Method for determining other amino acids in L-valine raw material by high performance liquid chromatography
A high-performance liquid chromatography and valine technology, which is applied in the field of determination of other amino acids in L-valine raw materials, can solve the problems of inability to clearly display the specific content of various other amino acids, expensive equipment costs and detection costs, and the service life of amino columns. Not long, etc., to solve the reversed-phase service life is not long, improve the effect of chromatographic peak shape, chromatographic peak shape and good resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
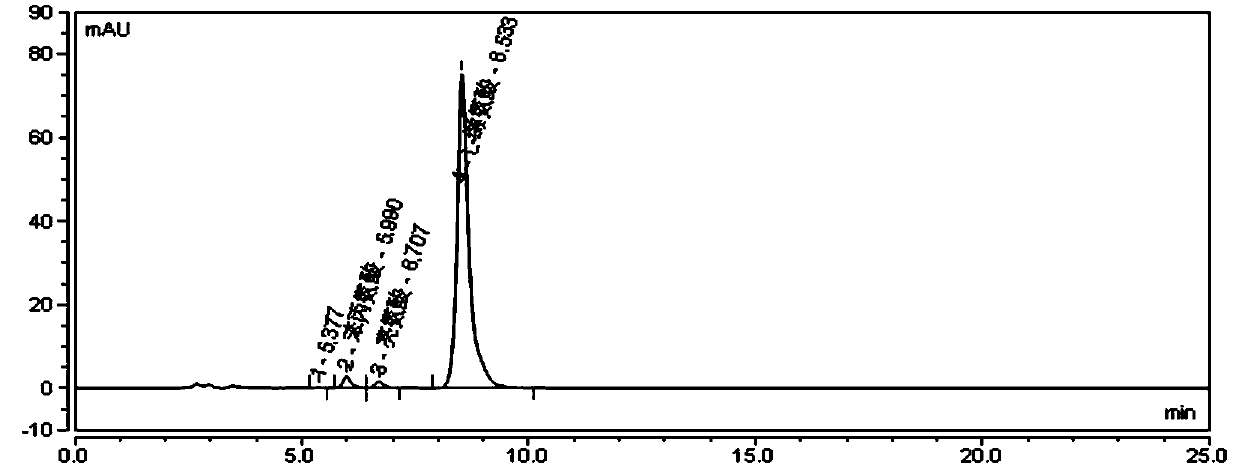
[0045] Take about 20 mg of L-valine raw material from batch S200308, add 10 ml of mobile phase to dissolve, and use it as the test solution, inject 20 μl of sample, and the chromatographic conditions are as follows:
[0046] Chromatographic column: aminopropyl bonded silica gel column, 250mm×4.6mm, 5μm, Phenomenex Luna NH 2 .
[0047] Mobile phase: a mixed solution of acetonitrile and phosphate buffer with a volume ratio of 72:28, wherein the concentration of phosphate buffer is 15 mmol / L, 0.1% triethylamine is added, and the pH is adjusted to 6.1 with phosphoric acid;
[0048] Phosphate buffer solution: Weigh 1.2g of potassium dihydrogen phosphate and 0.7g of disodium hydrogen phosphate and dissolve them in 1L of water, add 0.1% triethylamine, and adjust the pH to 6.1 with phosphoric acid;
[0049] Equilibrate the column with 50% acetonitrile for at least 30 minutes, then equilibrate the column with the mobile phase for at least 30 minutes.
[0050] The flow rate is 1.0ml / m...
Embodiment 2
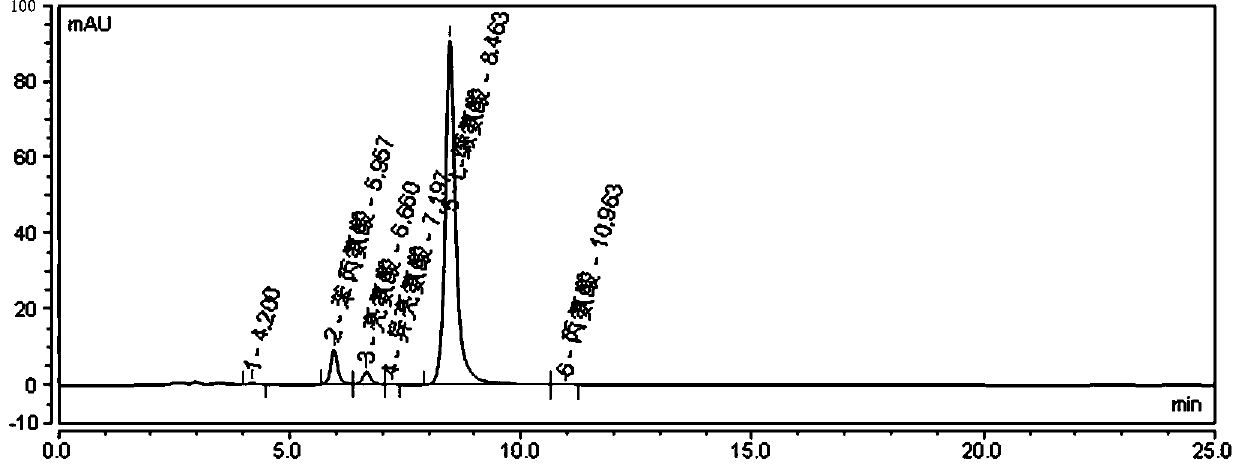
[0058] Take about 20 mg of L-valine raw material from batch S200301, add 10 ml of mobile phase to dissolve, and use it as the test solution, inject 20 μl of sample, and the chromatographic conditions are as follows:
[0059] Chromatographic column: aminopropyl bonded silica gel column, 250mm×4.6mm, 5μm, Phenomenex Luna NH 2 .
[0060] Mobile phase: a mixed solution of acetonitrile and phosphate buffer with a volume ratio of 74:26, wherein the concentration of phosphate buffer is 15 mmol / L, 0.3% triethylamine is added, and the pH is adjusted to 6.4 with phosphoric acid;
[0061] Phosphate buffer solution: Weigh 1.2g of potassium dihydrogen phosphate and 0.7g of disodium hydrogen phosphate and dissolve them in 1L of water, add 0.3% triethylamine, and adjust the pH to 6.4 with phosphoric acid;
[0062]Equilibrate the column with 50% acetonitrile for at least 30 minutes, then equilibrate the column with the mobile phase for at least 30 minutes.
[0063] The flow rate is 1.0ml / mi...
Embodiment 3
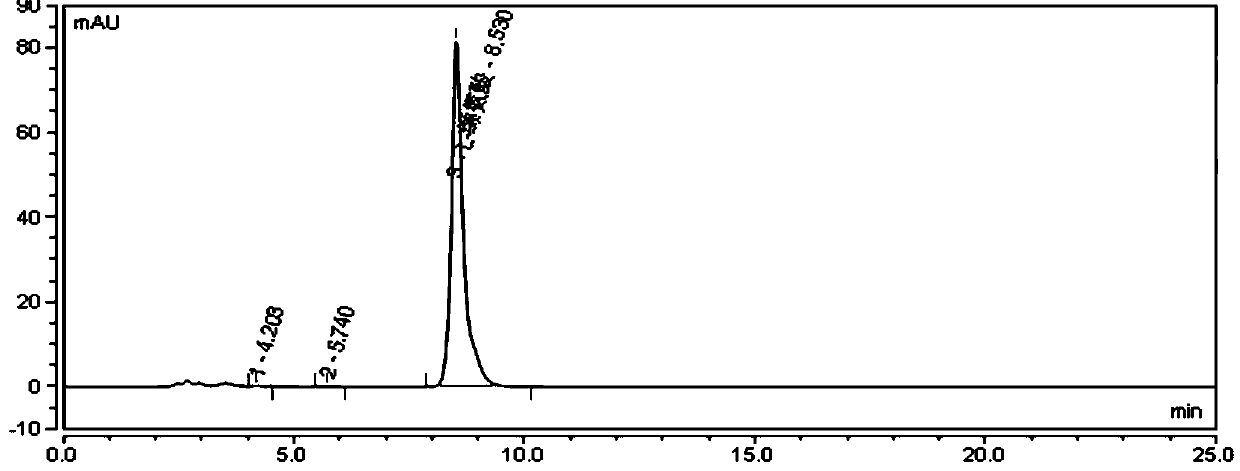
[0071] Take 200309 batches of L-valine raw materials about 20mg, add mobile phase 10ml to dissolve, as the test solution, inject 20μl, the chromatographic conditions are as follows:
[0072] Chromatographic column: aminopropyl bonded silica gel column, 250mm×4.6mm, 5μm, Phenomenex Luna NH 2 .
[0073] Mobile phase: a mixed solution of acetonitrile and phosphate buffer with a volume ratio of 72:28, wherein the concentration of phosphate buffer is 15 mmol / L, 0.2% triethylamine is added, and the pH is adjusted to 6.2 with phosphoric acid;
[0074] Phosphate buffer solution: Weigh 1.2g of potassium dihydrogen phosphate and 0.7g of disodium hydrogen phosphate and dissolve them in 1L of water, add 0.2% triethylamine, and adjust the pH to 6.2 with phosphoric acid;
[0075] Equilibrate the column with 50% acetonitrile for at least 30 minutes, then equilibrate the column with the mobile phase for at least 30 minutes.
[0076] The flow rate is 1.0ml / min;
[0077] Detection wavelength...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com