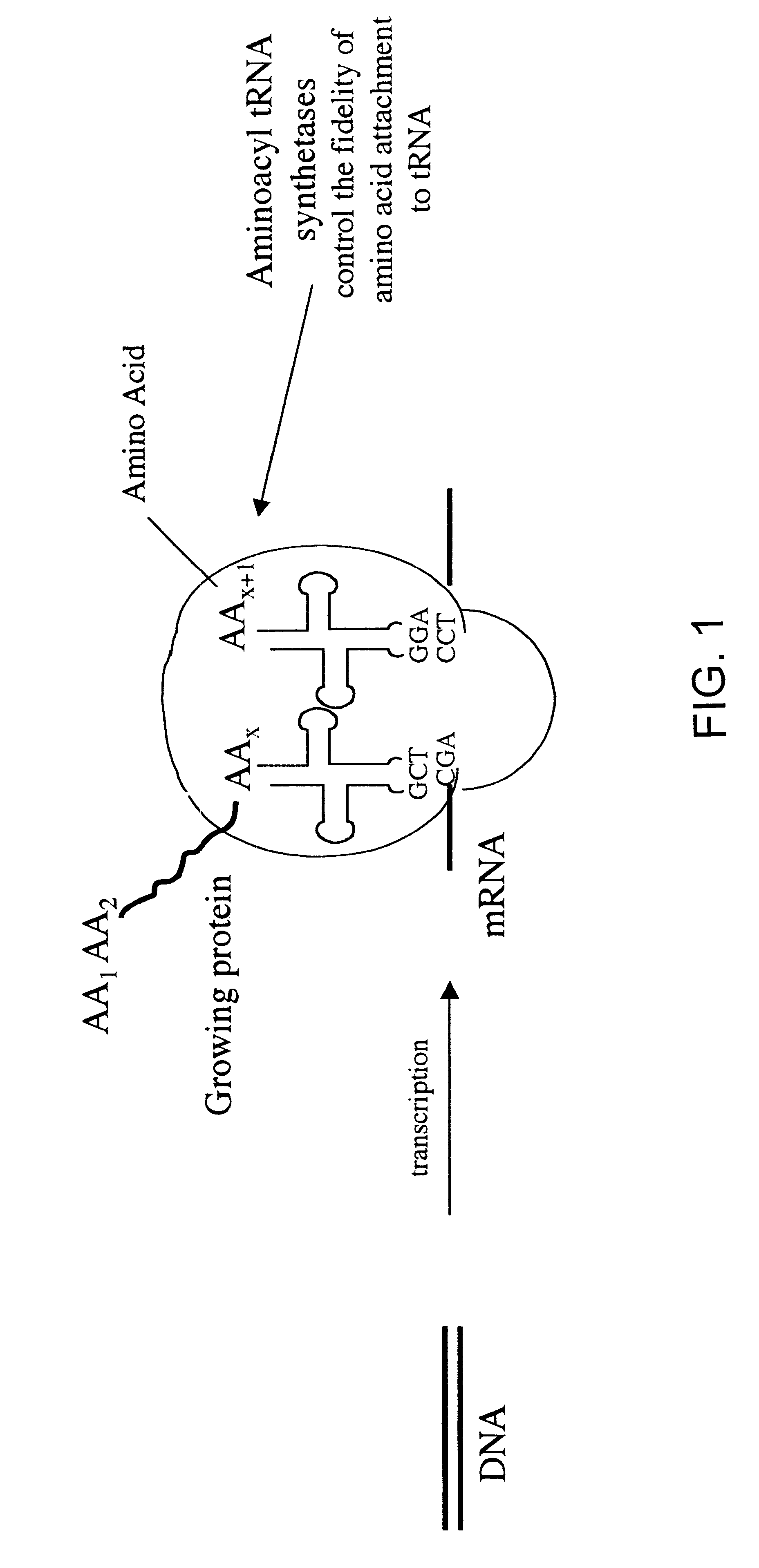
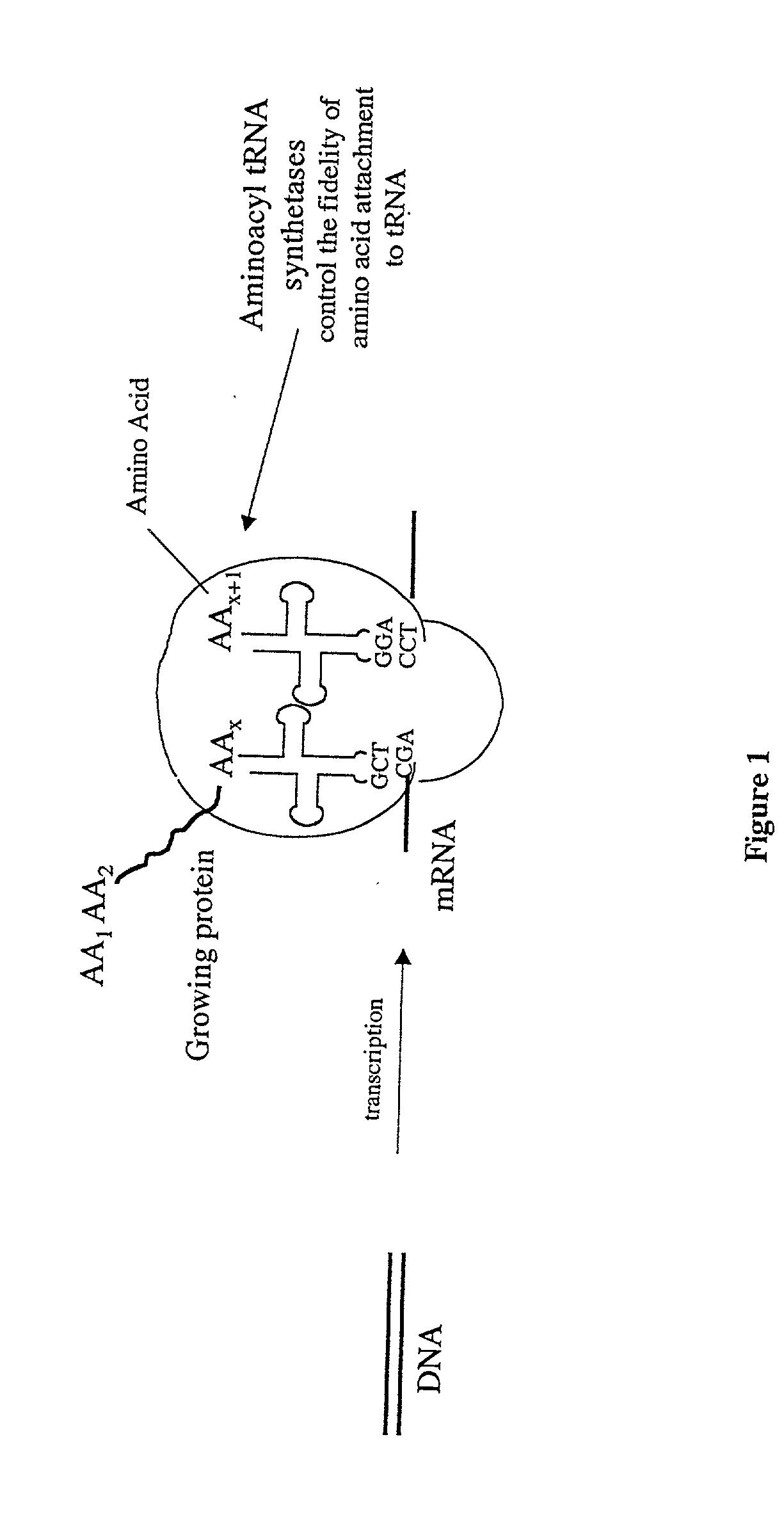
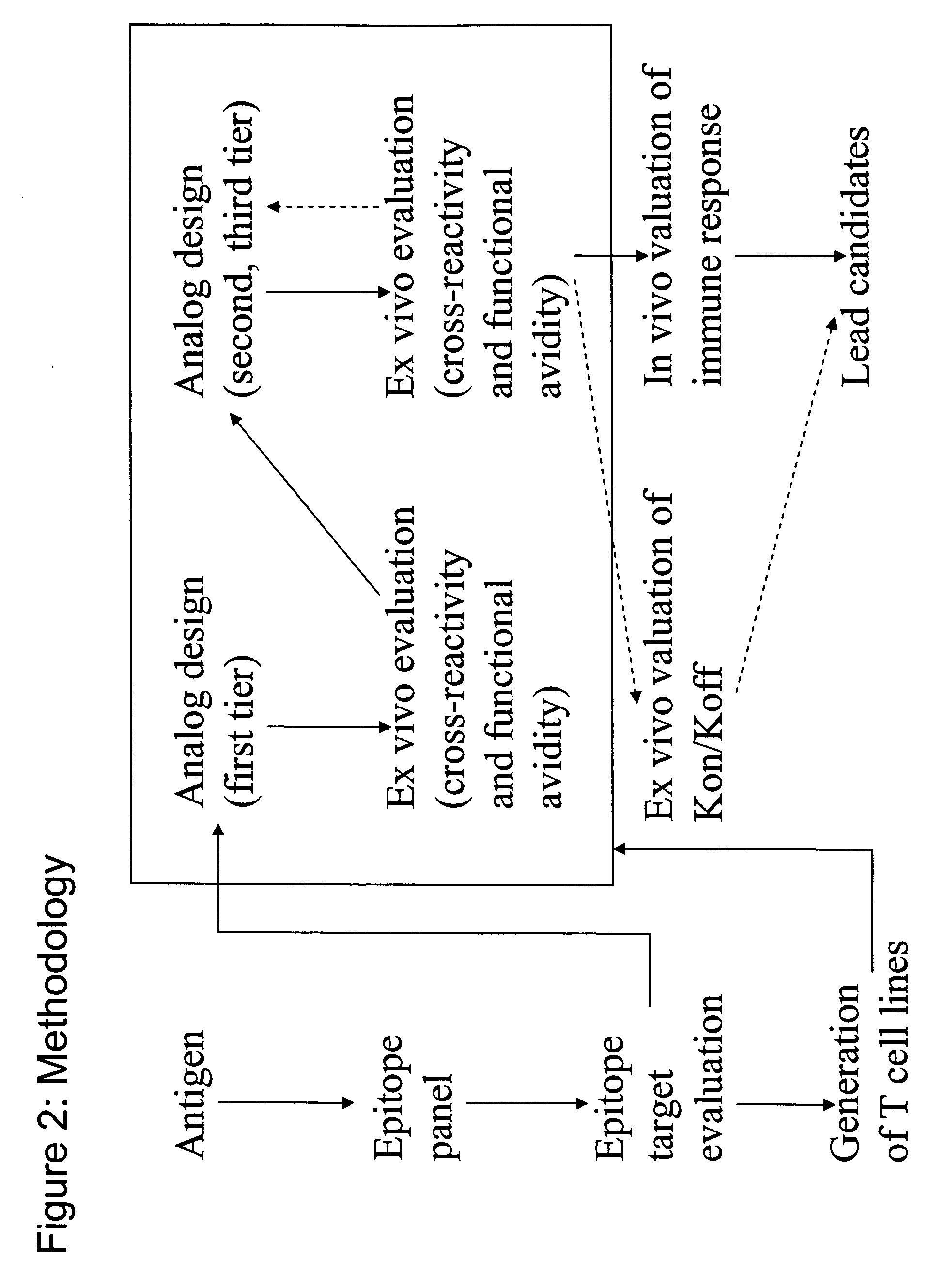
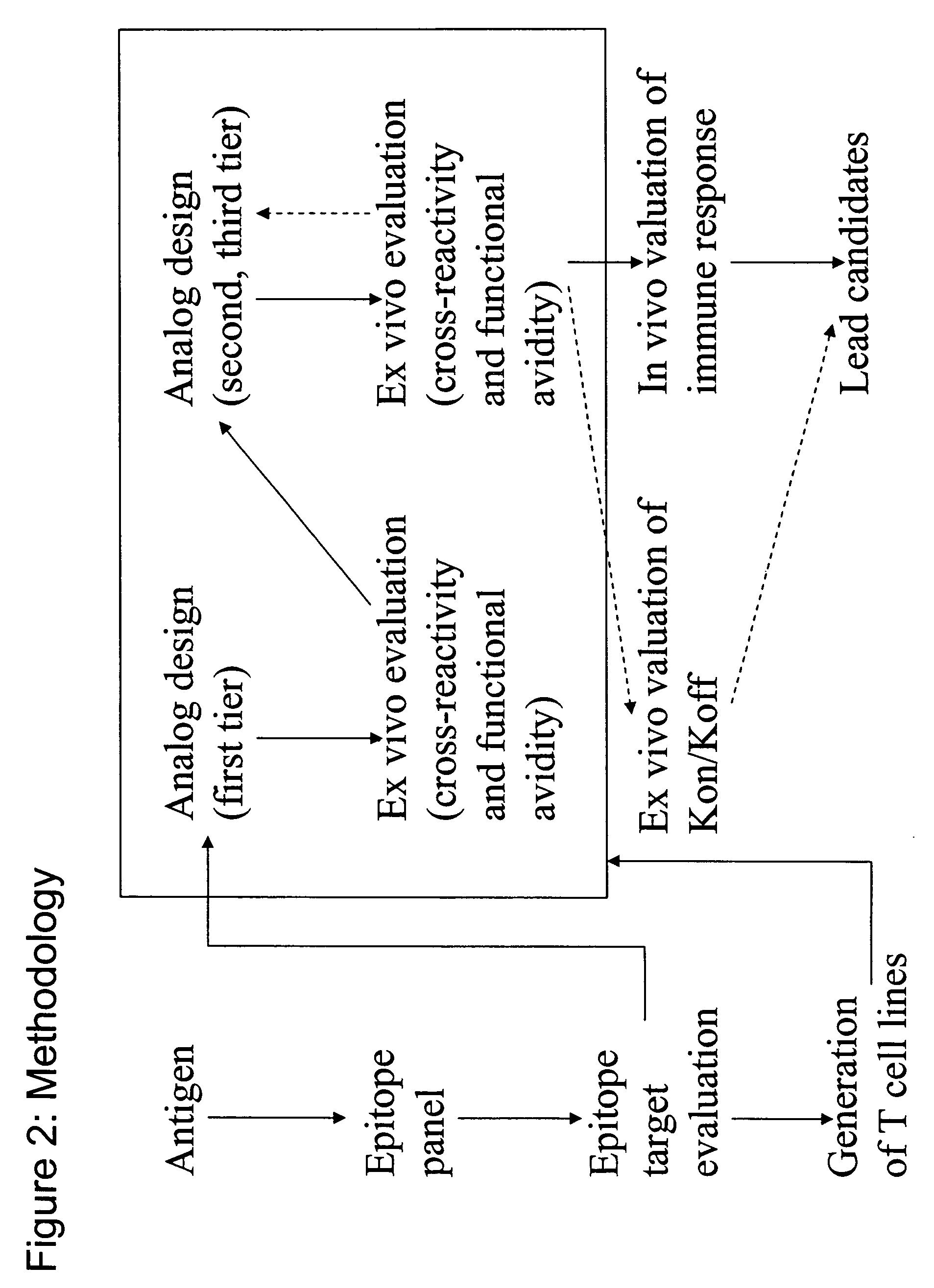
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS6586207B2High yieldRapid and predictable approachBacteriaOxidoreductasesMethionine biosynthesisDihydrofolate reductase
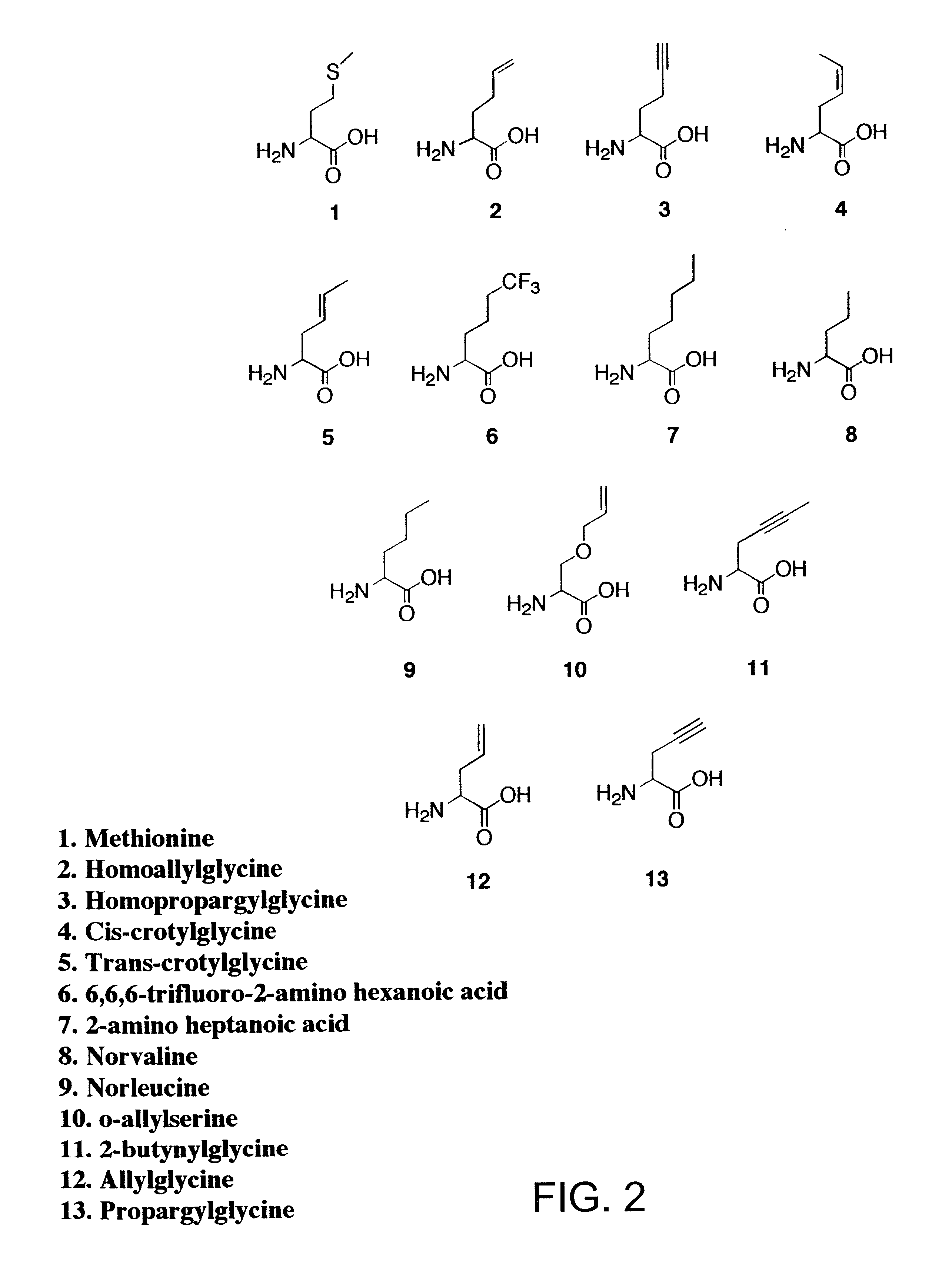
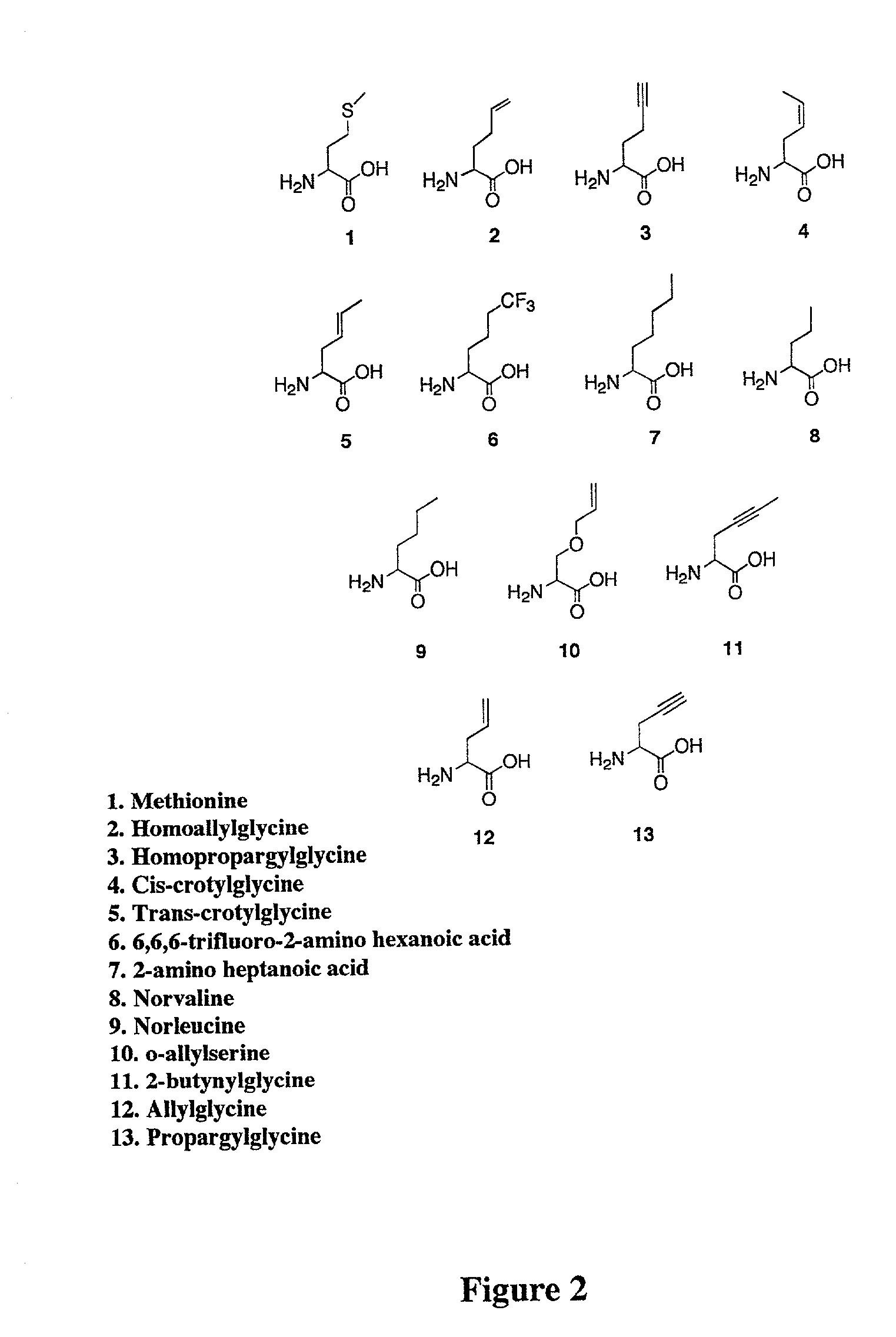
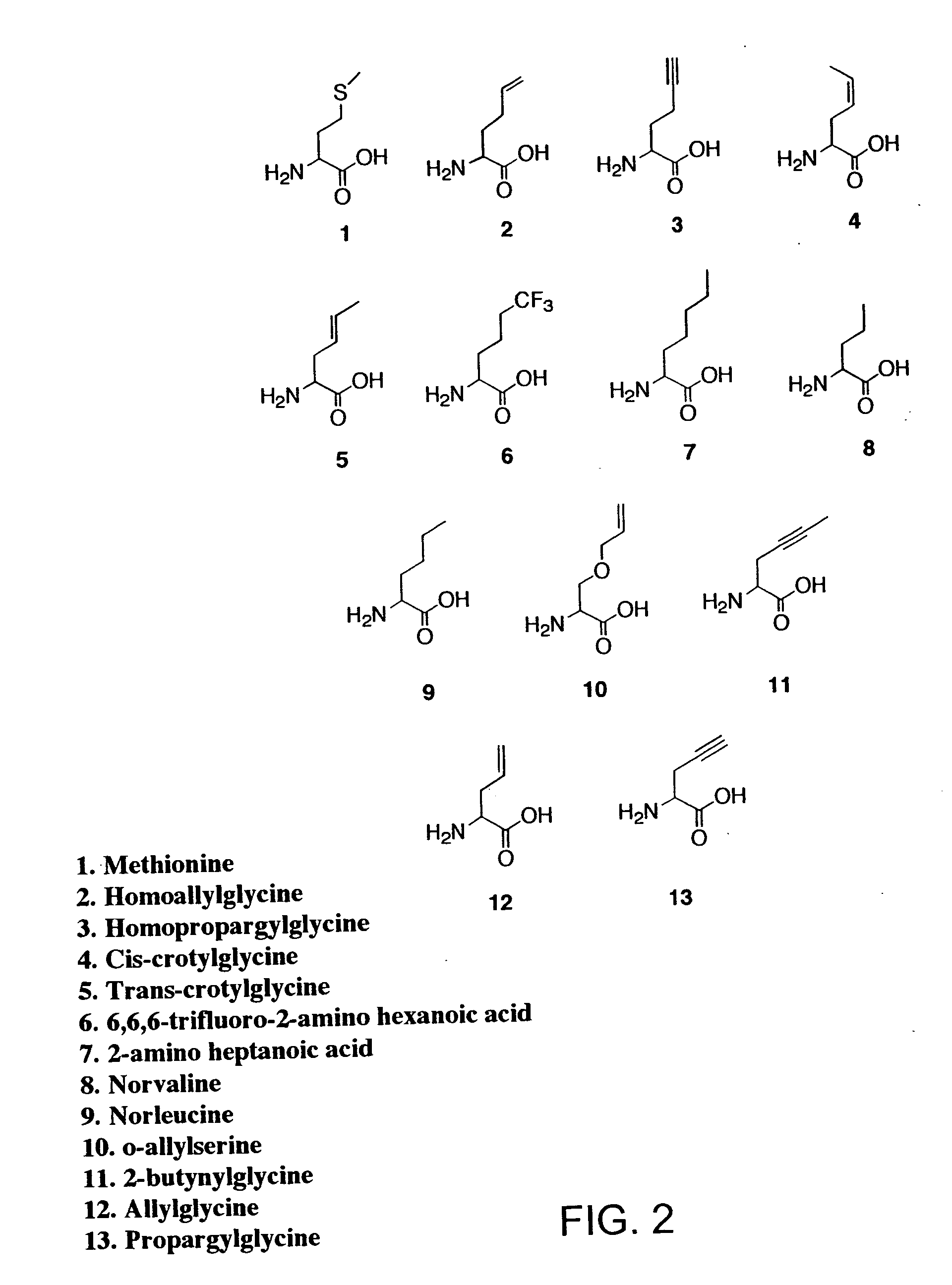
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS20020042097A1High yieldRapid and predictable approachFungiBacteriaMethionine biosynthesisDihydrofolate reductase
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
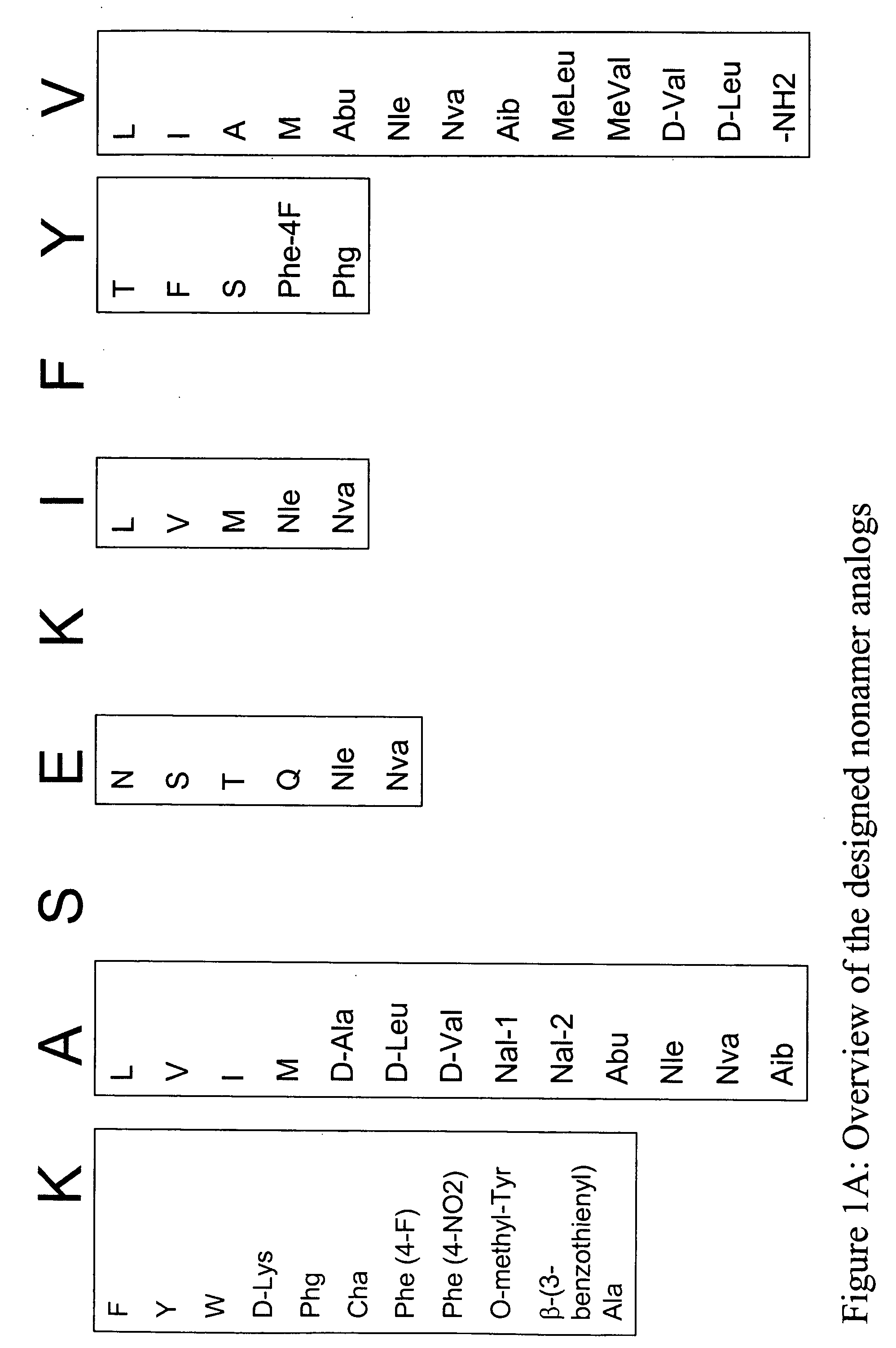
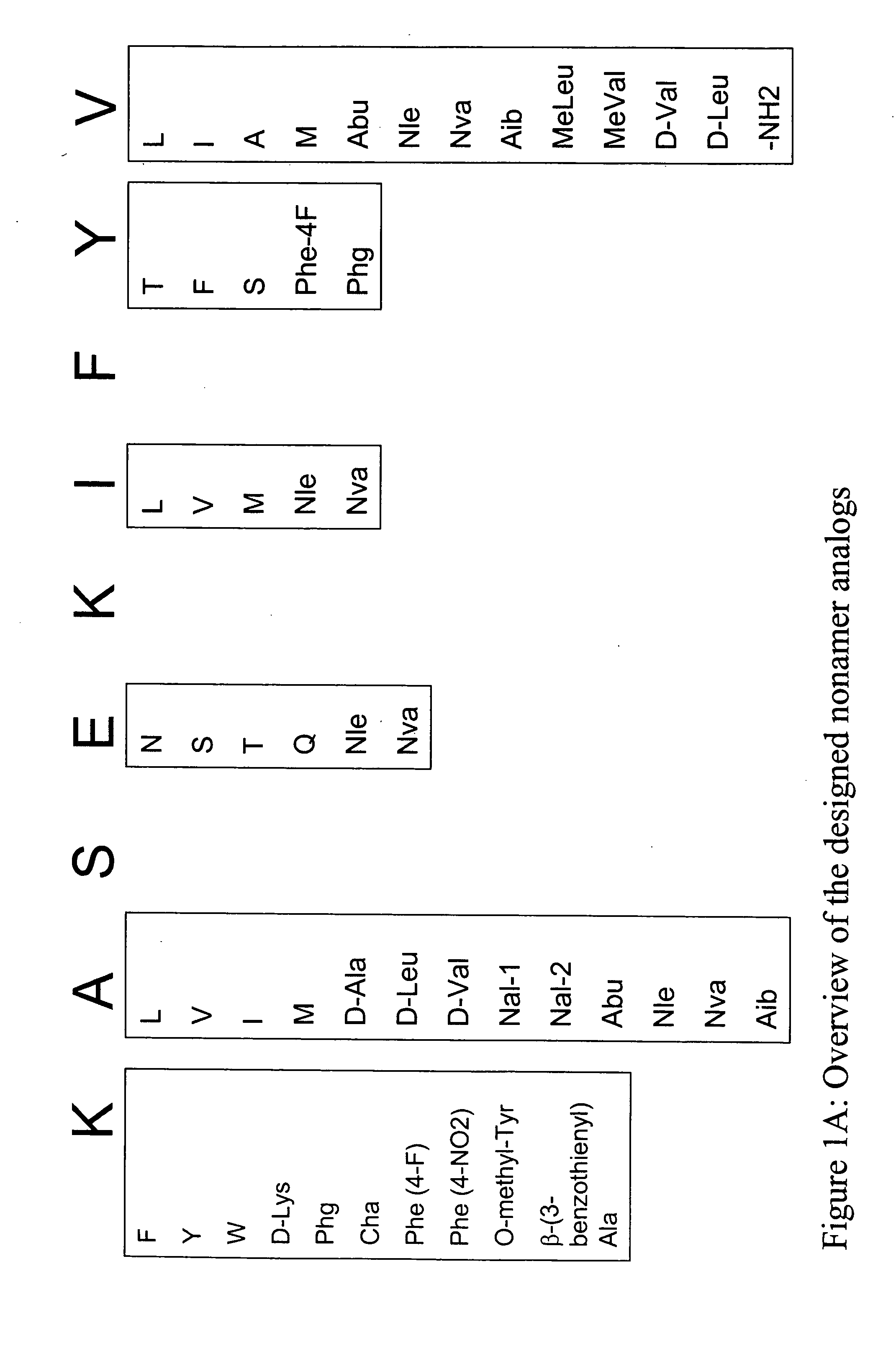
SSX-2 peptide analogs
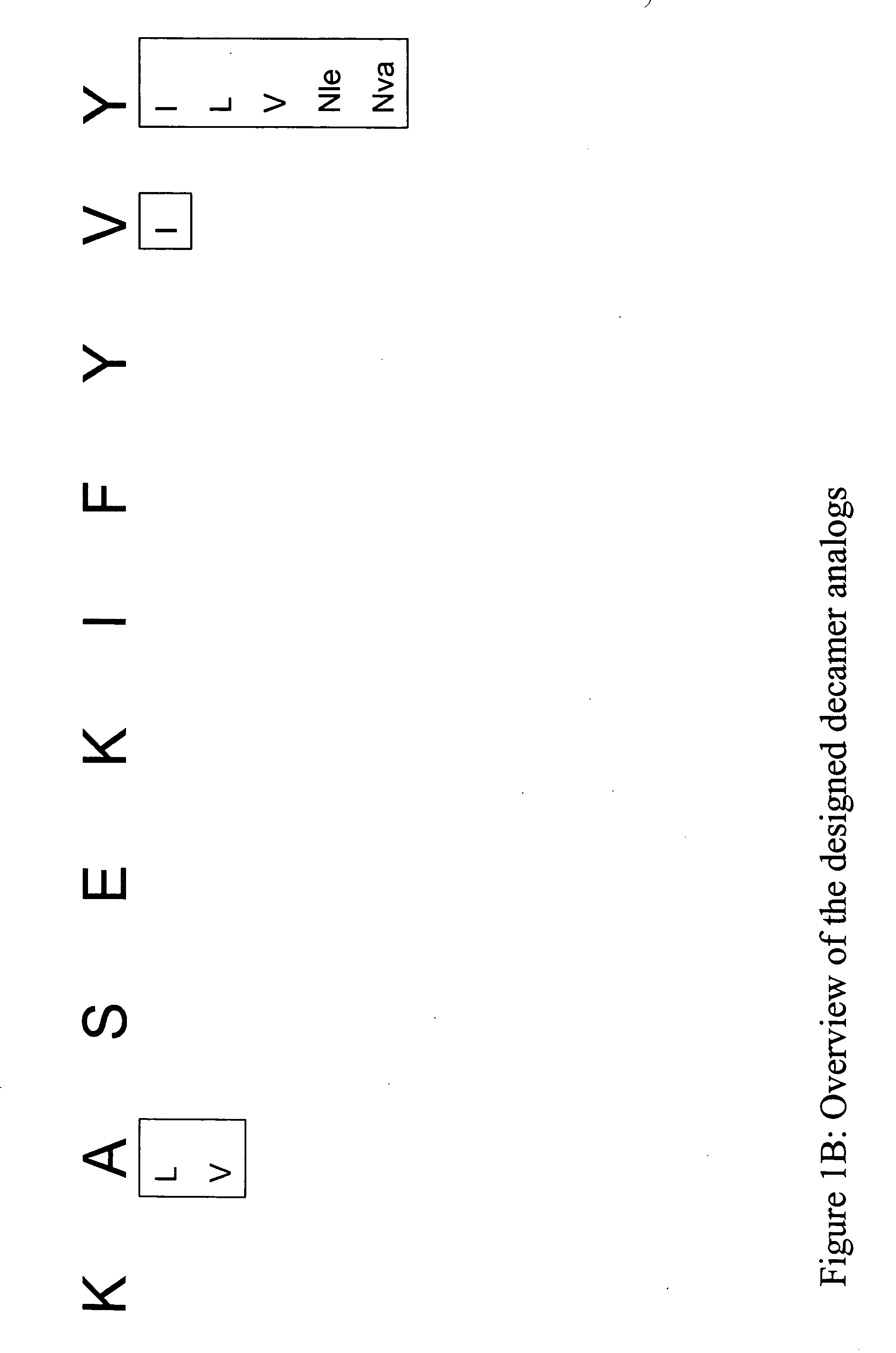
InactiveUS20060063913A1Tumor rejection antigen precursorsPeptide/protein ingredientsAmino acid substitutionAmino acid
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
Prevention of incorporation of non-standard amino acids into protein
ActiveUS20070009995A1Cellular level is reducedReducing, or substantially eliminating, endogenous cellular levels of norleucineBacteriaPeptide/protein ingredientsBeta-methylnorleucinePhenylalanine dehydrogenase
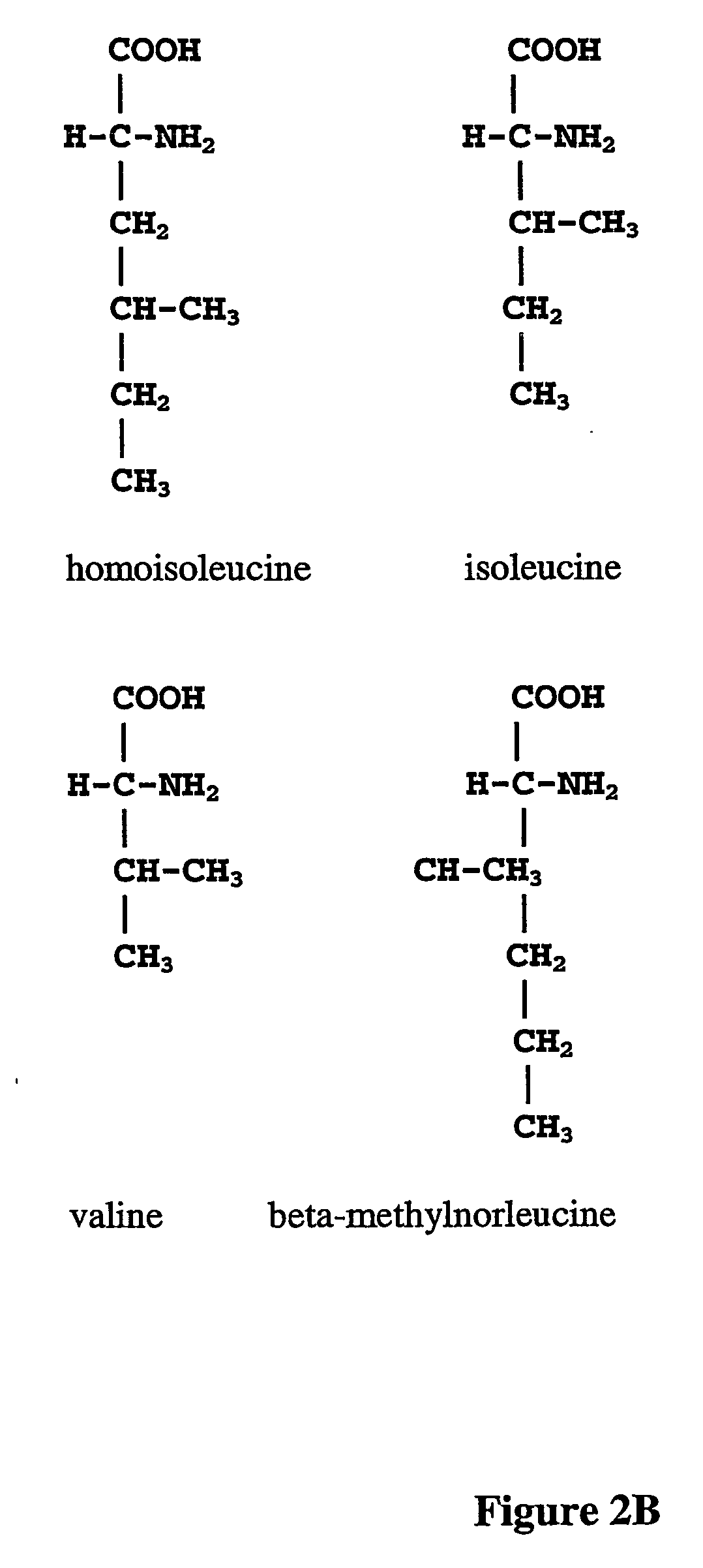
The instant invention is drawn to the methods and compositions necessary to provide recombinant proteins with a substantially reduced or eliminated content of norleucine or other non-standard amino acids. Various embodiments of the invention provide for the substantial elimination of the incorporation of non-standard amino acids into recombinant proteins by the co-expression or enhanced expression of a protein (or the enzymatically active portion thereof) capable of degrading norleucine or other non-standard amino acids, including norvaline, beta-methylnorleucine, and homoisoleucine. In certain particular embodiments of the invention, the norleucine is degraded by a glutamate dehydrogenase, a leucine dehydrogenase, a valine dehydrogenase, a phenylalanine dehydrogenase, a glutamate / leucine / phenylalanine / valine dehydrogenase, or an opine dehydrogenase. Also provided are the cells and DNA constructs for carrying out these methods.
Owner:MONSANTO TECH LLC
Overexpression of aminoacyl-tRNA synthetases for efficient production of engineered proteins containing amino acid analogues
InactiveUS20040058415A1High yieldRapid and predictable approachFungiBacteriaDihydrofolic acidAminoacid analog
Methods for producing modified polypeptides containing amino acid analogues are disclosed. The invention further provides purified dihydrofolate reductase polypeptides, produced by the methods of the invention, in which the methionine residues have been replaced with homoallyglycine, homoproparglycine, norvaline, norleucine, cis-crotylglycine, trans-crotylglycine, 2-aminoheptanoic acid, 2-butynylglycine and allylglycine.
Owner:CALIFORNIA INST OF TECH
Prame peptide analogues
InactiveUS20070060524A1Tumor rejection antigen precursorsPeptide/protein ingredientsMHC restrictionAmino acid substitution
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally, classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
Melanoma antigen peptide analogues
InactiveUS20070060518A1Peptide/protein ingredientsGenetic material ingredientsAntigenMelanoma antigen
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally, classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
PSMA peptide analogues
InactiveUS20070049533A1Tumor rejection antigen precursorsPeptide/protein ingredientsAmino acid substitutionPSMA Peptide
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally, classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
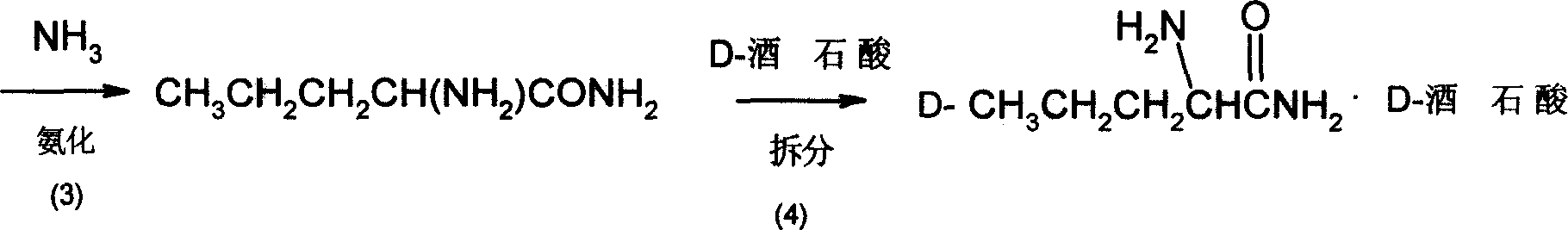
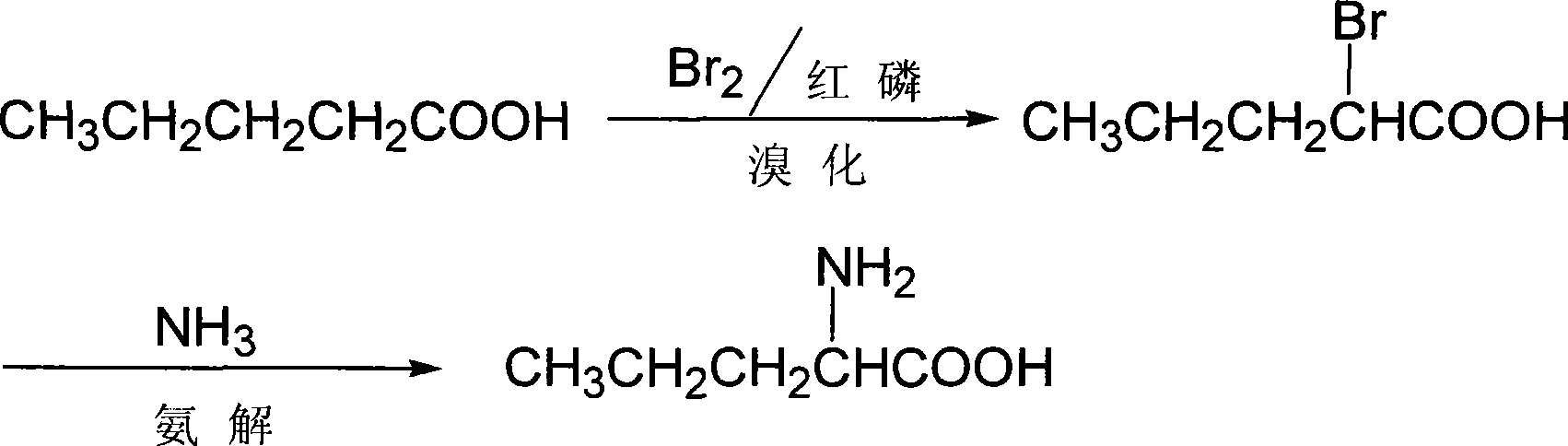
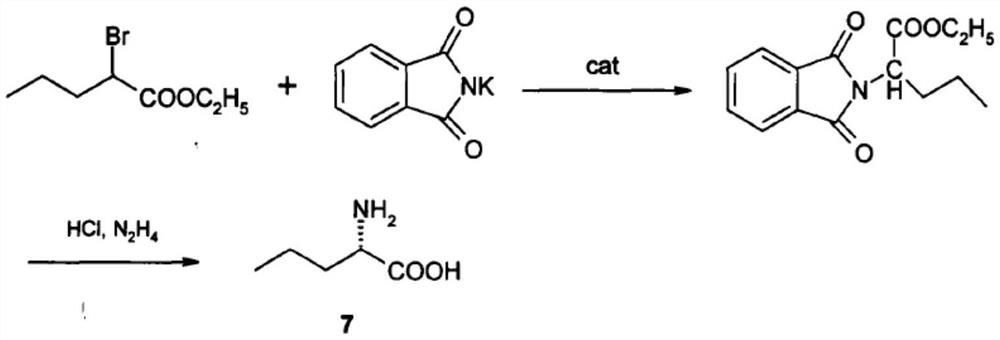
Method for synthesizing D-norvaline using n-pentanoic acid
InactiveCN101007774ASimple production processLow costOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisAmino acid
The invention discloses a synthesizing method of D-norvaline, which comprises the following steps: adopting n-pentatonic acid as main original material; acylating and chlorinating; bromining; ammonifying; detaching; recrystallizing; hydrolyzing.
Owner:ZHEJIANG UNIV
PSMA peptide analogues
InactiveUS7511118B2Tumor rejection antigen precursorsPeptide/protein ingredientsMHC restrictionAmino acid substitution
Some embodiments relate to analogs of peptides corresponding to class I MHC-restricted T cell epitopes and methods for their generation. These analogs can contain amino acid substitutions at residues that directly interact with MHC molecules, and can confer improved, modified or useful immunologic properties. Additionally, classes of analogs, in which the various substitutions comprise the non-standard residues norleucine and / or norvaline, are disclosed.
Owner:MANNKIND CORP
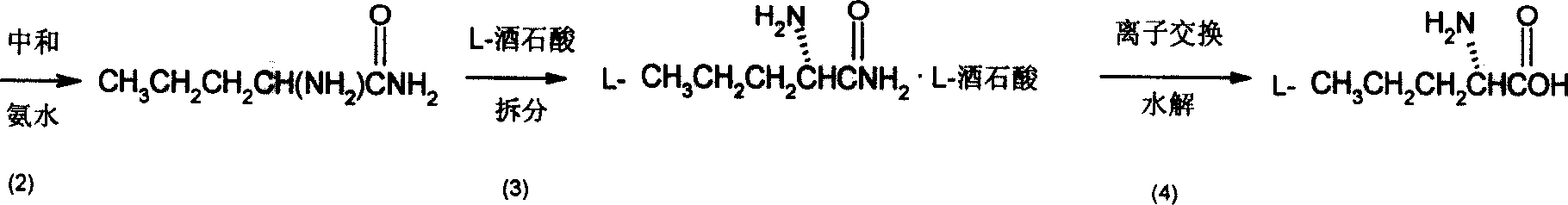
Synthesis method of chiral norvaline
InactiveCN101007772ASimple production processLow costOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisSynthesis methods
Owner:ZHEJIANG UNIV
Method for synthesis of L-norvaline
InactiveCN1962613ASimple production processLow costOrganic compound preparationAmino-carboxyl compound preparationBromineAminopentamide
The invention discloses a synthesizing method of L-norvaline based on valerianic acid as raw material, which comprises the following steps: 1) proceeding acylated chloride reaction for valerianic acid acted by sulfone chloride to obtain valeryl chloride; 2) reacting valeryl chloride and liquid bronmine; removing sulfone chloride and bromine to obtain the rough product of alpha-bromovaleryl chloride; 3) dissolving alpha-bromovaleryl chloride in the solvent to ammonolyze; washing; condensing to obtain racemic alpha-aminovaleramide; 4) detaching the racemic alpha-aminovaleramide to obtain L-aminovaleramide tartrate; 5) recrystallizing; 6) hydrolyzing.
Owner:ZHEJIANG UNIV
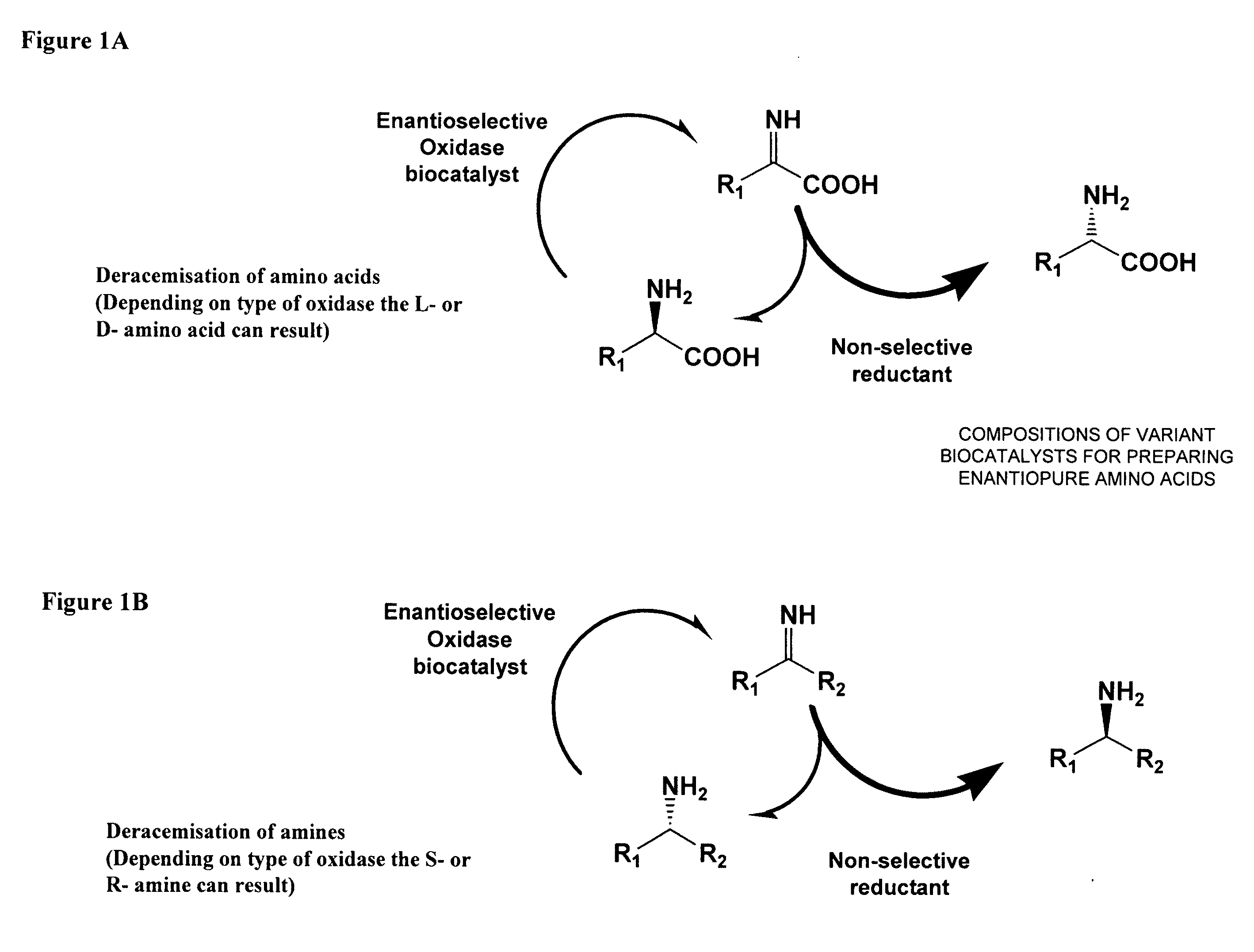
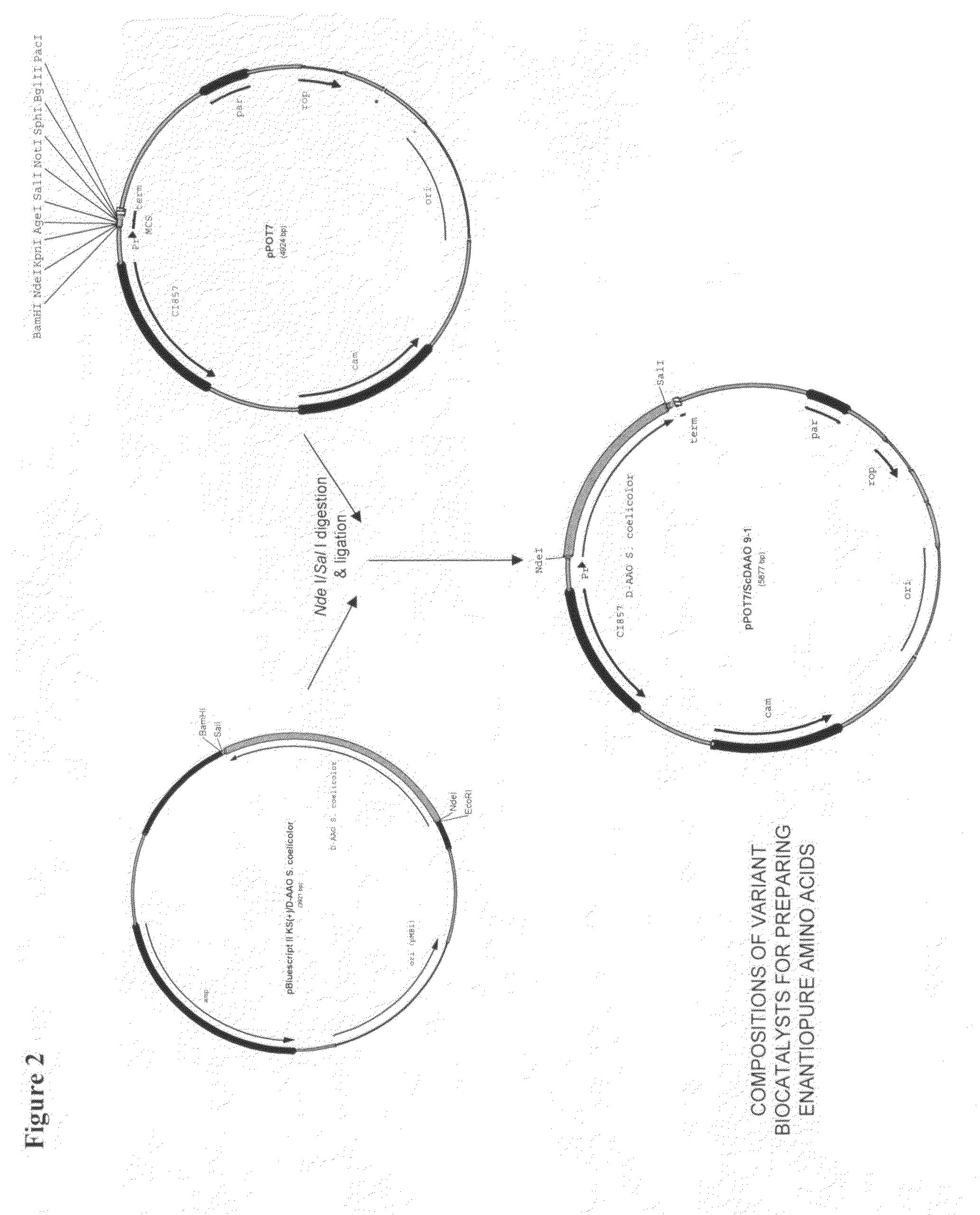
Compositions of variant biocatalysts for preparing enantiopure amino acids
InactiveUS20110059503A1Increased biocatalytic activityHigh activityOxidoreductasesFermentationPenicillamineEnantio selectivity
A composition of variant biocatalysts, specifically variants of D-amino acid oxidases, with improved biocatalytic activity towards D-amino acid substrates such as, but not limited to, D-tert-leucine, D-norvaline, D-2-aminobutyrate, D-alanine, D-isoleucine, D-valine, D-methionine, D-hydroxyadamantlyglycine, D-penicillamine, or D-norleucine is disclosed. Further disclosed is a method of preparing enantioselective amino acids using variant D-amino acid oxidases of the present invention.
Owner:RICHMOND CHEM CORP
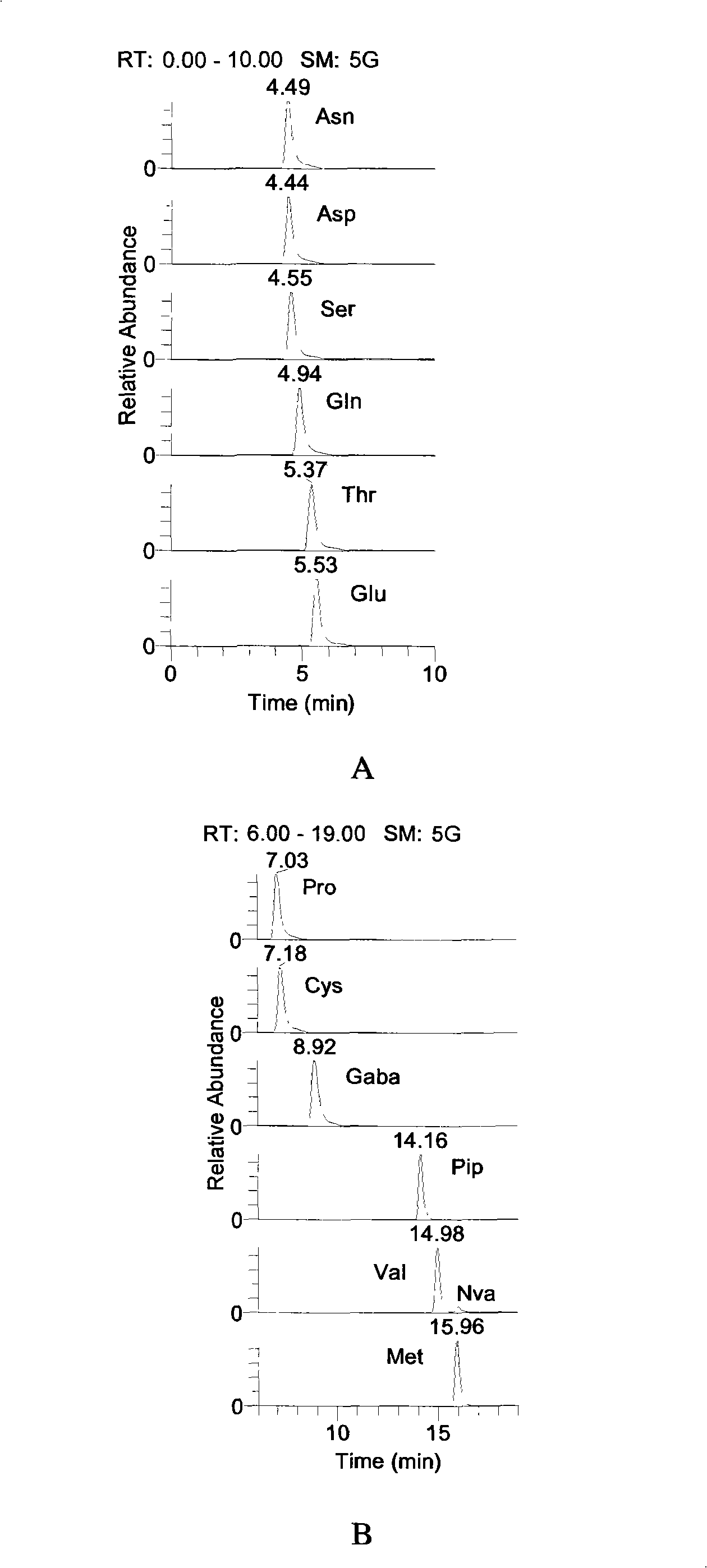
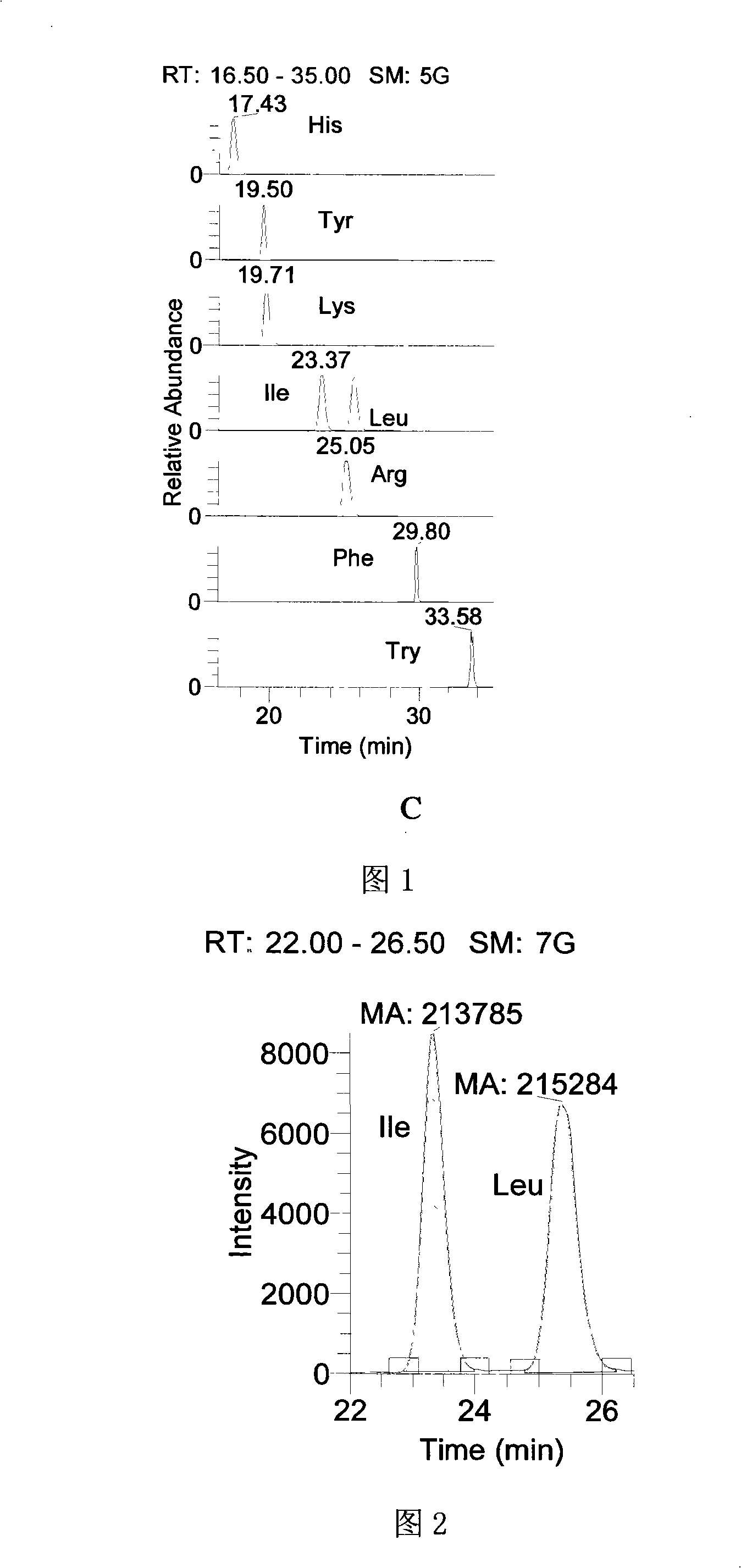
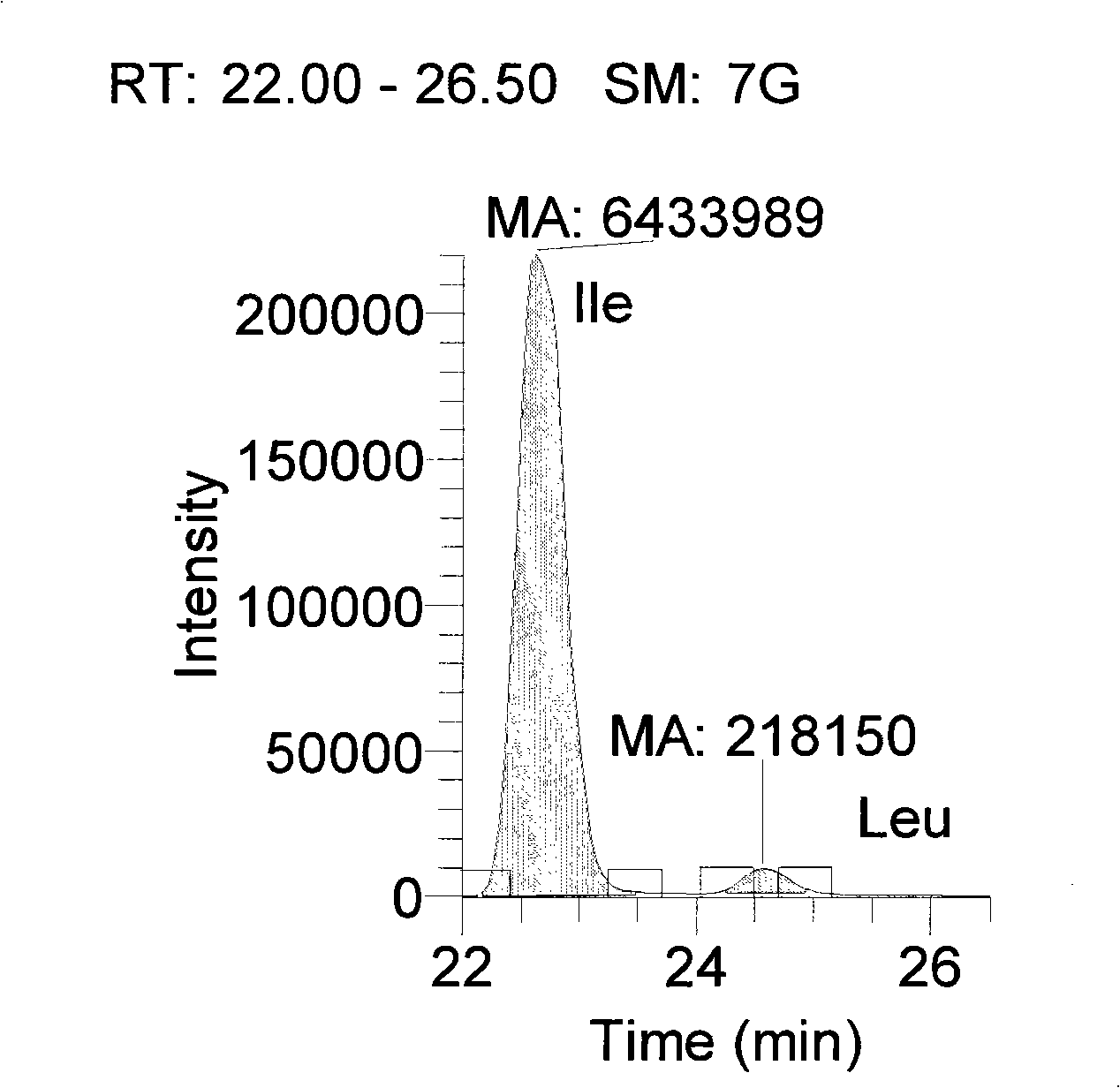
Preparation method of tobacco raffinate used for amino acid analysis
ActiveCN101344508AGood peak shapeOptimum chromatographic run timeComponent separationPost column derivatizationMass Spectrometry-Mass Spectrometry
The invention discloses a preparation method of a tobacco extracting solution that is used for amino acid analysis, takes HCl solution that contains norvaline as extracting solution, extracts tobacco ashes under ultrasonic oscillation, and obtains the extracting solution by filtering. The extracting solution is combined with a coupling technique of a liquid chromatogram and an electrospray tandom mass spectrometry, thus providing a complete experimental proposal which avoids fuzzy pre-column and post-column derivatization operations of tobacco samples, obtains good chromatogram peak form and proper chromatogram running time in the experiment, further leads the isomerides Leu and Ile to reach baseline separation, and consequently obtains accurate analyzing result.
Owner:CHINA TOBACCO GUANGDONG IND
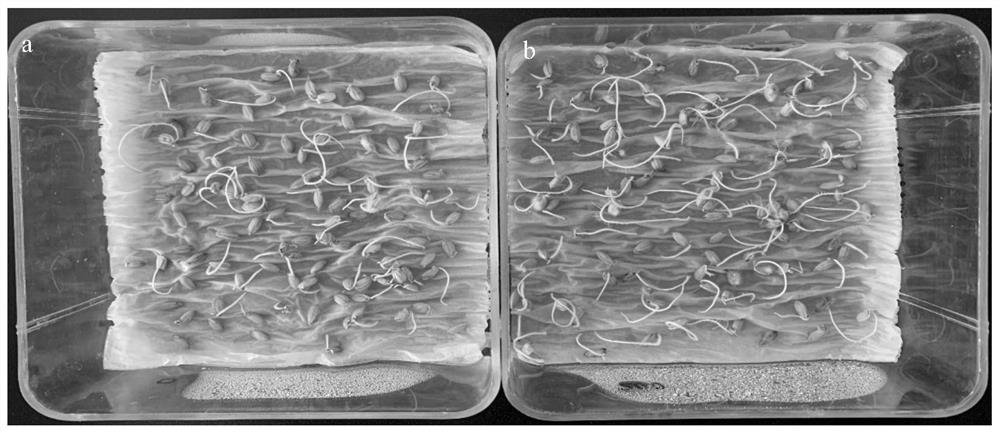
Method for inducing rice to improve salt tolerance
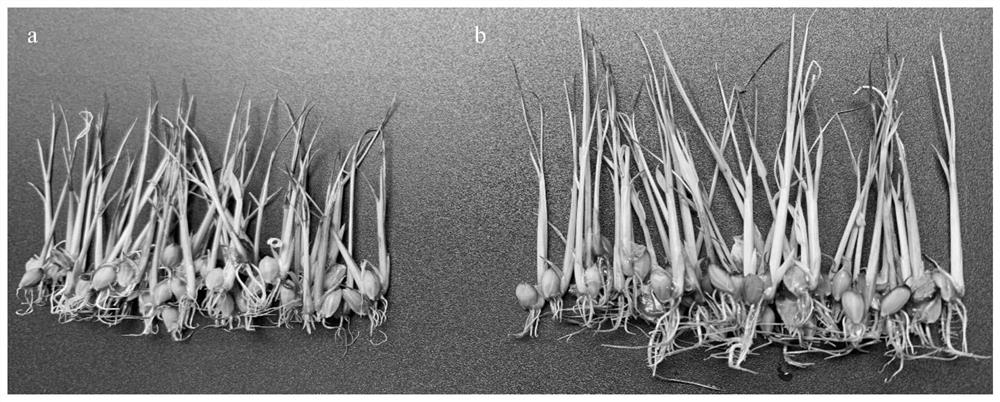
ActiveCN112020930AImprove salt toleranceImprove germination rateBiocidePlant growth regulatorsBiotechnologyDisease resistant
The invention discloses a method for inducing rice to improve salt tolerance, and belongs to the technical field of rice planting. The method comprises the following steps of 1, disinfecting the surfaces of rice seeds, and then dissolving 5-hydroxy-L-norvaline with a 0.3-1 wt.% NaCl solution to prepare a 5-hydroxy-L-norvaline solution with the concentration of 20-400 mg / L; and 2, soaking the riceseeds in the 5-hydroxy-L-norvaline solution prepared in the step 1 at the temperature of 20-28 DEG C for 16-24 hours to obtain treated rice seeds, and enabling the treated rice seeds to normally germinate and grow in a salt stress environment. According to the method, the exogenous 5-hydroxy-L-norvaline is used for soaking the rice seeds and irrigating, so that the germination rate and the growthrate of the induced rice seeds can be improved under the salt stress condition, and the rice can be effectively induced to improve the salt tolerance; and the method can meet the urgent requirements of coastal beaches in China for high-yield, high-quality, disease-resistant, salt-resistant and direct-seeding rice varieties, and has very high application potential and value.
Owner:JIANGSU COASTAL AREA AGRI SCI RES INST
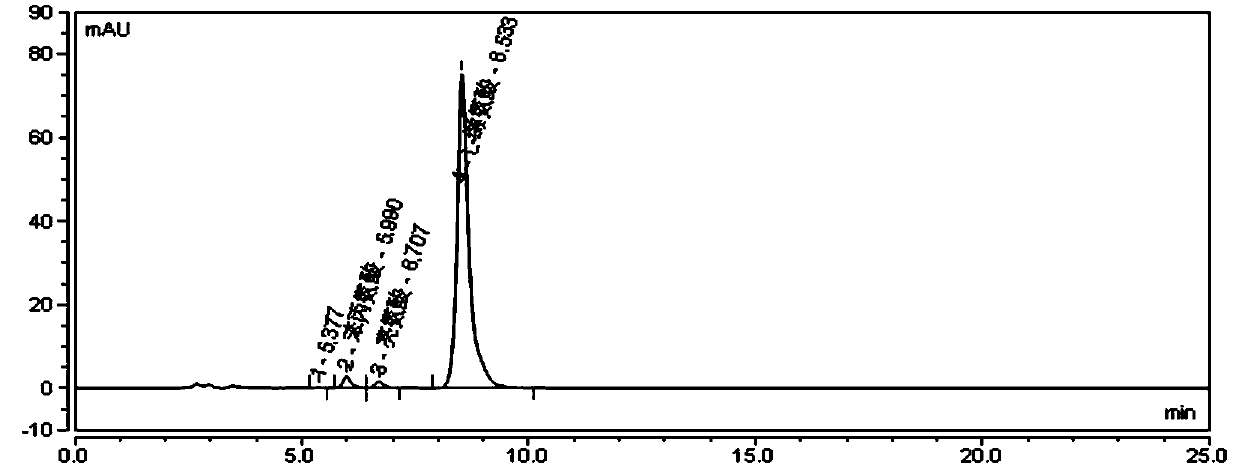
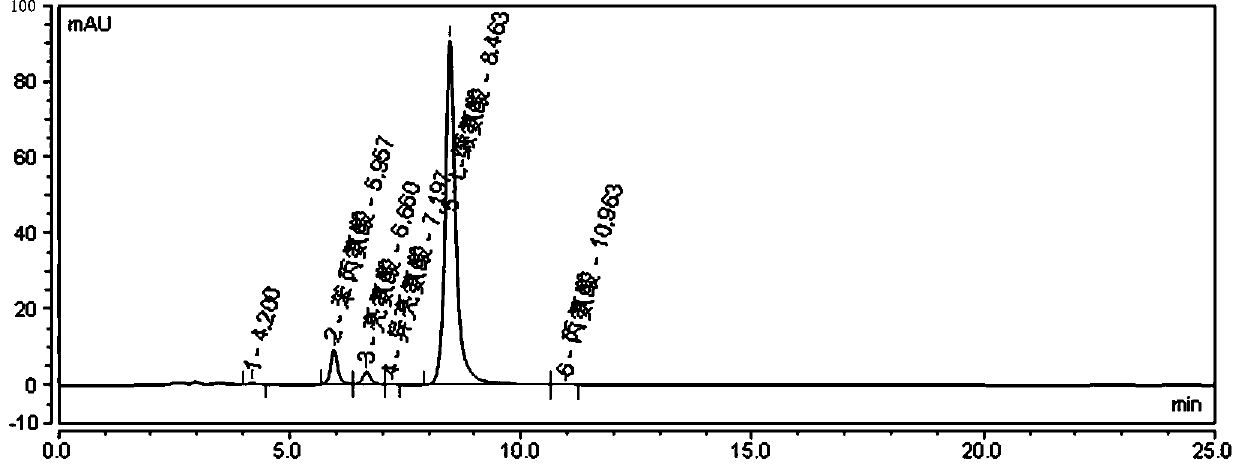
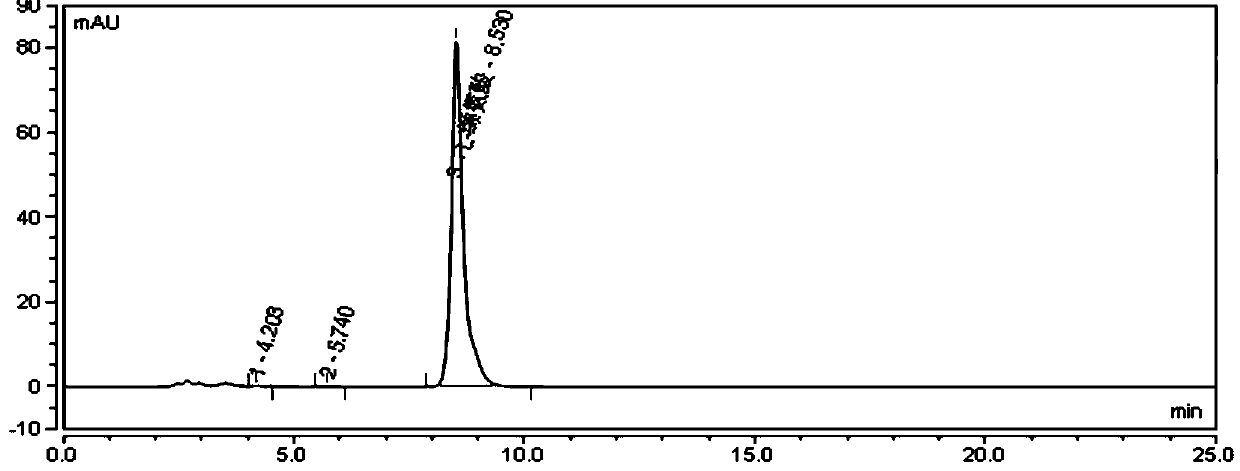
Method for determining other amino acids in L-valine raw material by high performance liquid chromatography
ActiveCN111505151ASolve the problem of short service life and unstable baseline of reversed phaseEasy to separateComponent separationSilica gelPhenylalanine
The invention provides a method for determining other amino acids in an L-valine raw material by high performance liquid chromatography. The method comprises the following steps of preparing L-valine,leucine, isoleucine, glycine, alanine and phenylalanine reference substance solutions; detecting through a high performance liquid chromatograph, comparing the appearance time and peak shape of L-valine, leucine, isoleucine, glycine, alanine and phenylalanine in a chromatogram of the test sample with those of L-valine, leucine, isoleucine, glycine, alanine and phenylalanine in a chromatogram of the reference substance solution, and judging whether the test sample solution contains other amino acids or not. According to the method, by screening an aminopropyl bonded silica gel chromatographiccolumn, the separation effects are found, and by screening the wavelengths, the maximum absorption of each amino acid is found, and the retention time of each amino acid peak on the chromatographic column is regulated and controlled by regulating the ratio of triethylamine, the pH value and the ratio of two phases by utilizing an acetonitrile / phosphate buffer solution binary mobile phase, so thatthe hydrolysis and shedding of bonded aminopropyl in the amino column are effectively prevented, and the problems of short reverse-phase service life and unstable baseline of the amino column are solved.
Owner:宜昌三峡普诺丁生物制药有限公司
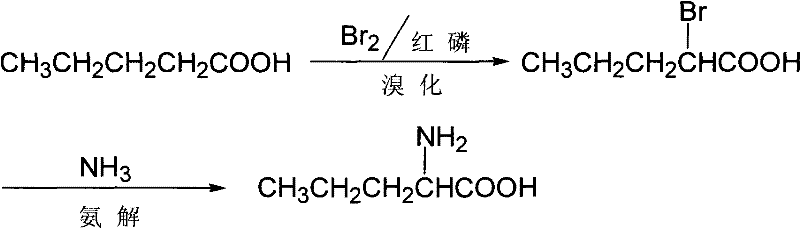
Synthesis of D,L-norvaline
InactiveCN101508654ARich sourcesSimple processOrganic compound preparationAmino-carboxyl compound preparationHexamethylenetetramineIon exchange
The invention discloses a method for synthesizing D, L-norvaline with n-pentanoic acid as a main initial raw material, orderly comprising the following steps: 1) bromo reaction: n-pentanoic acid is reacted with liquid bromine to form alpha-bromo n-pentanoic acid; 2) ammonolysis: the obtained alpha-bromo n-pentanoic acid is firstly neutralized by strong aqua ammonia under the protection of nitrogen and under ice bath cooling, and then is ammonolyzed for 0.5 to 12 hours at a temperature between 40 and 90 DEG C by using ammonia water or ammonia gas as an ammonolysis reagent and hexamethylenetetramine as a catalyst; then the obtained suspension is filtered, and the filtering cake is washed by methanol or ethanol and dried to form the D, L-norvaline; and 3) ion exchange to separate and recycle: the obtained filtrate in the step of ammonolysis and cation exchange resin are subjected to ion exchange, and materials on the resin are eluted with weak aqua ammonia, decolored, dewatered, washed by methanol or ethanol and dried to form the D, L-norvaline. The D, L-norvaline obtained in step 2) and step 3) is combined, and the ammonolysis yield is over 95 percent. The method for synthesizing the D, L-norvaline has advantages of simple process, few reaction steps, high yield, low cost, short production period and the like. The obtained D, L-norvaline can be further subjected to chemical resolution or enzymatic resolution to form chiral norvaline.
Owner:吕亮
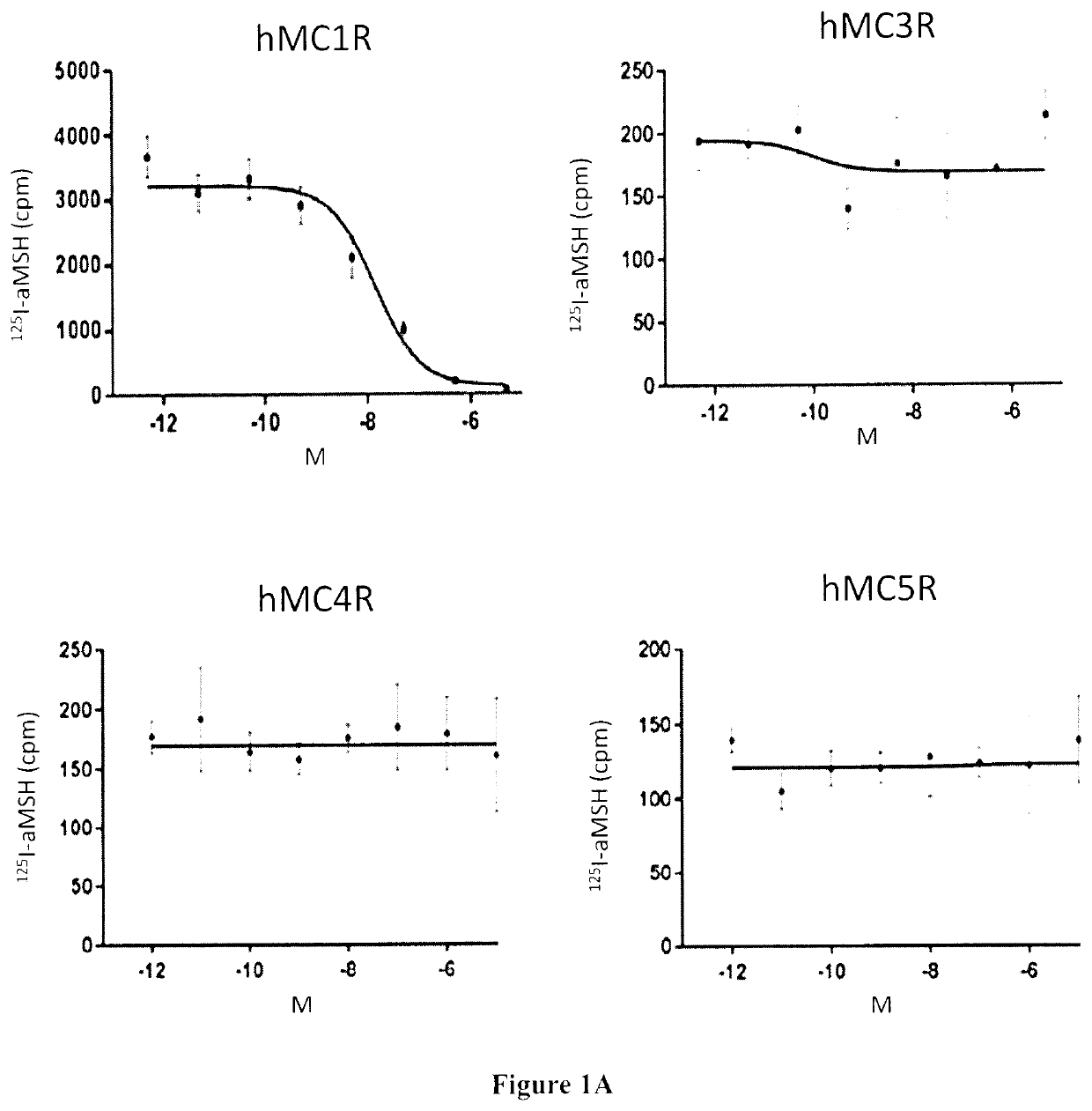
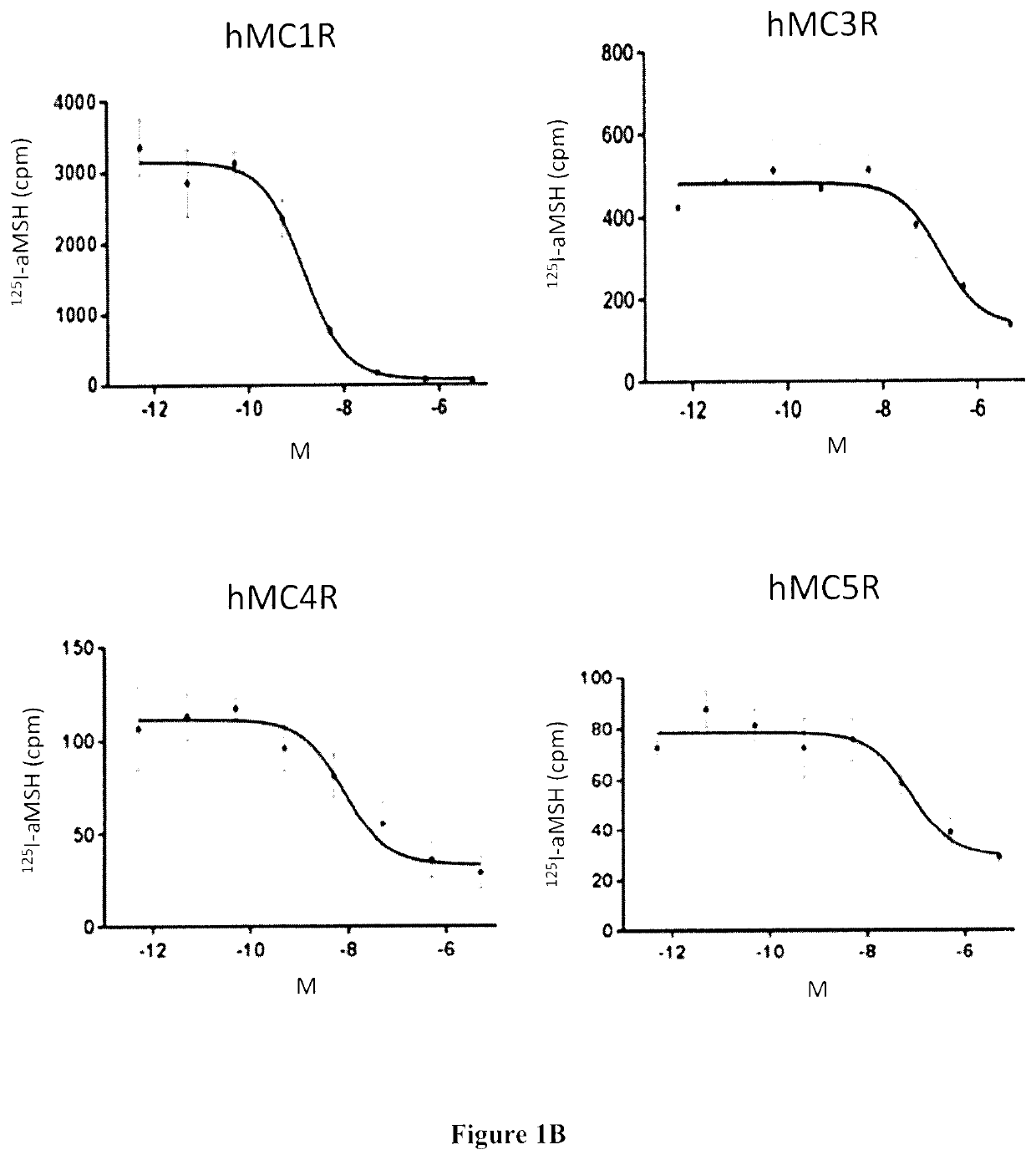
Radiolabeled melanocortin 1 receptor-specific alpha-melanocyte-stimulating hormone analogues for imaging or therapy
ActiveUS11395857B2Organic non-active ingredientsRadioactive preparation carriersTarget peptideMelanocortin receptor
A compound is provided comprising a melanocortin 1 receptor (MC1R) targeting peptide (MC1RTP), a radiolabeling group, and a linker joining the MC1RTP to the radio labeling group. The MC1RTP is linear or cyclized, and comprises a sequence of Formula I or Formula II: Xaa1-Xaa2a-Xaa3-Xaa4-Xaa5-Xaa6-Xaa7a (I) or Xaa1-Xaa2b-Xaa3-Xaa4-Xaa5-Xaa6-Xaa7b (II). Xaa1 is L- / D-Nle, L- / D-Nle, L- / D-Ala, L- / D-Leu, L- / D-Ile, D-Ile, L- / D-Cys, L- / D-Met, L- / D-Phe, L- / D-Trp, L- / D-Val, L- / D-Nal, L- / D-2-Nal, Gly, L- / D-α-aminobutryic acid, L- / D-norvaline, or L- / D-homonorleucine. Xaa2a and Xaa7b are L- / D-Cys, L- / D-Asp, L- / D-Glu, L- / D-2-Aad, L- / D-3-Aad, L- / D-Pra, L- / D-Hpg, or L- / D-Bpg. Xaa2b and Xaa7a are L- / D-Cys, L- / D-Lys, L- / D-Orn, L- / D-Dab, L- / D-Dap, L- / D-Lys(N3), L- / D-Orn(N3), L- / D-Dab(N3), L- / D-Dap(N3), L- / D-2-(5′-azidopentyl)alanine, or L- / D-2-(6′-azidohexyl)alanine. Xaa3 is L- / D-His, Pro, beta-(1,2,3-triazol-4-yl)-L-alanine, beta-(1,2,3-triazol-4-yl)-D-alanine, 1,2,4-triazole-3-alanine, or 1,2,4-triazole-3-D-alanine. Xaa4 is L- / D-Phe, L- / D-2-Nal, L- / D-Phe(4-F), L- / D-Phe(4-Cl), L- / D-Phe(4-Br), L- / D-Phe(4-I), L- / D-Phe(4-NH2), or L- / D-Phe(4-NO2). Xaa5 is L- / D-Arg, L- / D-hArg), Leu, L- / D-Agb, or L- / D-Agp. Xaa6 is L- / D-Trp, L- / D-Phe, L- / D-Trp(5-Br), L- / D-Trp(5-OCH3), L- / D-Trp(6-F), L- / D-Trp(5-OH) or L- / D-Trp(CHO). One or more amino acid residues of the MC1RTP is alpha N-methylated, wherein 1, 2, 3 or 4 of Xaa3, Xaa5, Xaa6 and Xaa7a is alpha N-methylated or wherein 1, 2, 3 or 4 of Xaa3, Xaa5, Xaa6 and Xaa7b is alpha N-methylated. The linker comprises an albumin-binding group.
Owner:PROVINCIAL HEALTH SERVICES AUTHORITY
Method for synthesizing L-norvaline
ActiveCN105237419AFew reaction stepsEasy to operateOrganic compound preparationAmino-carboxyl compound preparationAcetic acidSalicylaldehyde
The invention provides a method for synthesizing L-norvaline. The method comprises the following steps: 1, adding acetic acid into a reaction kettle, adding DL-norvaline and D-tartaric acid while stirring, heating to 80DEG C, adding salicylaldehyde, and maintaining the temperature in a range of 80-85DEG C for 10h; 2, cooling to 20-25DEG C after temperature maintenance, keeping the decreased temperature for 1h, and centrifuging to obtain crude L-norvaline and D-tartaric acid; 3, adding petroleum ether to the crude L-norvaline and D-tartaric acid, stirring and washing for 30min, and centrifuging to obtain a purified L-norvaline and D-tartaric acid double salt; 4, heating the double salt in water to 70DEG C in order to completely dissolve the double salt, adjusting the pH value to 7 by using ammonia water, cooling to 20-25DEG C, crystallizing for 2h, centrifuging, and rinsing by using methanol to obtain crude L-norvaline; and 5, heating the crude L-norvaline in water to 80-85DEG C in order to completely dissolve the crude L-norvaline, adding active carbon, decoloring for 1h, filtering, concentrating, cooling to 20-25DEG C, crystallizing for 3h, centrifuging, rinsing with water to obtain wet L-norvaline, and carrying out 60DEG C vacuum drying to obtain dry L-norvaline. The method has the advantages of few steps, good environmental protection property and high yield.
Owner:NANJING REDWOOD FINE CHEM CO LTD
Radiolabeled melanocortin 1 receptor-specific alpha-melanocyte-stimulating hormone analogues for imaging or therapy
PendingUS20210205483A1Organic non-active ingredientsRadioactive preparation carriersTarget peptideMelanocortin receptor
A compound is provided comprising a melanocortin 1 receptor (MC1R) targeting peptide (MC1RTP), a radiolabeling group, and a linker joining the MC1RTP to the radiolabeling group. The MC1RTP is linear or cyclized, and comprises a sequence of Formula I or Formula II: Xaa1-Xaa2a-Xaa3-Xaa4-Xaa5-Xaa6-Xaa7a (I) or Xaa1-Xaa2b-Xaa3-Xaa4-Xaa5-Xaa6-Xaa7b (II). Xaa1 is L- / D-Nle, L- / D-Nle, L- / D-Ala, L- / D-Leu, L- / D-Ile, D-Ile, L- / D-Cys, L- / D-Met, L- / D-Phe, L- / D-Trp, L- / D-Val, L- / D-Nal, L- / D-2-Nal, Gly, L- / D-α-aminobutryic acid, L- / D-norvaline, or L- / D-homonorleucine. Xaa2a and Xaa7b are L- / D-Cys, L- / D-Asp, L- / D-Glu, L- / D-2-Aad, L- / D-3-Aad, L- / D-Pra, L- / D-Hpg, or L- / D-Bpg. Xaa2b and Xaa7a are L- / D-Cys, L- / D-Lys, L- / D-Orn, L- / D-Dab, L- / D-Dap, L- / D-Lys(N3), L- / D-Orn(N3), L- / D-Dab(N3), L- / D-Dap(N3), L- / D-2-(5′-azidopentyl)alanine, or L- / D-2-(6′-azidohexyl)alanine. Xaa3 is L- / D-His, Pro, beta-(1,2,3-triazol-4-yl)-L-alanine, beta-(1,2,3-triazol-4-yl)-D-alanine, 1,2,4-triazole-3-alanine, or 1,2,4-triazole-3-D-N alanine. Xaa4 is L- / D-Phe, L- / D-2-Nal, L- / D-Phe(4-F), L- / D-Phe(4-Cl), L- / D-Phe(4-Br), L- / D-Phe(4-I), L- / D-Phe(4-NH2), or L- / D-Phe(4-NO2). Xaa5 is L- / D-Arg, L- / D-hArg), Leu, L- / D-Agb, or L- / D-Agp. Xaa6 is L- / D-Trp, L- / D-Phe, L- / D-Trp(5-Br), L- / D-Trp(5-OCH3), L- / D-Trp(6-F), L- / D-Trp(5-OH) or L- / D-Trp(CHO). One or more amino acid residues of the MC1RTP is alpha N-methylated, wherein 1, 2, 3 or 4 of Xaa3, Xaa5, Xaa6 and Xaa7a is alpha N-methylated or wherein 1, 2, 3 or 4 of Xaa3, Xaa5, Xaa6 and Xaa7b is alpha N-methylated. The linker comprises an albumin-binding group.
Owner:PROVINCIAL HEALTH SERVICES AUTHORITY
Method for synthesis of L-norvaline
InactiveCN100427460CSimple production processLow costOrganic compound preparationAmino-carboxyl compound preparationBromineAminopentamide
The invention discloses a synthesizing method of L-norvaline based on valerianic acid as raw material, which comprises the following steps: 1) proceeding acylated chloride reaction for valerianic acid acted by sulfone chloride to obtain valeryl chloride; 2) reacting valeryl chloride and liquid bronmine; removing sulfone chloride and bromine to obtain the rough product of alpha-bromovaleryl chloride; 3) dissolving alpha-bromovaleryl chloride in the solvent to ammonolyze; washing; condensing to obtain racemic alpha-aminovaleramide; 4) detaching the racemic alpha-aminovaleramide to obtain L-aminovaleramide tartrate; 5) recrystallizing; 6) hydrolyzing.
Owner:ZHEJIANG UNIV
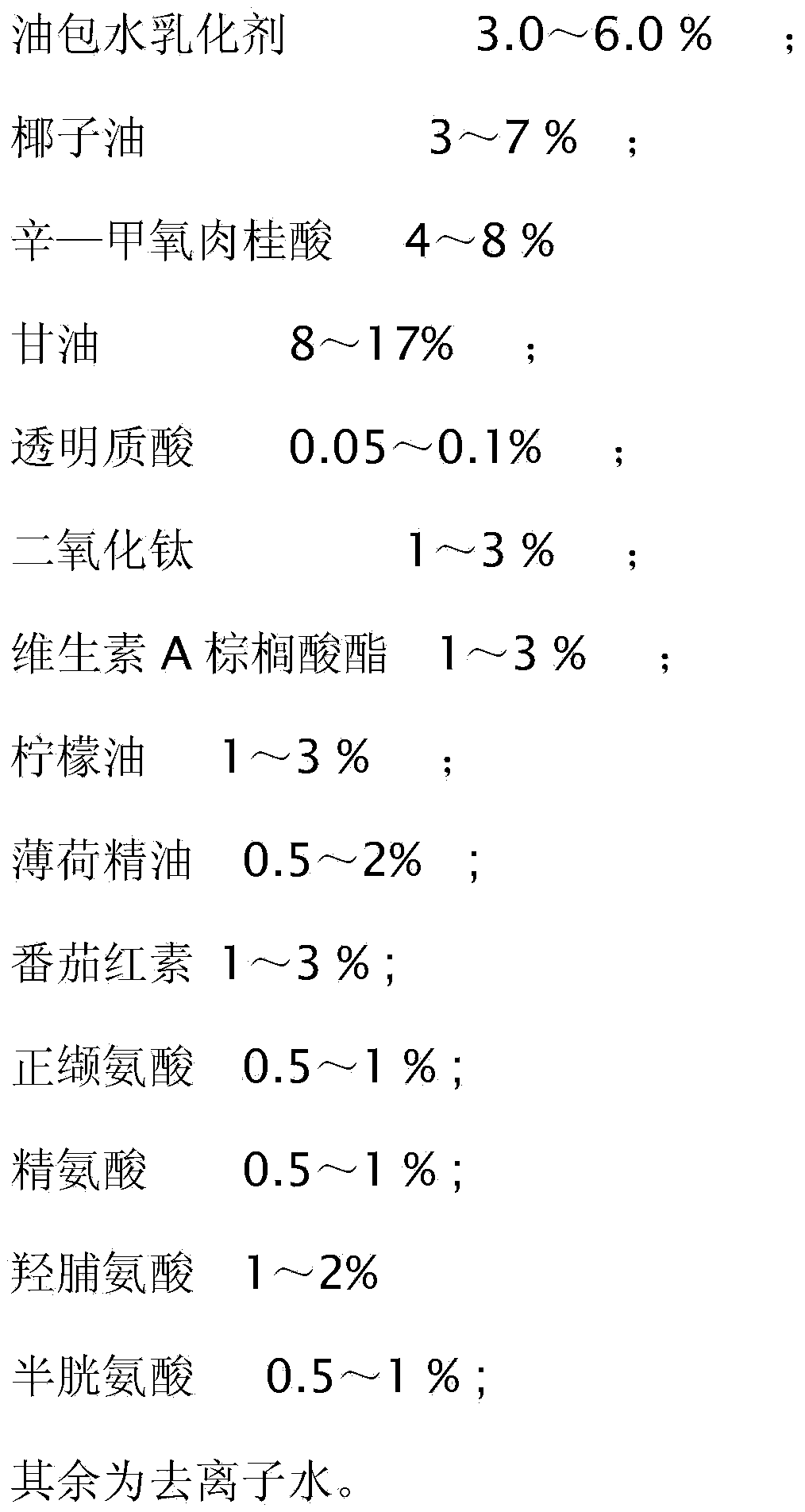
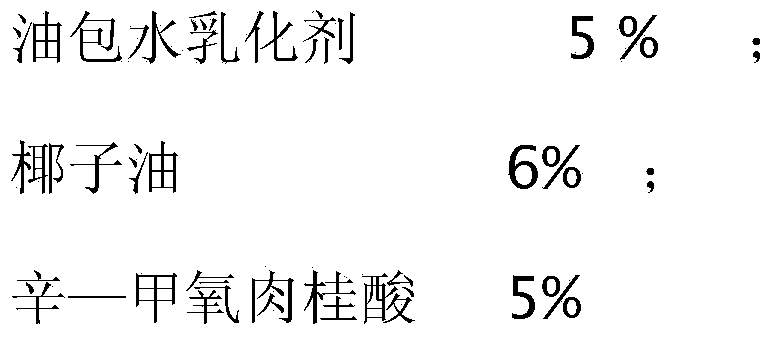
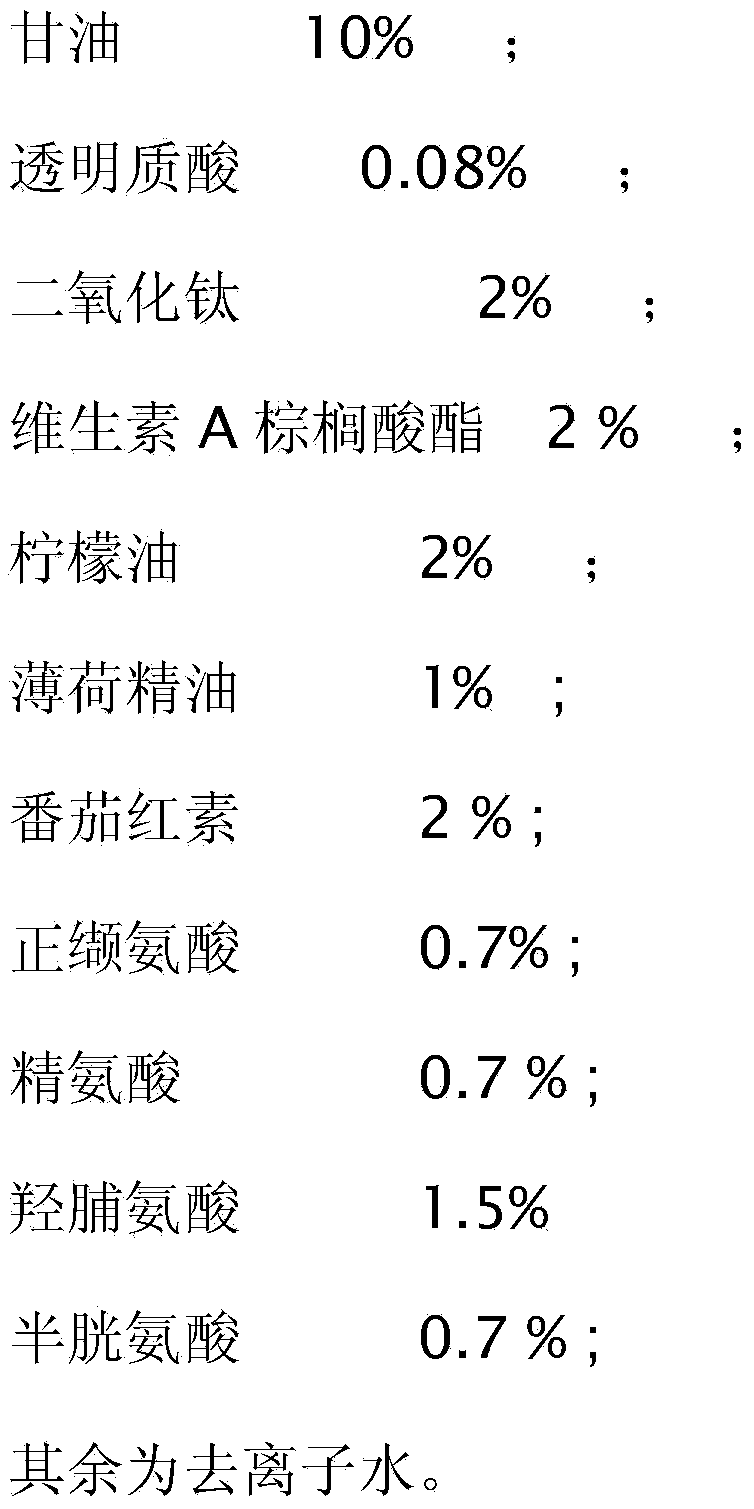
Physical sunscreen cream
InactiveCN104188812ALong lastingEasy to cleanCosmetic preparationsToilet preparationsHydroxyprolineArginine
The invention relates to daily cosmetics, and particularly relates to physical sunscreen cream. The cream is prepared from water-in-oil emulsion, coconut oil, octyl methoxycinnamate, glycerinum, hyaluronic acid, titanium dioxide, vitamin A palmitate, peppermint essential oil, lemon oil, lycopene, norvaline, arginine, hydroxyproline, cysteine and deionized water. The physical sunscreen cream does not contain harmful chemical components or heavy metal components, does not irritate skin, is long in duration, contains multiple amino acids, is easy to clean, and has a good moistening effect.
Owner:QINGDAO YONGTONG ELEVATOR ENG
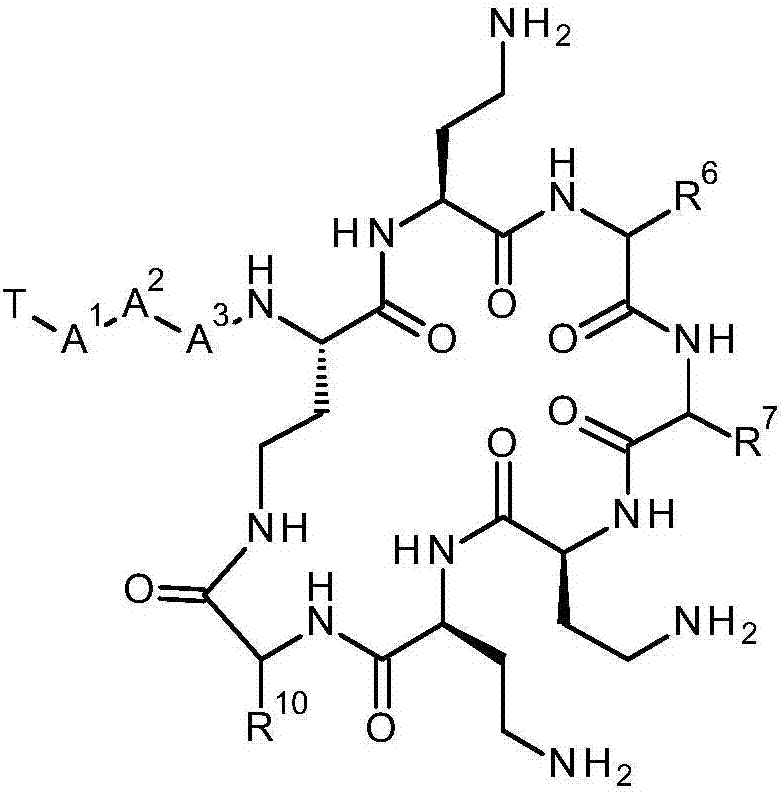
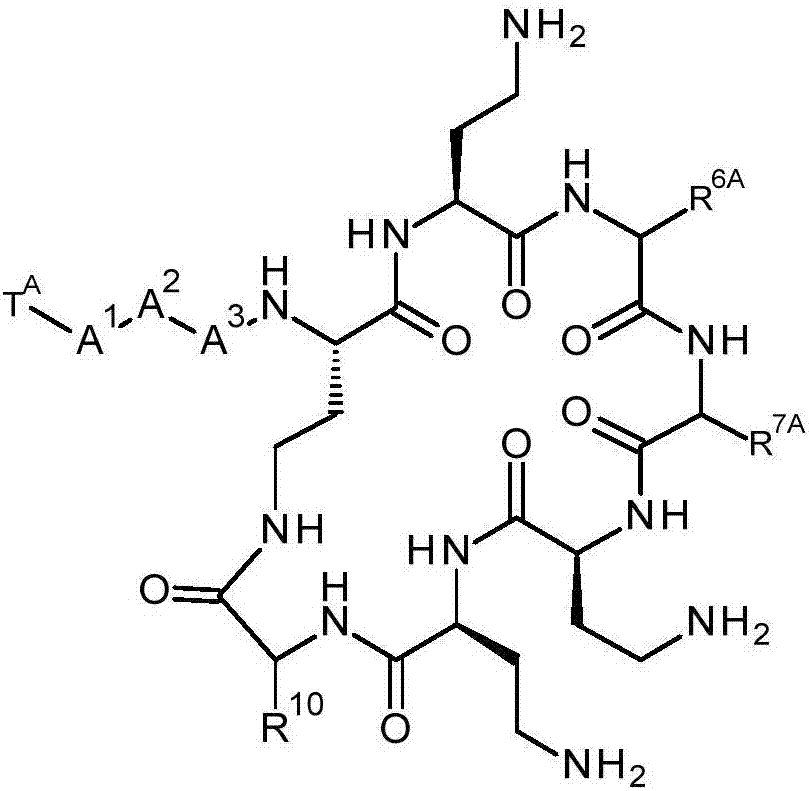
Compounds
The present invention provides a compound of formula (I), and its use in methods of treatment, including the treatment of bacterial infections. Methods for the preparation of the compound of formula (I) are also provided. The compound of formula (I) has the structure shown below, where -R6 and -R7 are each together with the carbonyl group and nitrogen alpha to the carbon to which it is attached an amino acid residue, except that R6 together with the carbonyl group and nitrogen alpha to the carbon to which it is attached is not a phenylalanine, leucine or valine residue and / or -R7 together with the carbonyl group and nitrogen alpha to the carbon to which it is attached is not a leucine, iso-leucine, phenylalanine, threonine, valine or nor-valine residue, and -T, -A1, -A2, -A3 and -R10 are as discussed in the application:
Owner:SPERO THERAPEUTICS INC
Prevention of incorporation of non-standard amino acids into protein
ActiveUS8603781B2Reducing, or substantially eliminating, endogenous cellular levels of norleucineLower Level RequirementsBacteriaPeptide/protein ingredientsBeta-methylnorleucinePhenylalanine dehydrogenase
The instant invention is drawn to the methods and compositions necessary to provide recombinant proteins with a substantially reduced or eliminated content of norleucine or other non-standard amino acids. Various embodiments of the invention provide for the substantial elimination of the incorporation of non-standard amino acids into recombinant proteins by the co-expression or enhanced expression of a protein (or the enzymatically active portion thereof) capable of degrading norleucine or other non-standard amino acids, including norvaline, beta-methylnorleucine, and homoisoleucine. In certain particular embodiments of the invention, the norleucine is degraded by a glutamate dehydrogenase, a leucine dehydrogenase, a valine dehydrogenase, a phenylalanine dehydrogenase, a glutamate / leucine / phenylalanine / valine dehydrogenase, or an opine dehydrogenase. Also provided are the cells and DNA constructs for carrying out these methods.
Owner:MONSANTO TECH LLC
Preparation method of L-norvaline
PendingCN113025669ARaw materials are easy to obtainImprove responseOrganic compound preparationOrganic chemistry methodsPhosphoric acidPyridoxine phosphate
The invention discloses a preparation method of L-norvaline. The method takes pentanamide hydrochloride, pyridoxal phosphate and cobalt chloride hexahydrate as raw materials, and the L-norvaline is prepared by reaction under the catalysis of enzyme. The method is easy to operate and high in repeatability, the purity of the obtained product reaches 99.5% or above, and the recovery rate reaches 90% or above.
Owner:连云港杰瑞药业有限公司
Synthesis of D,L-norvaline
InactiveCN101508654BRich sourcesSimple processOrganic compound preparationAmino-carboxyl compound preparationHexamethylenetetramineIon exchange
Owner:吕亮
Waterproof sunscreen cream
InactiveCN107536785ANot easily soluble inEasy to washCosmetic preparationsToilet preparationsArginineGlycerol
The invention discloses a waterproof sunscreen, which comprises the following components: 1-5 parts of zinc oxide, 0.2-2 parts of citric acid, 0.1-1 parts of sodium hydroxymethyl glycinate, 0.5-0.8 parts of octyl cyanodiphenyl acrylate, Butylmethoxydibenzoylmethane 0.5-2 parts, polyglycerol 1-8 parts, arginine 1-3 parts, cysteine 0.1-0.5 parts, norvaline 0.1-0.5 parts. The waterproof sunscreen disclosed in the invention is not easily soluble in water, and has better effects of sunburn and tanning.
Owner:赖婷婷
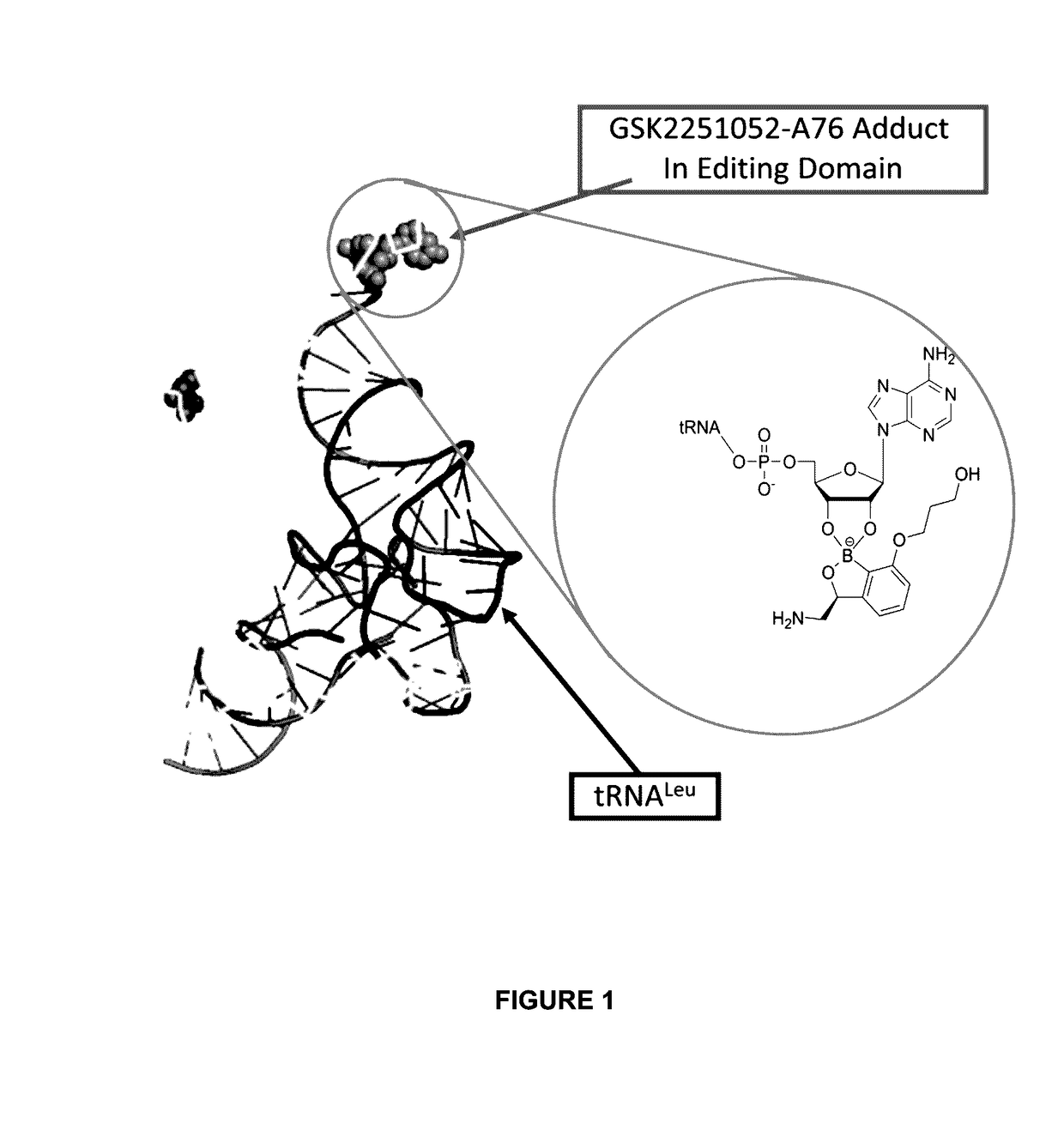
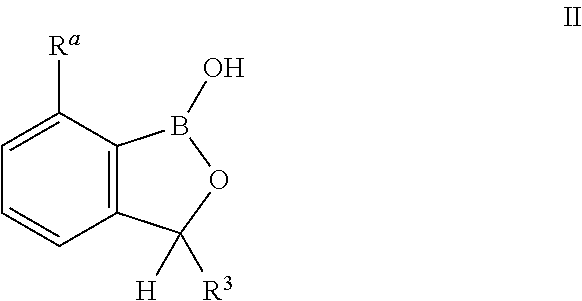
Benzoxaborole compounds and uses thereof
ActiveUS20170071961A1Reduce probabilityReduce frequencyBoron compound active ingredientsAgainst vector-borne diseasesOrganic chemistryAmino acid
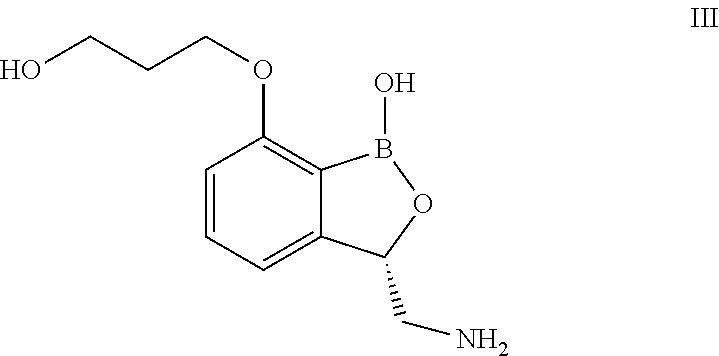
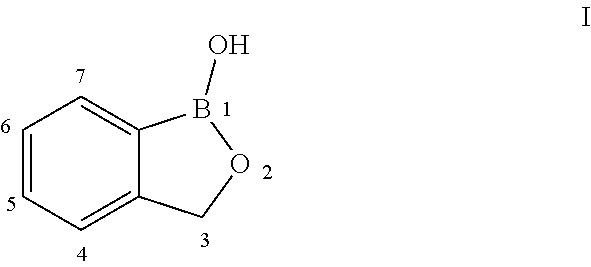
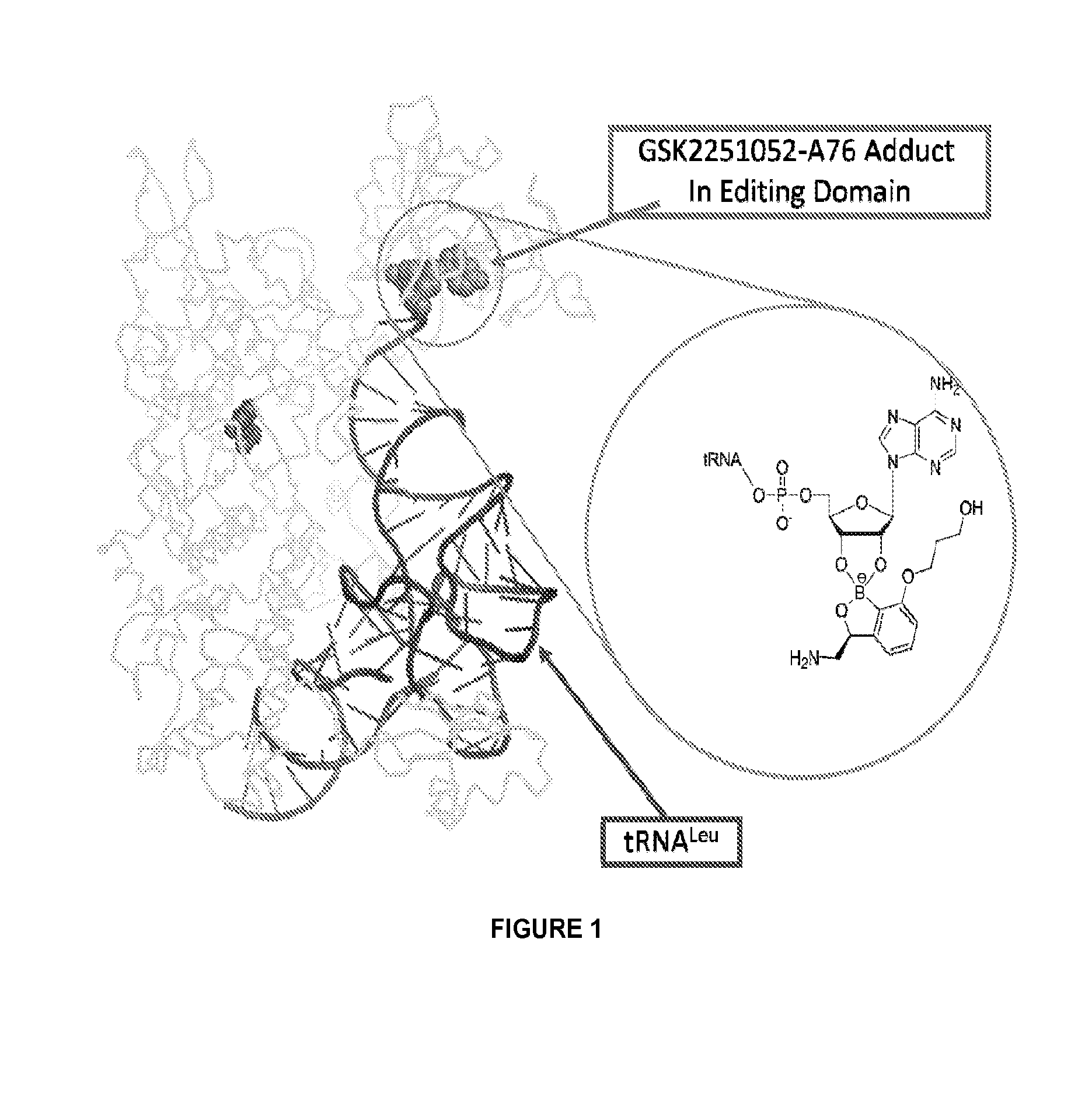
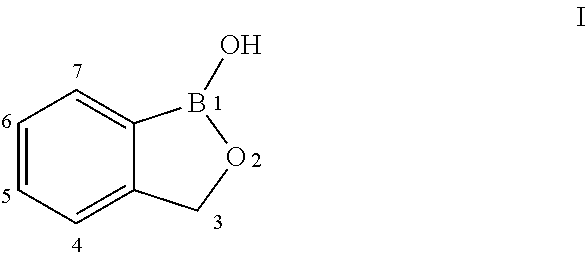
Norvaline and / or other amino acids that are capable of being acylated onto tRNALeu by LeuRS, in combination with substituted benzoxaboroles, such as a compound having a structure according to formula III:and methods for decreasing the frequency of resistance and / or reducing the rate of resistance and / or suppressing the emergence of resistance that develops in bacteria exposed to a substituted benzoxaborole or salt thereof by administering a combination of a substituted benzoxaborole such as a compound of formula III or salt thereof and an amino acid or a salt thereof.
Owner:ANACOR PHARMA INC +1
Benzoxaborole compounds and uses thereof
InactiveUS20150080342A1Reduce probabilityReduce frequencyBiocideBoron compound active ingredientsOrganic chemistryAmino acid
Norvaline and / or other amino acids that are capable of being acylated onto tRNALeu by LeuRS, in combination with substituted benzoxaboroles, such as a compound having a structure according to formula III: and methods for decreasing the frequency of resistance and / or reducing the rate of resistance and / or suppressing the emergence of resistance that develops in bacteria exposed to a substituted benzoxaborole or salt thereof by administering a combination of a substituted benzoxaborole such as a compound of formula III or salt thereof and an amino acid or a salt thereof.
Owner:ANACOR PHARMA INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com