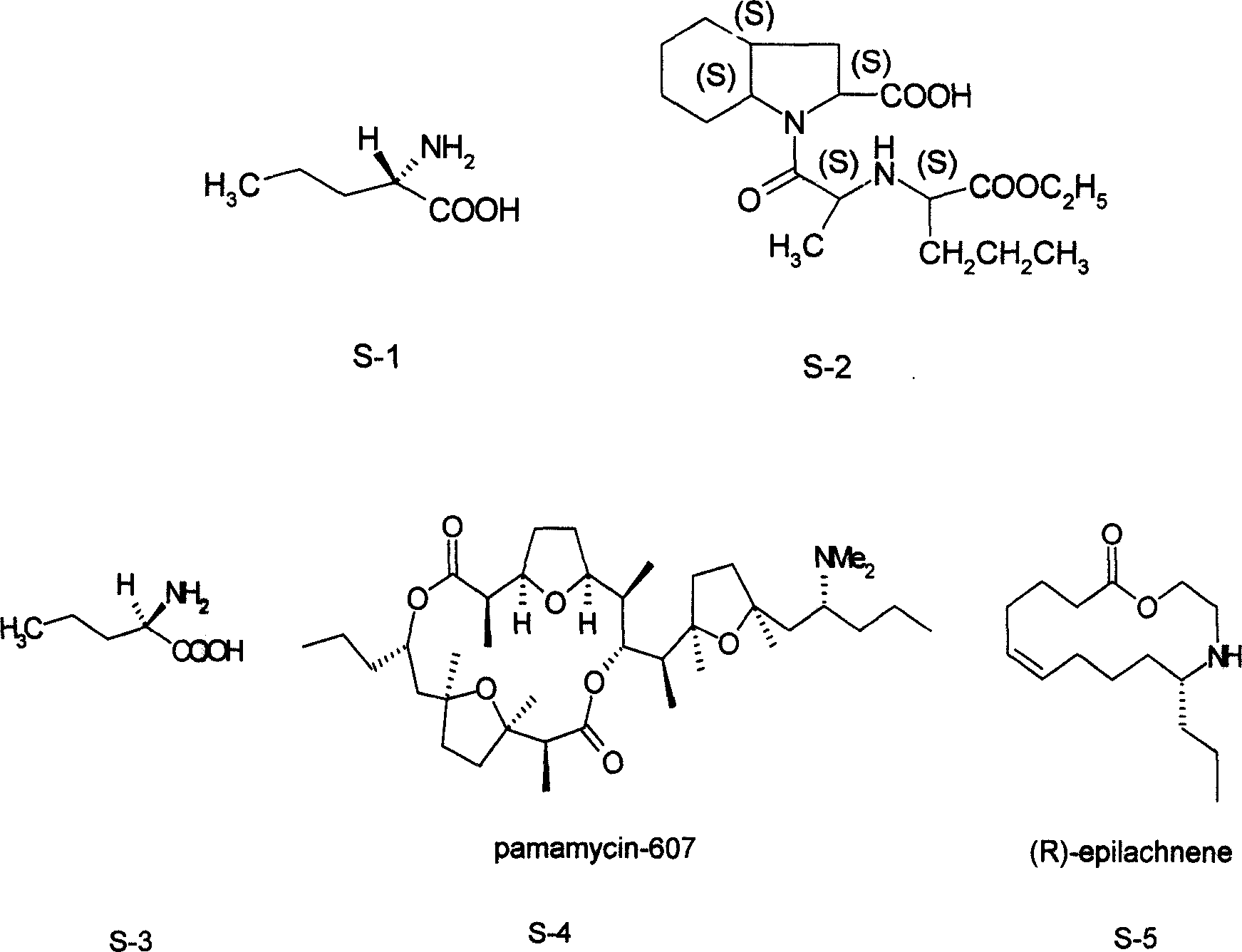
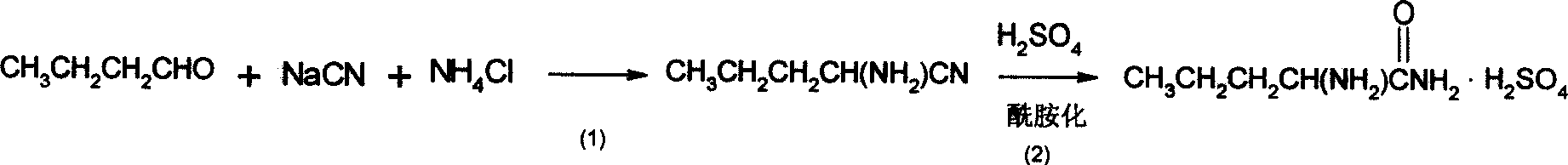Synthesis method of chiral norvaline
A norvaline and synthesis method technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as complex synthesis process, high cost, and complex starting materials, and achieve simple production process, The effect of low cost and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthetic method of L-norvaline, take n-butyraldehyde and sodium cyanide, ammonium chloride as main starting raw material, make through following steps successively:
[0023] (1) Preparation of aminovaleronitrile:
[0024] In the mixed solvent that 50ml methanol and 200ml water are made into, add n-butyraldehyde (14.4g, 0.2mol), sodium cyanide (10.8g, 0.22mol), ammonium chloride (11.8g, 0.22mol), stir to dissolve, At this time, the solution was not separated; the temperature was controlled at about 70°C for 6 hours. After stopping the reaction, the reaction solution was extracted twice with 50 ml of ethyl acetate respectively, the organic phases were combined, and the crude product of aminovaleronitrile (12.6 g, 0.13 mol) was obtained after removing the solvent.
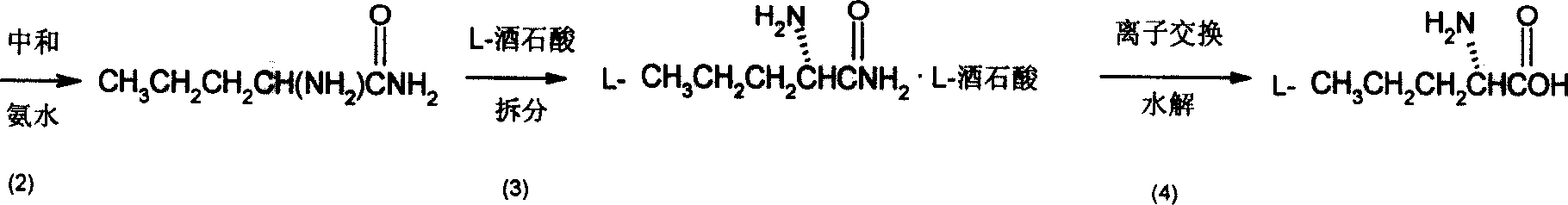
[0025] (2) cyano amidation, i.e. the preparation of racemic aminovaleramide:
[0026] The aminovaleronitrile crude product obtained in the previous step (9.8g, 0.1mol) was added dropwise to 32g of 98% co...
Embodiment 2
[0034] The synthetic method of L-norvaline, take n-butyraldehyde and sodium cyanide, ammonium chloride as main starting raw material, make through following steps successively:
[0035] (1) Preparation of aminovaleronitrile:
[0036]In the mixed solvent that 50ml ethanol and 200ml water are made into, add n-butyraldehyde (14.4g, 0.2mol), sodium cyanide (14.7g, 0.3mol), ammonium chloride (16.1g, 0.3mol), stir to dissolve, At this time, the solution was not separated; the reaction temperature was controlled at about 80°C for 8 hours. After stopping the reaction, the reaction solution was extracted twice with 50 ml of ethyl acetate respectively, the organic phases were combined, and the crude product of aminovaleronitrile (10.3 g, 0.11 mol) was obtained after removing the solvent.
[0037] (2) cyano amidation, i.e. the preparation of racemic aminovaleramide:
[0038] The aminovaleronitrile crude product obtained in the previous step (9.8g, 0.1mol) was added dropwise in 38g of 1...
Embodiment 3
[0046] The synthetic method of L-norvaline, take n-butyraldehyde and sodium cyanide, ammonium chloride as main starting raw material, make through following steps successively:
[0047] (1) Preparation of aminovaleronitrile:
[0048] In the mixed solvent that 66ml methanol and 200ml water are made into, add n-butyraldehyde (14.4g, 0.2mol), sodium cyanide (19.6g, 0.4mol), ammonium chloride (21.4g, 0.4mol), stir to dissolve, At this time, the solution was not separated; the reaction temperature was controlled at about 50° C. for 4 hours. After stopping the reaction, the reaction solution was extracted twice with 50 ml of ethyl acetate, the organic phases were combined, and the crude product of aminovaleronitrile (7.3 g, 0.07 mol) was obtained after removing the solvent.
[0049] (2) cyano amidation, i.e. the preparation of racemic aminovaleramide:
[0050] The aminovaleronitrile crude product obtained in the previous step (9.8g, 0.1mol) was added dropwise to 38g of 99% concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com