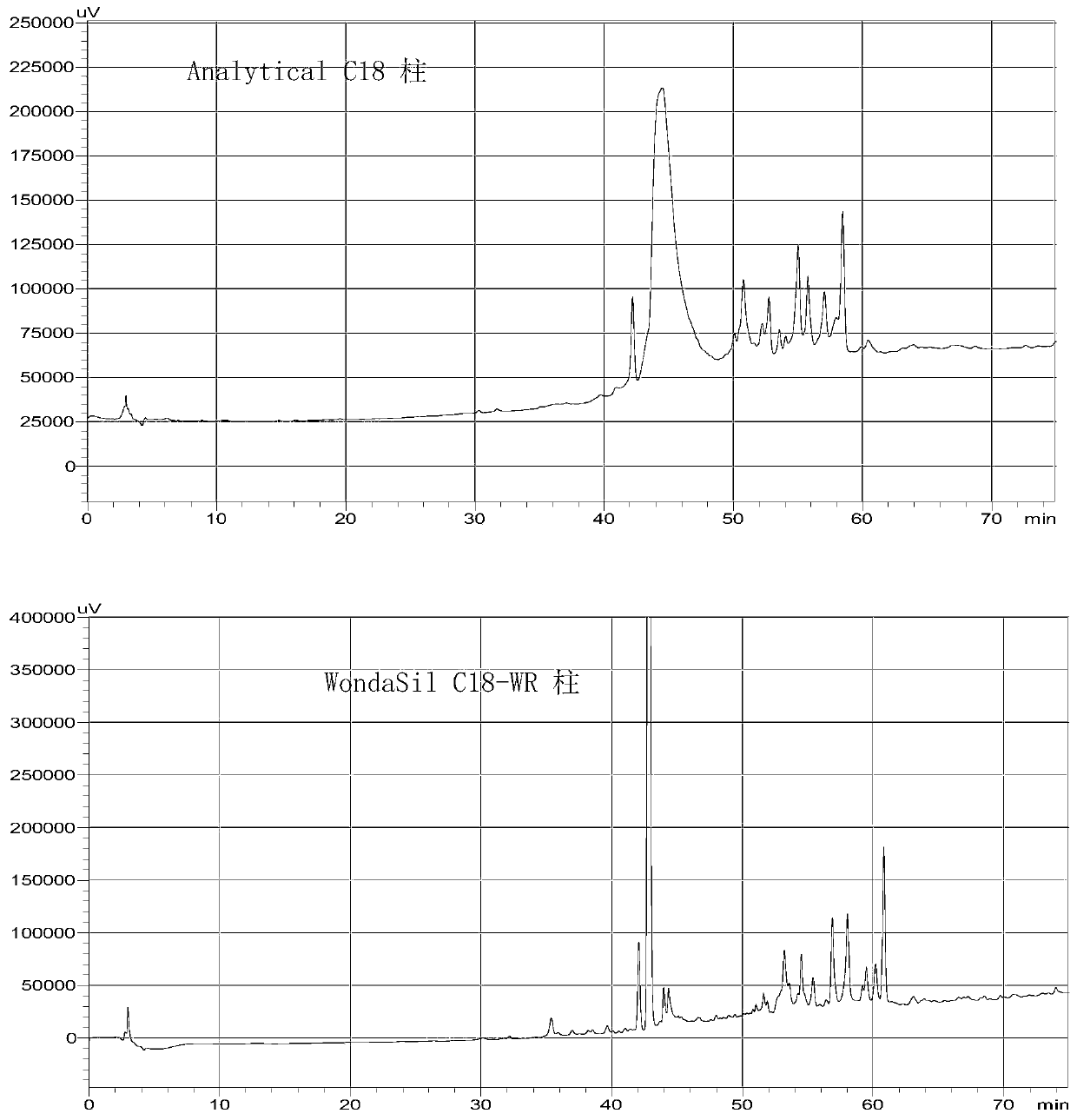
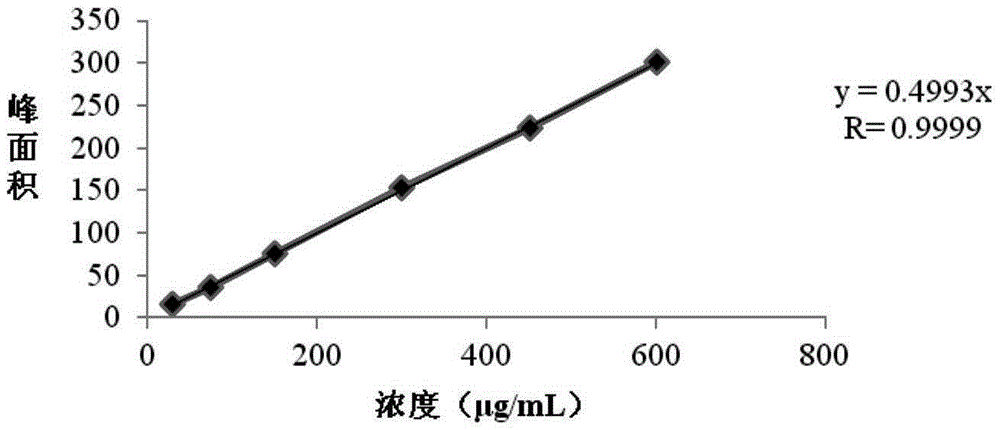
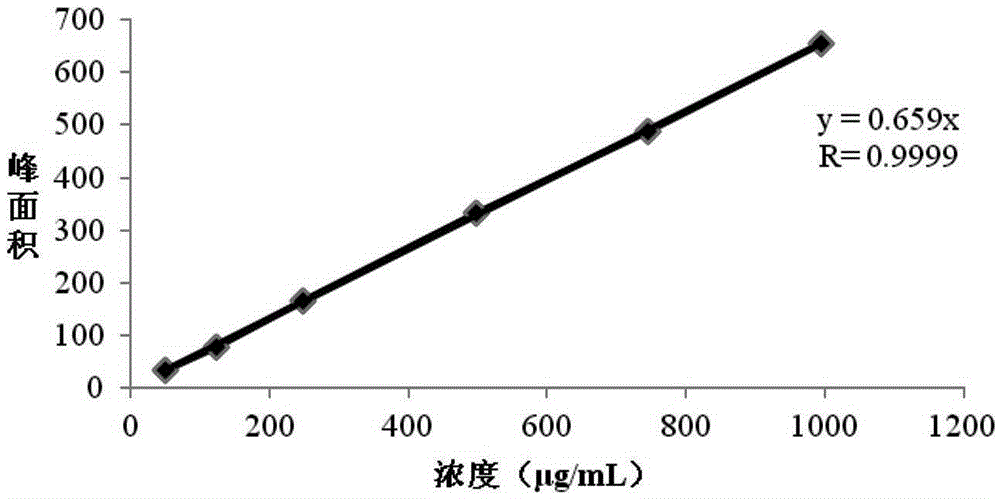
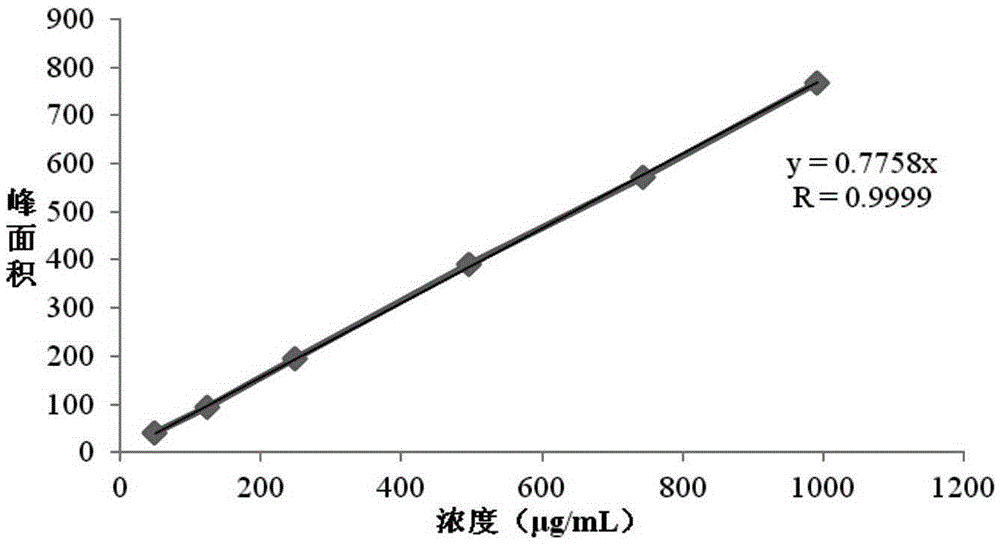
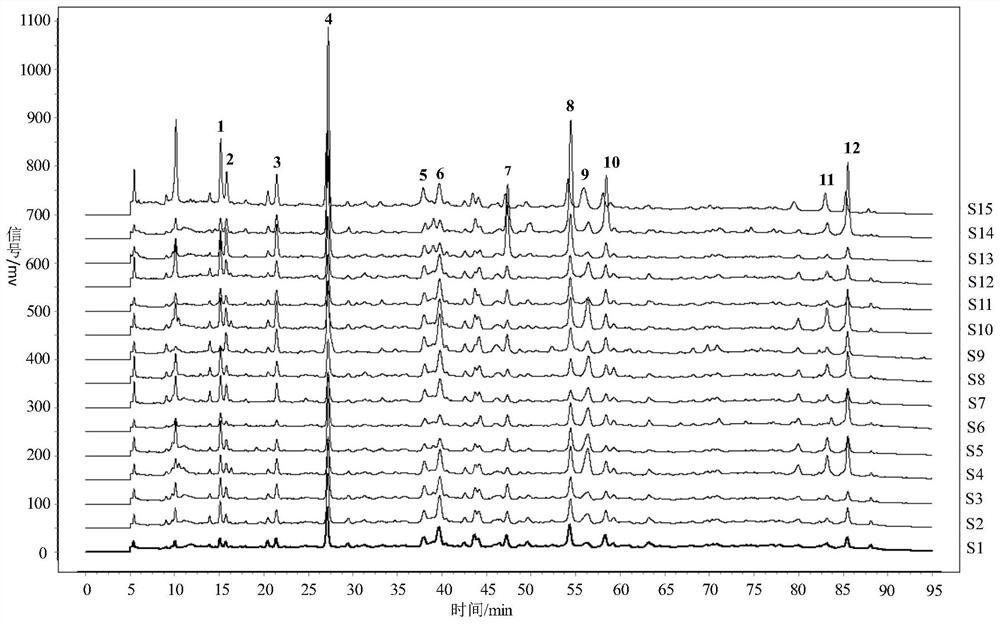
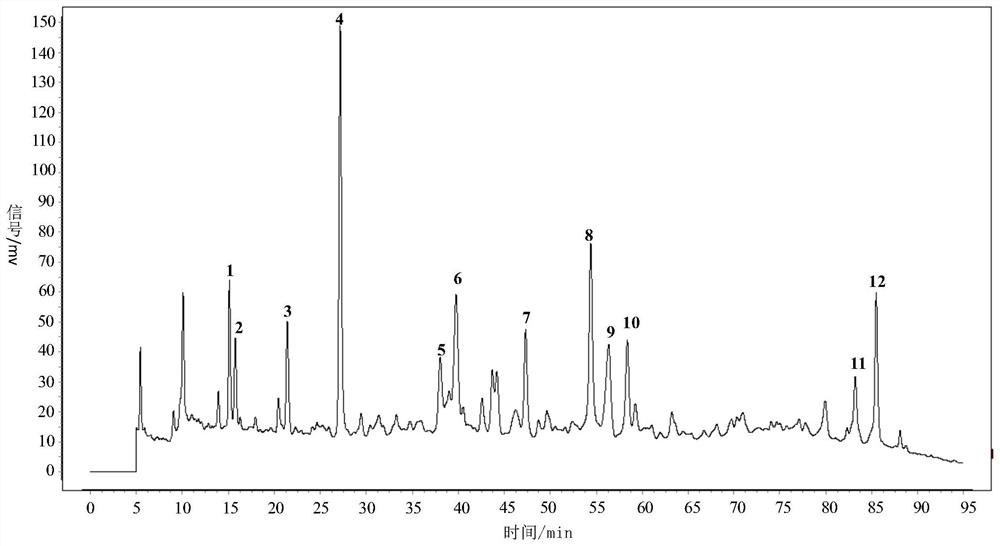
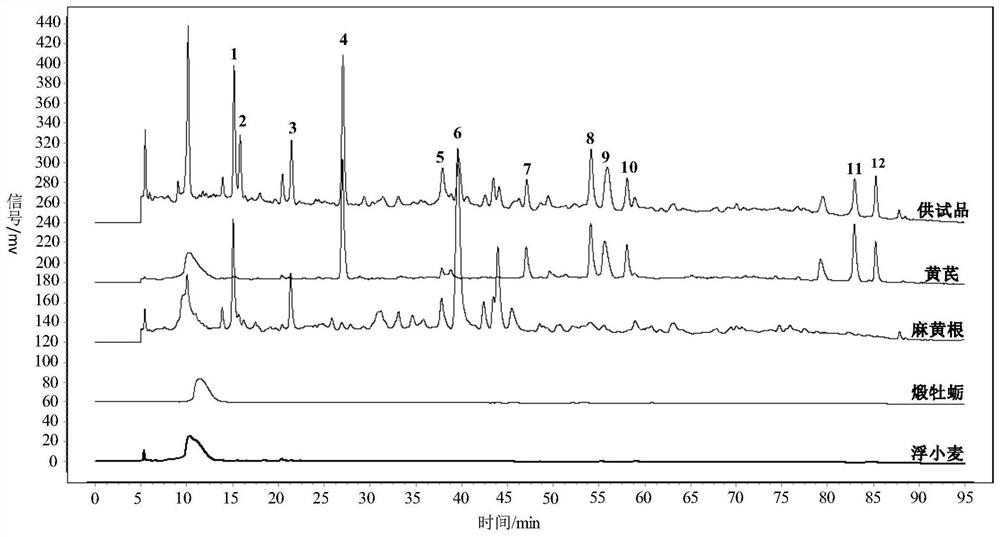
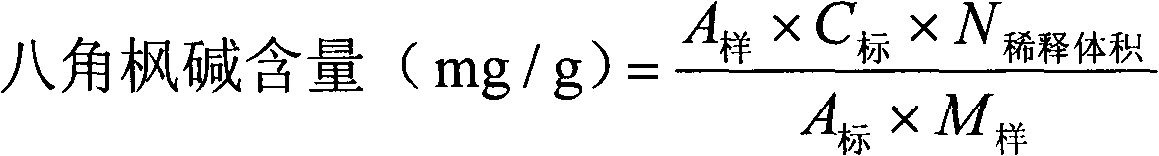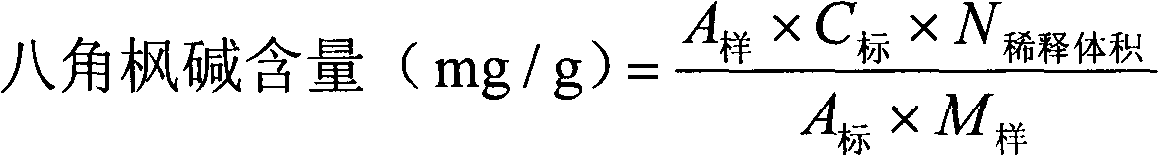Patents
Literature
54results about How to "Moderate retention time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
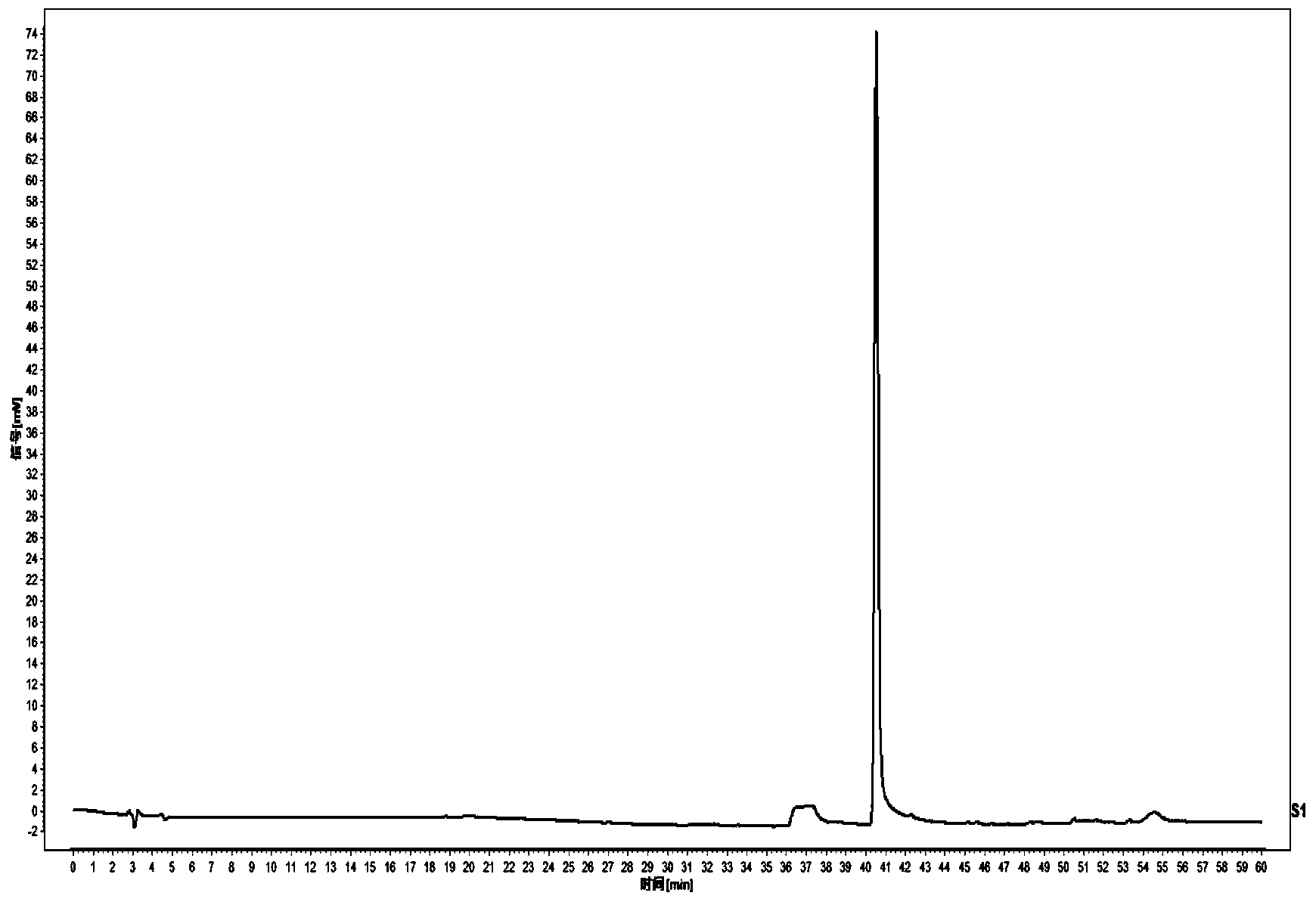
Blood-production preparation quality test method and construction method of standard fingerprint spectrum thereof
ActiveCN103926351AThe pretreatment method is simpleImprove stabilityComponent separationTest samplePhosphoric acid
The invention discloses a blood-production preparation quality test method which comprises the following steps: (1) preparation of a comparison product solution; (2) preparation of a test sample solution; (3) chromatographic conditions: a chromatographic column is C18 reversion phase chromatographic column; gradient elution is adopted, and a flowing phase is a gradient elution solution composed of acetonitrile and 0.1-0.2 percent of a phosphoric acid solution; the detection wavelength is 200-230 nanometers; the column temperature is 25-40 DEG C; the flowing speed is 0.8ml / min-1.2ml / min; the analysis lasts for 60 min; and (4) testing: the fingerprint spectrum is obtained through efficient liquid phase chromatography. The testing method provided by the invention is simple to operate, characteristic components are completely retained, and the testing method is good in repeatability and stability, high in precision and strong in specificity.
Owner:KANGSHOU PHARMACY CO LTD HUNAN
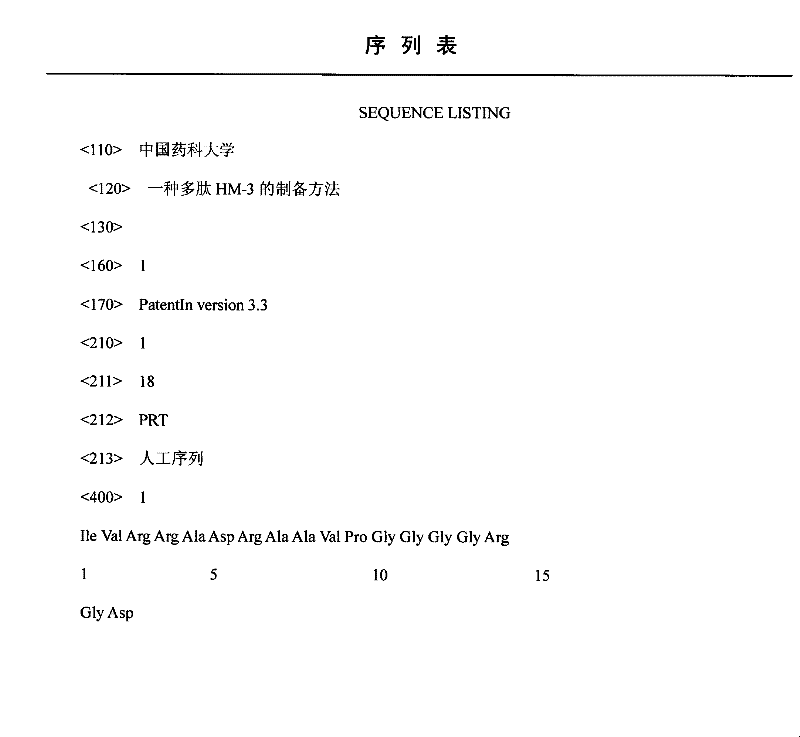
A kind of preparation method of polypeptide hm-3
InactiveCN102286078AMild conditionsLess side effectsPeptide preparation methodsDipeptideCombinatorial chemistry
The invention discloses a method for preparing HM-3 polypeptide, which belongs to the field of polypeptide technology in the field of biochemistry, and uses Fmoc-Asp(Otbu)-wang resin or Fmoc-Asp(Otbu)-CTC resin as a starting material , and then use protected amino acids to inoculate dipeptide to octadecapeptide sequentially, fully wash after the peptide incorporation work is completed, then cut the peptide, and post-process to obtain the crude product of HM-3. The crude product was dissolved, purified twice by preparative high performance liquid phase, and finally concentrated and freeze-dried to obtain the pure product. The method not only ensures the synthesis efficiency, but also improves the purity.
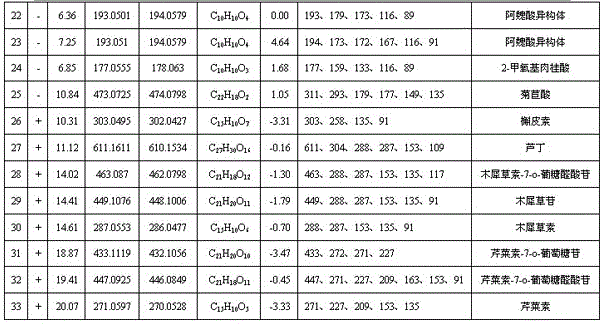
Owner:CHINA PHARM UNIV
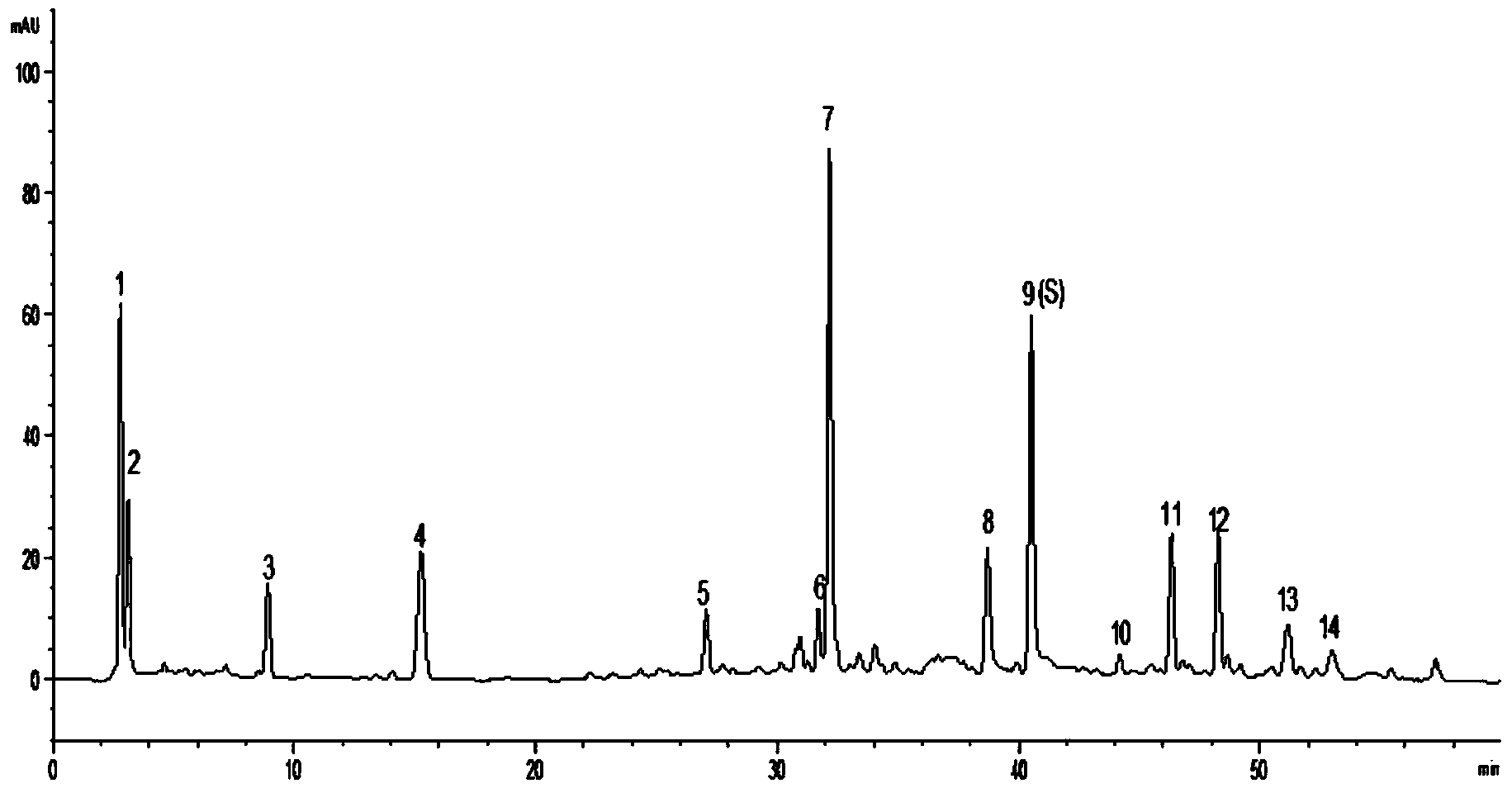
Callicarpa nudiflora medicine, intermediate and fingerprint detection method and standard fingerprint of preparation
ActiveCN104215698AThe pretreatment method is simpleCharacteristic ingredients remain intactComponent separationClinical efficacyCallicarpa nudiflora
The invention discloses a callicarpa nudiflora medicine, an intermediate and a fingerprint detection method of a preparation. The method comprises the following steps: (a) preparation of a reference solution; (b) preparation of a test solution; (c) chromatographic condition; (d) formulation of a standard fingerprint of isoacteoside as a reference peak; and (e) quality control of the fingerprint. According to the above method, a pretreatment method of each tested object is simple, characteristic constituents are retained completely, stability is good, precision is high, and there is certain specificity. The separation effect of each characteristic peak in the fingerprint is good. The callicarpa nudiflora medicine, the intermediate and the fingerprint of the preparation have good correlation. The method can be used for identifying quality of the callicarpa nudiflora medicine and increasing production process controllability of the callicarpa nudiflora preparation, and is helpful for guaranteeing quality stability and clinical efficacy of the callicarpa nudiflora preparation. The invention also discloses the callicarpa nudiflora medicine obtained according to the above fingerprint detection method, the intermediate and the standard fingerprint of the preparation.
Owner:JIUZHITANG +2
Method for detecting blood concentration of multiple antiepileptic drugs simultaneously
InactiveCN1971270ASuitable for routine testingDo not interfere with determinationTesting dairy productsTesting medicinal preparationsEpoxyPretreatment method
The invention belongs to field of medical examination and relates to assay determination method of internal medicine, specially a detecting method of six antiepileptics medicines of primidone, phenylethylmalonylurea, diphenylhydantoin, carbamazepine, Larmortriazine, okazepine and the active product monohydroxy okazepine of okazepine and the active product epoxy carbamazepine of carbamazepine in human plasma. The sample to be measured is eluted equicontinuously in condition of acidic mobile phase via the pretreatment of protein precipitation, and separated by chromatographic column, and detected by ultraviolet detector. The quantity of sample in the invention is small; the pretreatment method is simple, fast and sensitive, free of expensive apparatus and agents, applicability is wide, cost is low, it fits for the detection of normal blood concentration in clinic.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Research for realizing quick classification and identification of chemical components in ixeris sonchifolia hance injection based on UPLC-Q-TOF-MS technology
ActiveCN104359968AEasy to separateSolve key problemsComponent separationMaterial analysis by electric/magnetic meansOrganic acidChemical compound
The invention discloses a research for realizing quick classification and identification of chemical components in an ixeris sonchifolia hance injection based on a UPLC-Q-TOF-MS technology, and aims to take flavonoids, organic acids, amino acids and nucleosides in the ixeris sonchifolia hance injection as research objects to realize quick classification and identification of the chemical components in the ixeris sonchifolia hance injection based on a UPLC-Q-TOF-MS technical platform. The research comprises the following steps: firstly, performing information integration on components of flavonoids, organic acids, amino acids and nucleosides in the ixeris sonchifolia hance injection to discover and summarize a rule for diagnosing fragments and neutral losses of the four types of substances; meanwhile, performing mass spectrographic analysis on reference substances of different types of compounds by adopting the UPLC-Q-TOF-MS technology, and performing verification; and constructing a method for realizing quick classification and identification of chemical components in the ixeris sonchifolia hance injection by using a method for diagnosing fragments and neutral losses as a screening and identifying tool.
Owner:TONGHUA HUAXIA PHARMA
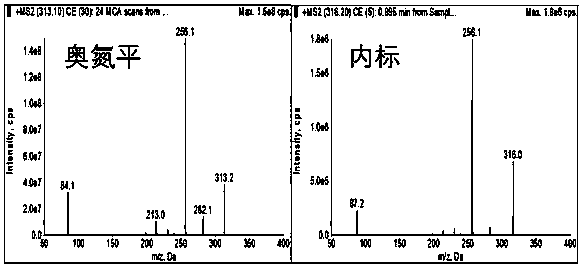
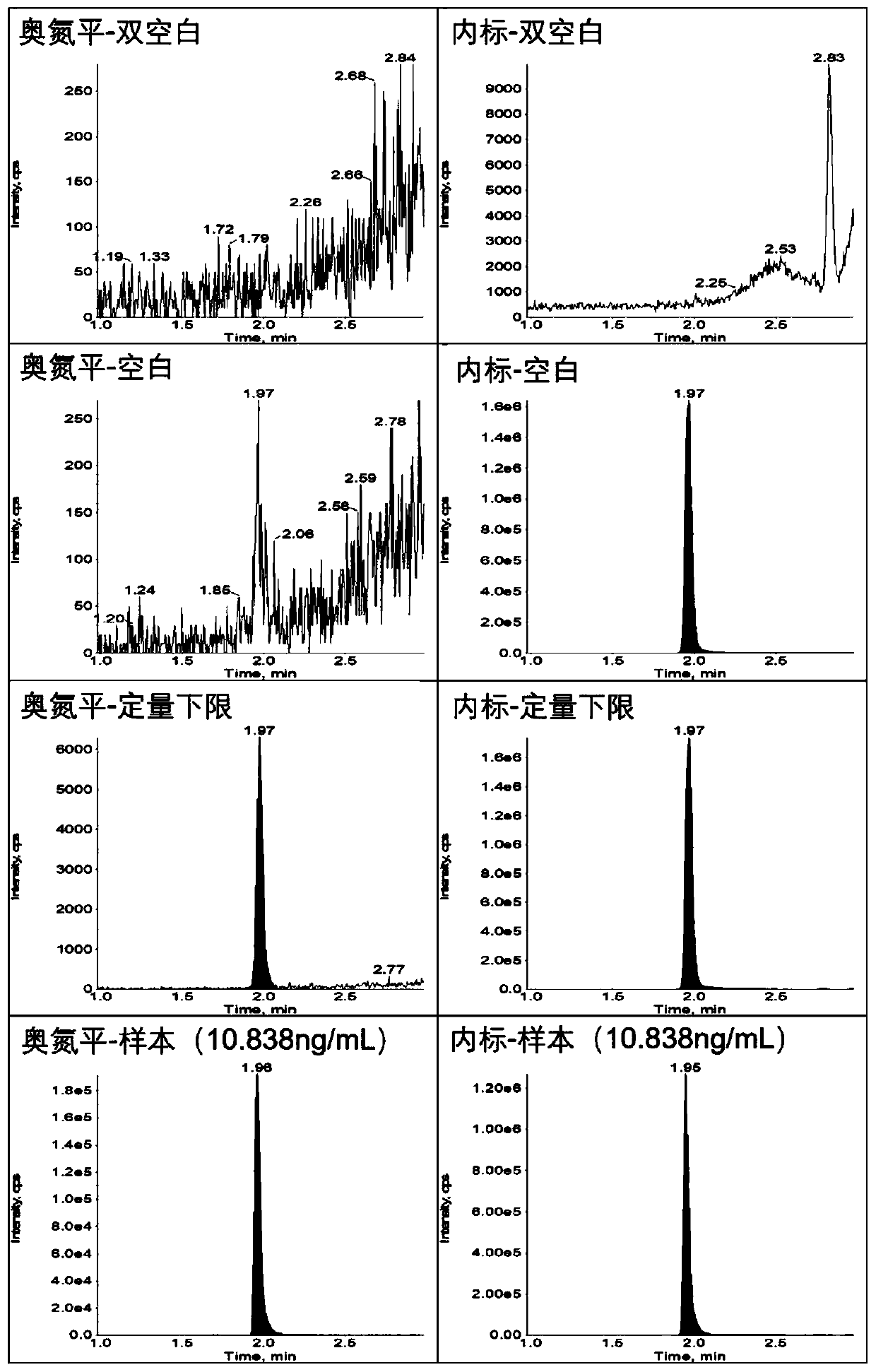
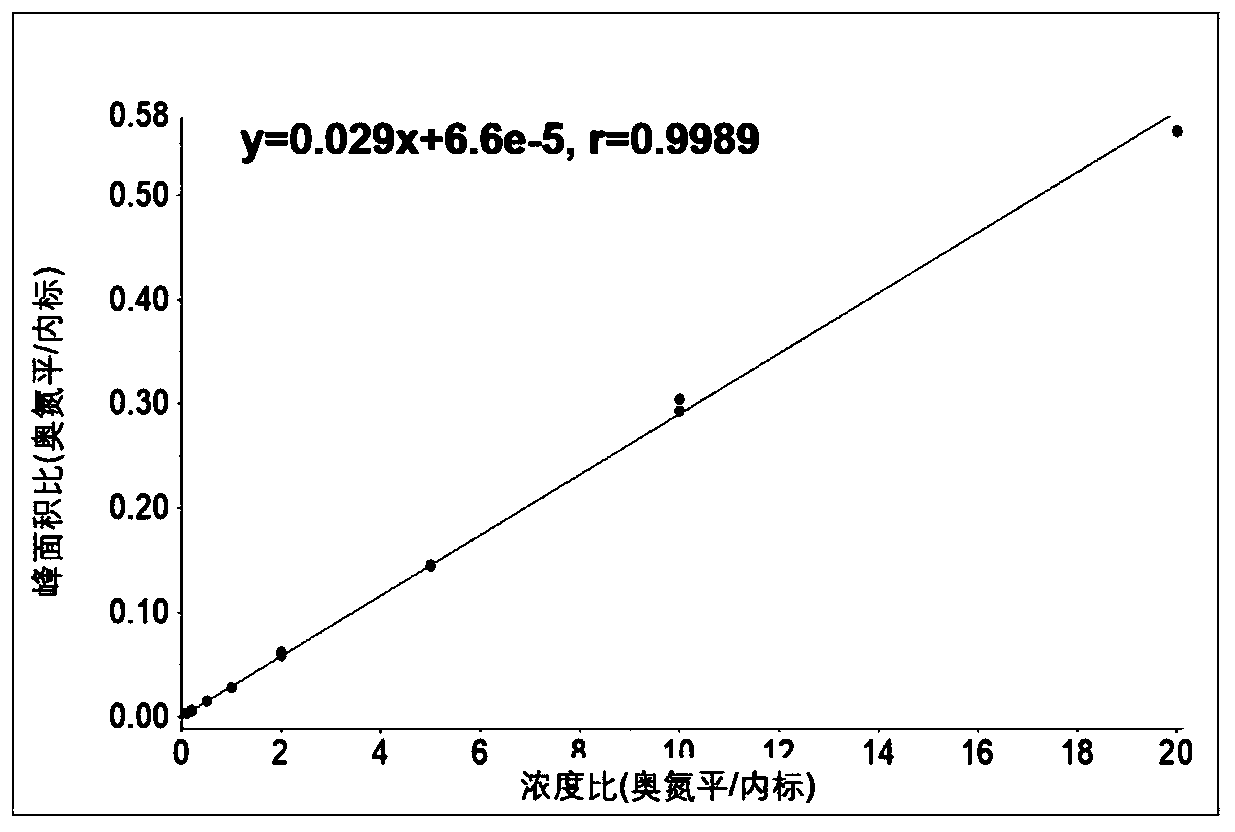
Method for determining concentration of olanzapine in blood plasma through high-performance liquid chromatography-tandem mass spectrometry
ActiveCN110068644AReduce distractionsAchieving peak symmetryComponent separationWorking fluidCentrifugation
The invention discloses a method for determining concentration of olanzapine in blood plasma through high-performance liquid chromatography-tandem mass spectrometry. The method comprises the followingsteps: preparing standard curve working fluid with a variety of concentrations by dissolving olanzapine with methanol, and preparing internal standard working fluid by dissolving stable isotope internal standard substances with the methanol; separately adding 0 microliter to 5 microliters of the standard curve working fluid with the variety of concentrations into blank plasma and complementing to100 microliters; swirling to prepare standard curve blood plasma samples with the variety of concentrations; adding the internal standard working fluid into the standard curve blood plasma samples, carrying out centrifugation, taking supernatant liquid and carrying out LC-MS / MS quantitative analysis, and drawing the standard curve; precisely sucking 100 microliters of to-be-tested blood plasma, adding the internal standard working fluid into the to-be-tested blood plasma, carrying out centrifugation, taking supernatant liquid and carrying out LC-MS / MS quantitative analysis, and reading the concentration of the olanzapine on the standard curve. The method disclosed by the invention has the characteristics of simpleness and convenience in sample pre-processing, short analysis time, small volumes of required samples, high sensitivity, high recycling ratio, small matrix effect and the like.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV
Method for rapidly detecting content of EGCG in tea polyphenol
PendingCN110161166AImprove washing abilityAvoid dissociationComponent separationGradient elutionPolyphenol
The invention belongs to the field of analytical chemistry, and particularly relates to an ultra-high performance liquid chromatography rapid detection technology, in particular to a method for efficiently and rapidly detecting the content of EGCG in tea polyphenol as a raw material by utilizing the ultra-high performance liquid chromatography detection technology. A C18 chromatographic column isadopted; water-glacial acetic acid-methanol with a certain proportion is used as a mobile phase; the content of the EGCG in the tea polyphenol is rapidly detected in a gradient elution mode; and the theoretical plate number of the EGCG under the chromatographic condition is greater than 16,000, and the separation degree is greater than 3. By adopting the detection method, the EGCG, EC and caffeinerelatively difficult to separate are effectively separated; and the method is short in detection time, high in sensitivity, good in reproducibility, efficient and rapid, and can be applied to the EGCG content detection and quality control.
Owner:广西轻工业科学技术研究院有限公司
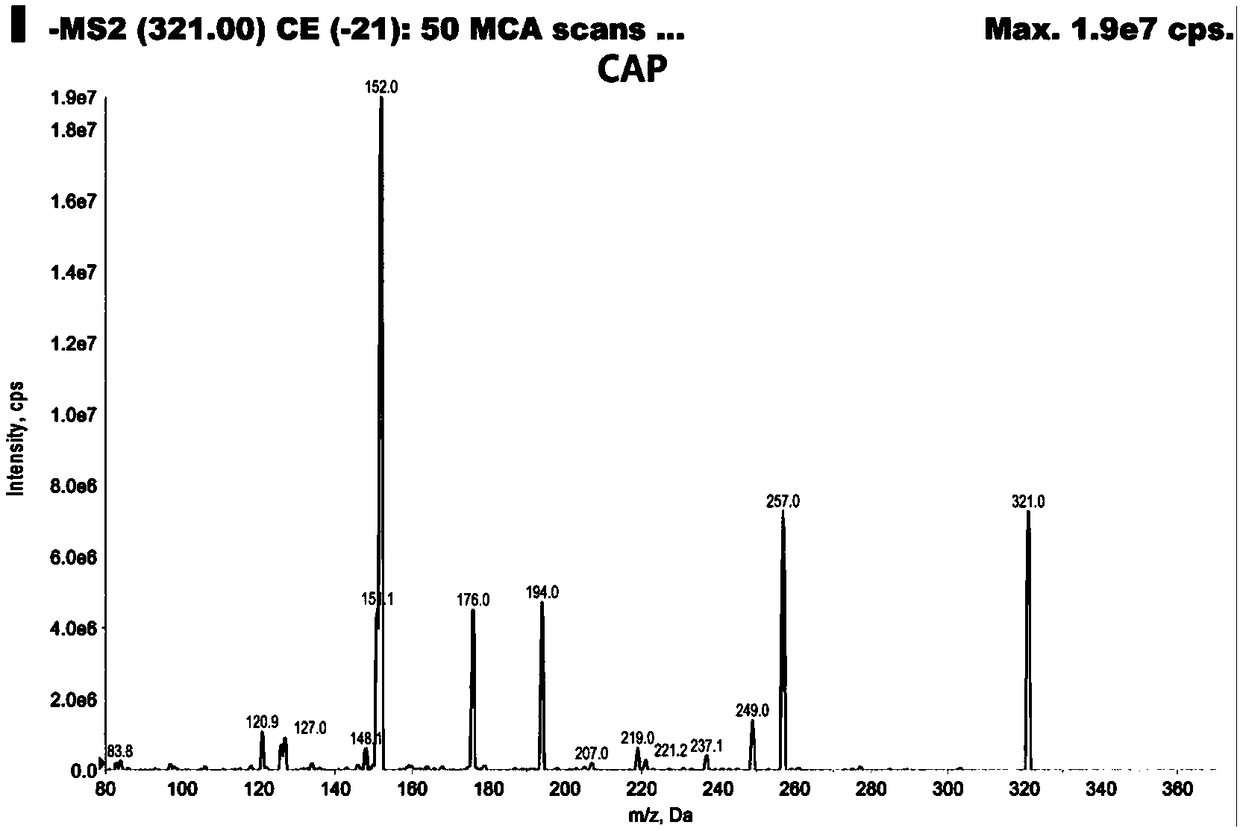
Analysis method for efficiently detecting multiple residues of chloramphenicol, thiamphenicol, florfenicol and metabolite florfenicol amine of florfenicol in eggs
InactiveCN108760938AHigh sensitivityShort peak timeComponent separationMetaboliteRelative standard deviation
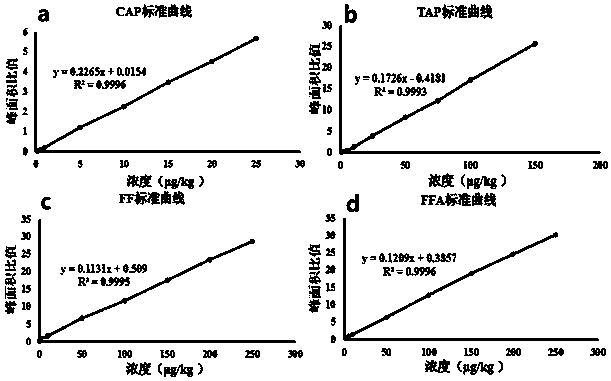
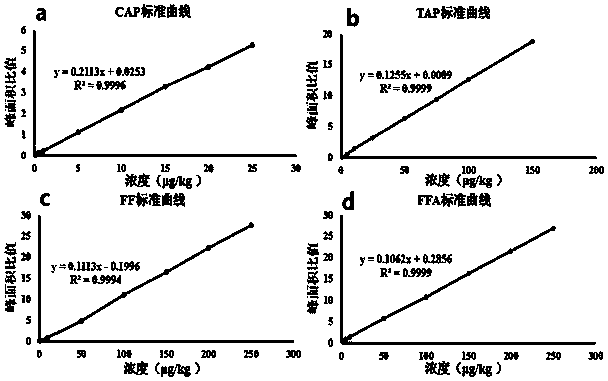
The invention relates to the field of veterinary drug residue detection, in particular to an analysis method for efficiently detecting multiple residues of chloramphenicol, thiamphenicol, florfenicoland metabolite florfenicol amine of the florfenicol in eggs. An ultra-high performance liquid chromatography-tandem mass spectrum detection method for the multiple residues of the chloramphenicol, thethiamphenicol, the florfenicol and the metabolite florfenicol amine of the florfenicol in eggs is established for the first time; the appearance time of target compounds is short (1 min about), the sensitivity is high (the LOD of CAP, the LOD of TAP, the LOD of FF and the LOD of FFA are 0.03 microgram / kg, 0.3 microgram / kg, 0.1 microgram / kg and 0.4 microgram / kg in eggs respectively, and the LOQ ofthe CAP, the LOQ of the TAP, the LOQ of the FF and the LOQ of the FFA are 0.08 microgram / kg, 0.8 microgram / kg, 0.27 microgram / kg and 1.2 micrograms / kg in eggs respectively), the recovery rates when the adding concentrations of the CAP, the TAP, the FF and the FFA are LOQ, 0.5 MRL, 1.0 MRL and 2.0 MRL in eggs respectively are larger than or equal to 90.31%, 93.40%, 92.32% and 92.35% respectively,and the within-day relative standard deviation and the daytime relative standard deviation are lower than 4.33% and 5.77 % respectively. Meanwhile, the analysis method is simple in elution program, small in solvent consumption, higher in analysis efficiency, short in time consumption (each sample needs 4 min) and more suitable for application and popularization in mass sample analysis.
Owner:YANGZHOU UNIV
Analysis method for efficiently detecting piperazine residues in chicken tissue, eggs and pork
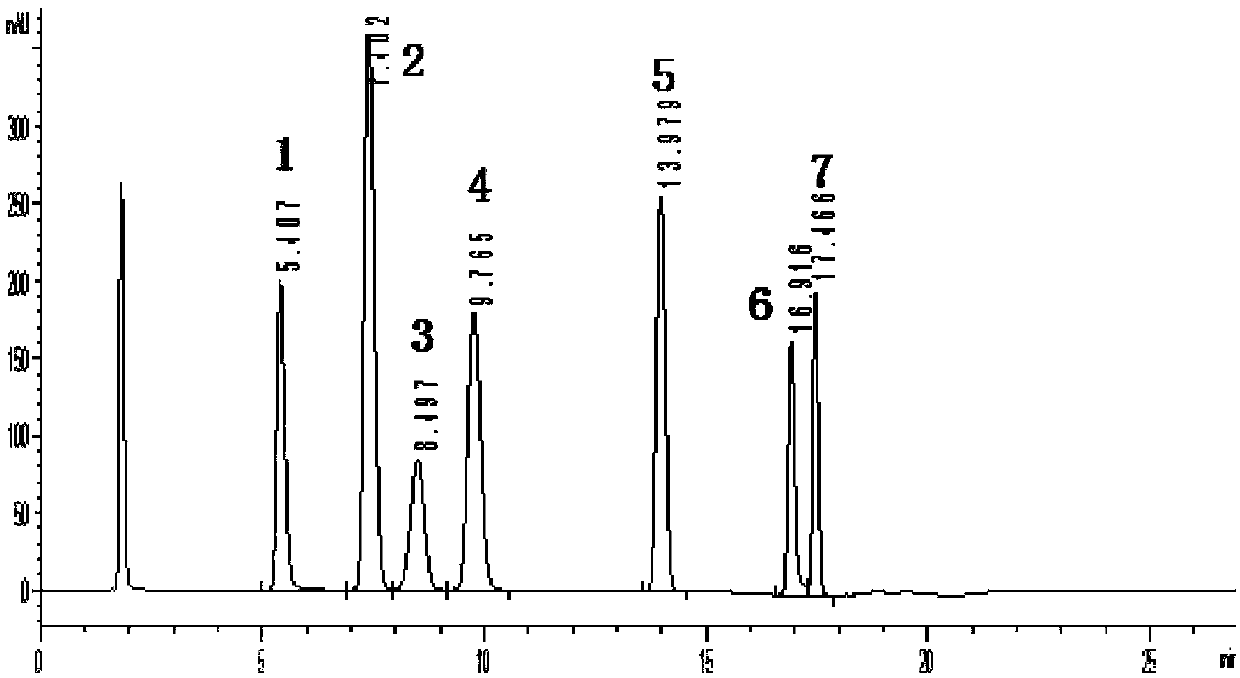
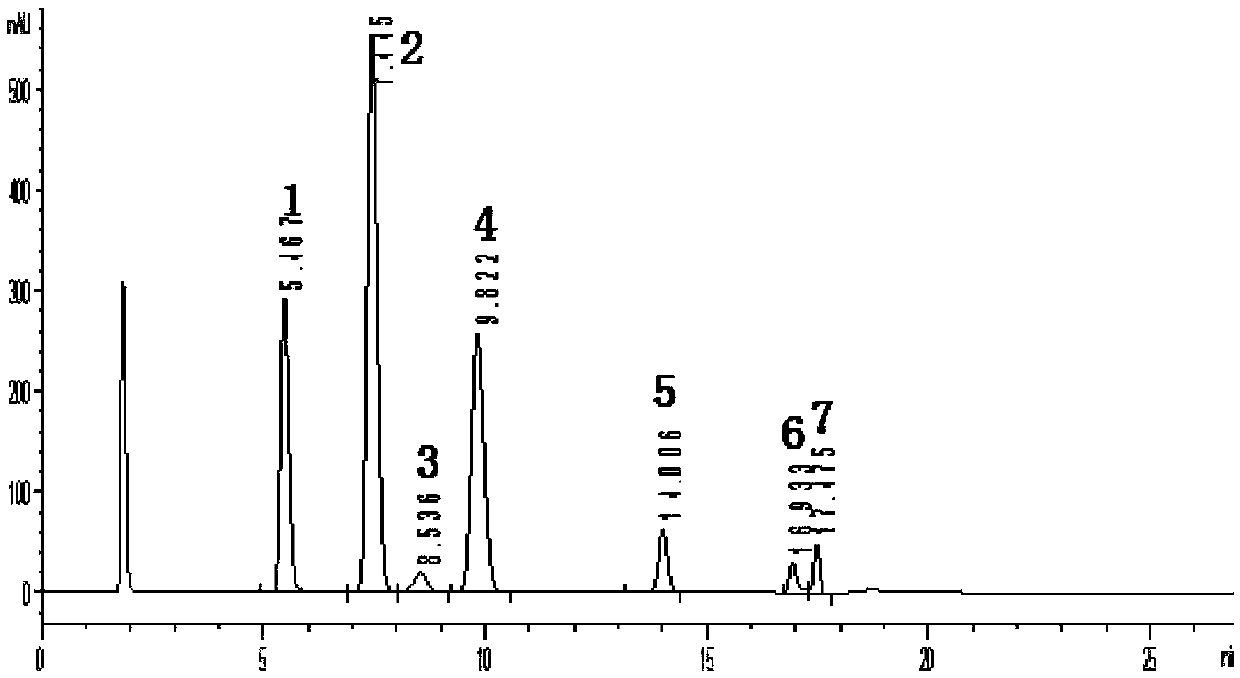
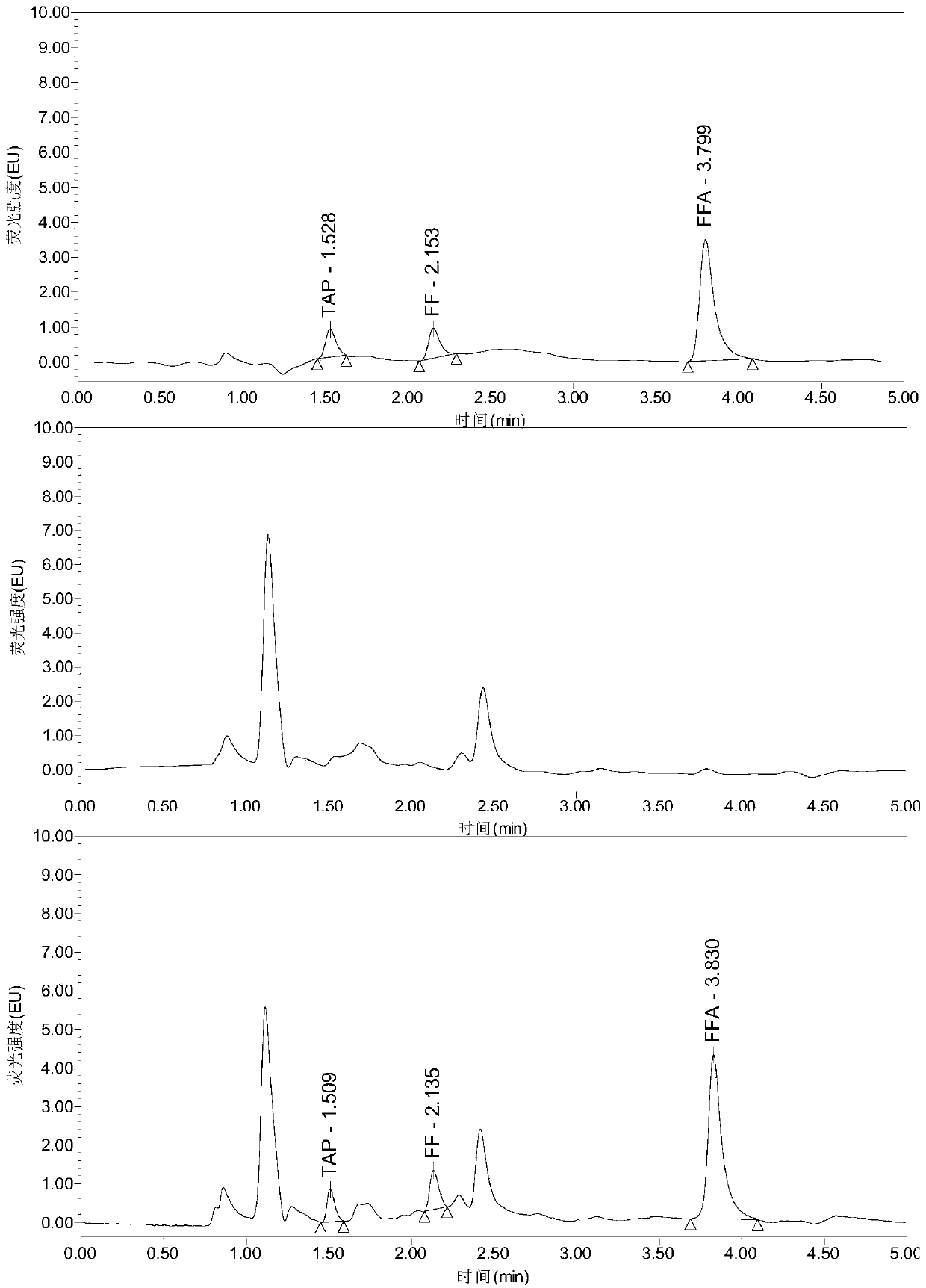
InactiveCN108287204AModerate retention timeNo distractionComponent separationLiquid Chromatography-FluorescenceDansyl chloride
The invention relates to the field of veterinary drug residue detection, in particular to an analysis method for efficiently detecting piperazine residues in chicken tissue, eggs and pork. The methodcomprises the steps of extracting and purifying the chicken tissue, eggs or pork samples through an accelerated solvent extraction method and a solid phase extraction method, performing derivatizationwith dansyl chloride and then performing detection by using an ultra-high performance liquid chromatography fluorescence detection method. Compared with other detection methods, the method has the advantages of higher analysis efficiency, less mobile phase consumption, a high recovery rate, high accuracy, high sensitivity and good reproducibility.
Owner:YANGZHOU UNIV
Method for assaying glucosamine and chondroitin sulfate
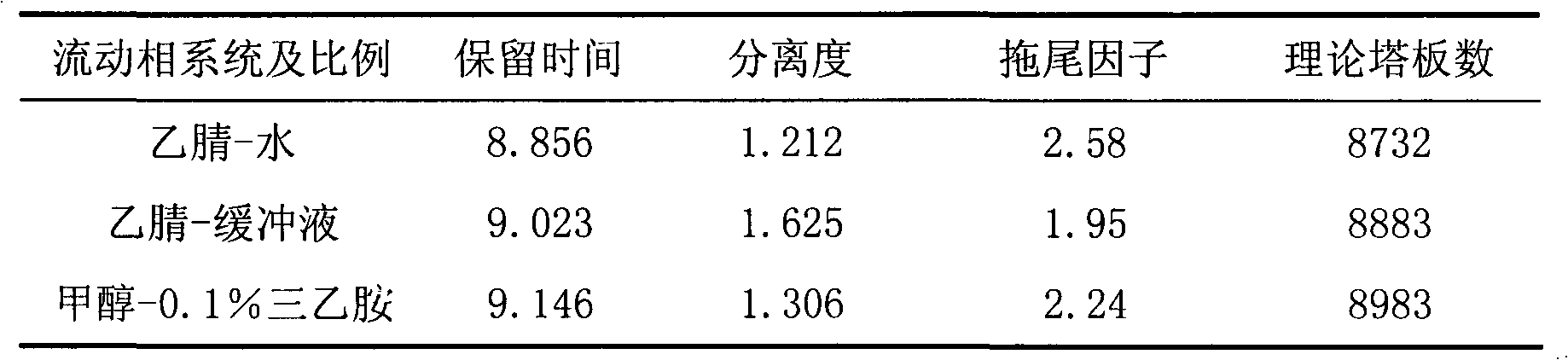
ActiveCN108872441AHigh sensitivityImprove efficiencyComponent separationRetention timeLinear relationship
The invention relates to the technical field of analytical chemistry and particularly relates to a method for simultaneously assaying glucosamine and chondroitin sulfate in healthy foods. According tothe method, by adopting an evaporative light scattering detector, the sensitivity of detection on the glucosamine and the chondroitin sulfate is improved; by adopting an amide chromatographic column,the retention time is moderate, the degree of separation is good, the separation of all chromatographic peaks is relatively good, the reference line is stable, the peak form is good, and the repeatability is relatively good. The method has good linear relationship, precision, stability and recovery rate, can be used for accurately assaying the content of the glucosamine and the chondroitin sulfate in the healthy foods simultaneously, is applicable to the assaying of the glucosamine and the chondroitin sulfate in the healthy foods and is also applicable to the assaying of glucosamine or chondroitin sulfate raw materials.
Owner:JIANGSU KANION PHARMA CO LTD
Method for measuring content of dimethylbenzene oxidation products by using HPLC external standard method
InactiveCN107907615AEfficient separationShort analysis timeComponent separationBenzoic acidUv vis absorbance
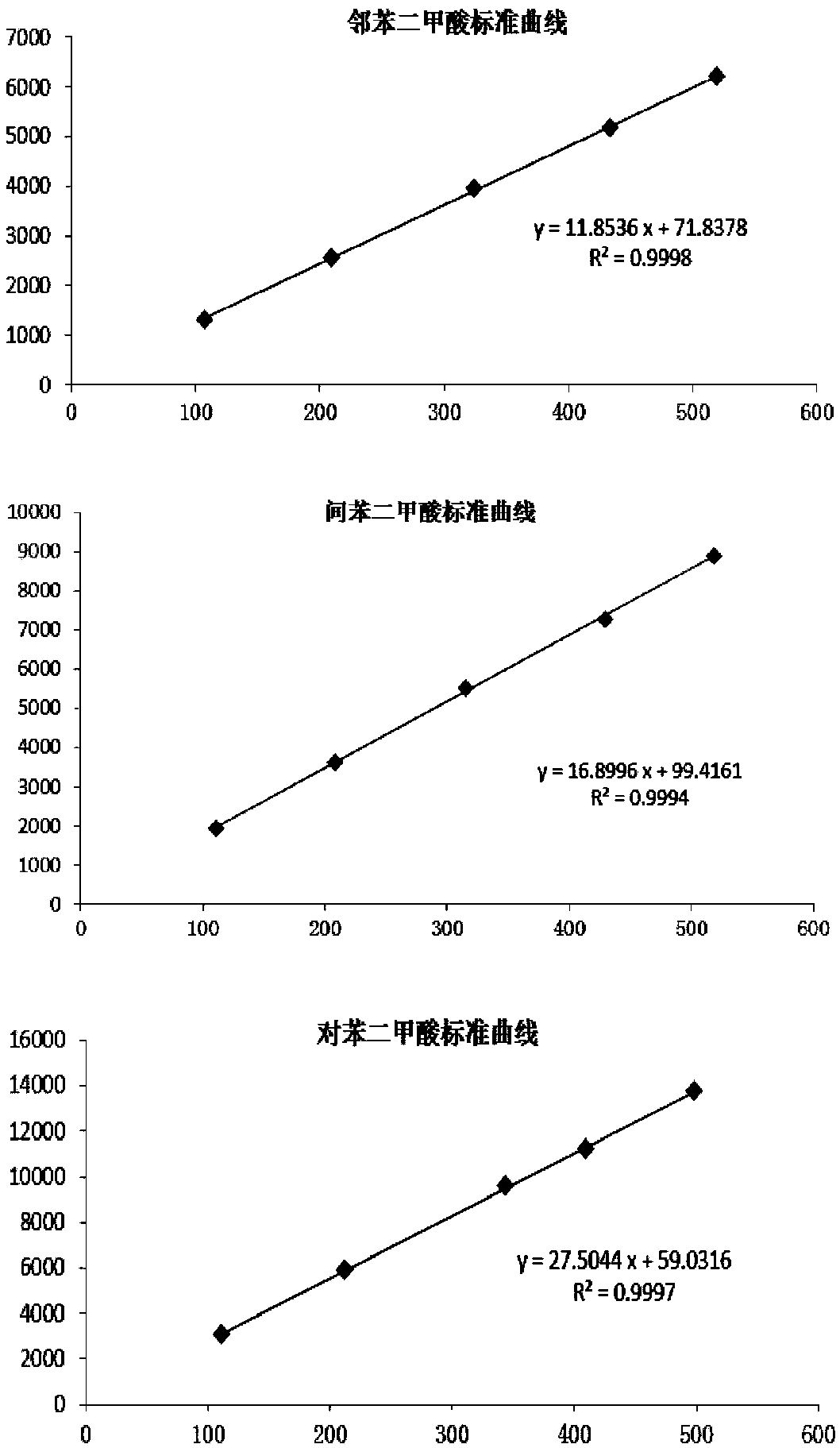
The invention discloses a method for measuring content of dimethylbenzene oxidation products by using an HPLC external standard method. According to the method, a high performance reversed-phase liquid chromatograph instrument is adopted, a C18 silica gel chromatographic column is taken as a separation column, an ultraviolet visible absorption detector is adopted, methanol and an acid aqueous solution are taken as mobile phases, phthalate, isophthalic acid and terephthalic acid are taken as standard samples, a concentration-peak area standard curve of a target component is drawn by using the external standard method so as to obtain a linear regression equation, and contents of the phthalate, isophthalic acid and terephthalic acid are calculated by using the regression equation. Through adoption of the method, three main products with similar polarity and four byproducts including 3-formylbenzoic acid, benzoic acid, paratoluic acid and p-tolualdehyde can be separated effectively, and the method takes short time in analysis, is easy and feasible, and is convenient and reliable.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
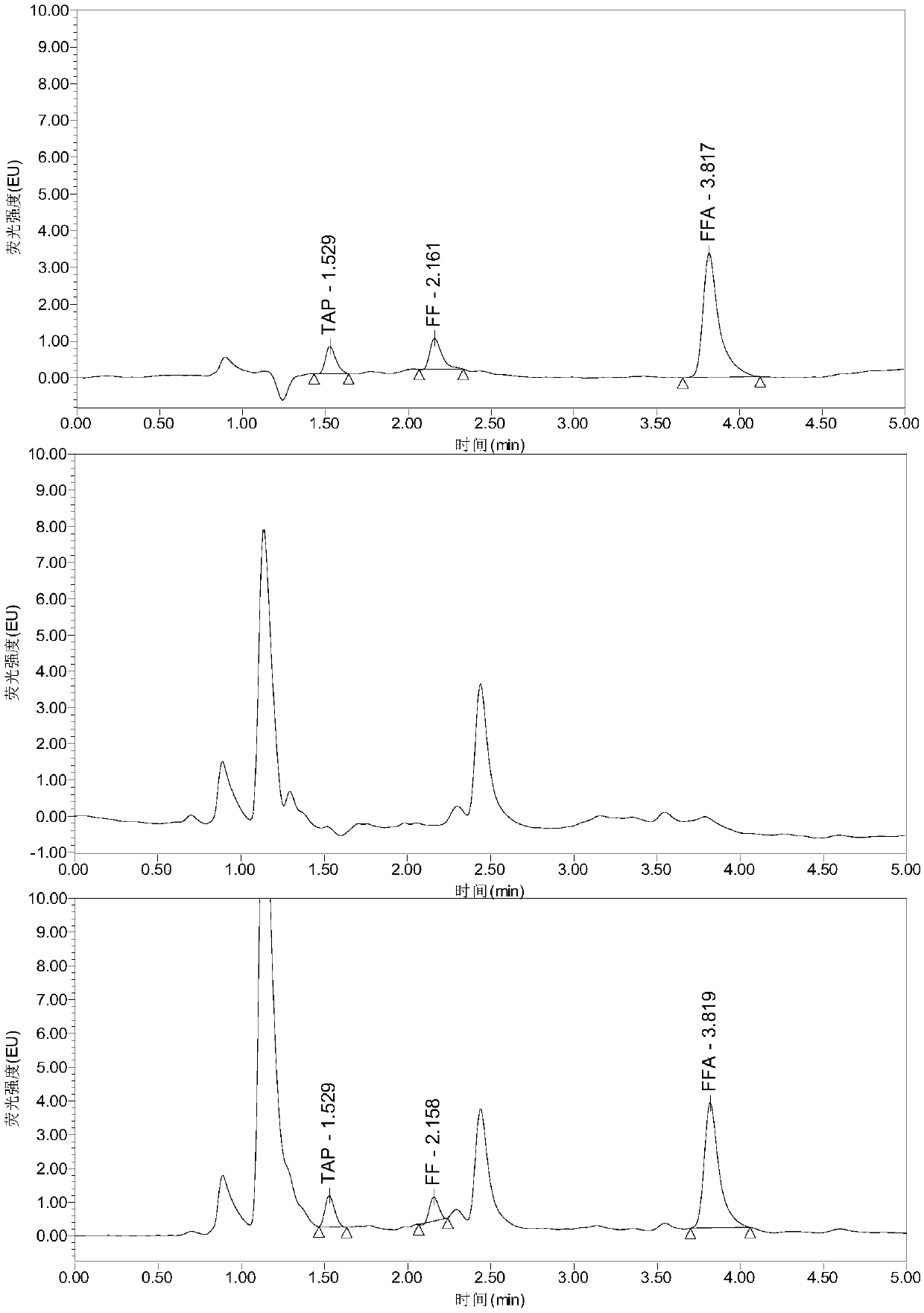
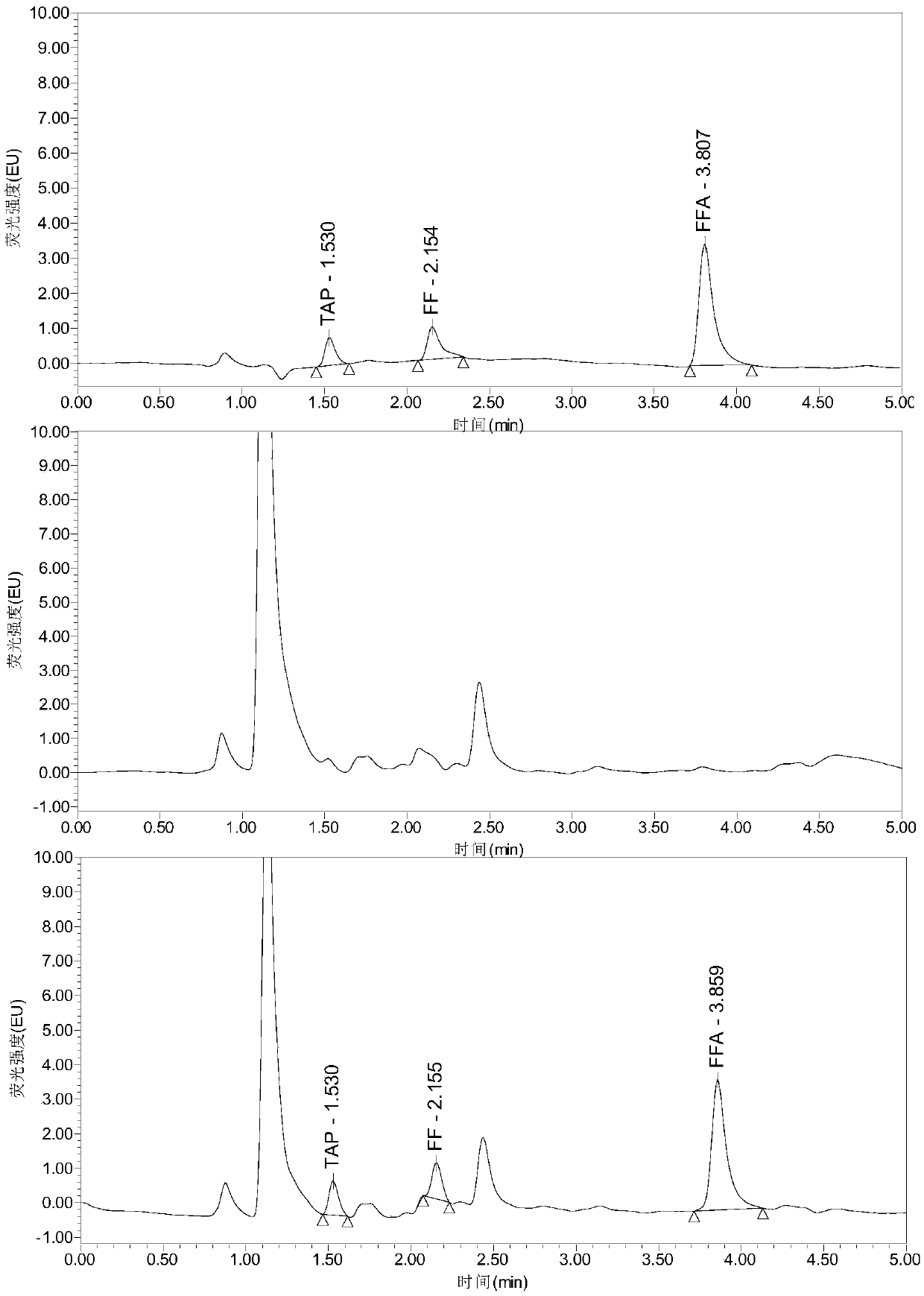
Analysis method for efficiently simultaneously detecting thiamphenicol, florfenicol and florfenicol amine multiple residues in poultry eggs, poultry meat and pork
InactiveCN109655551AModerate retention timeNo distractionComponent separationLiquid Chromatography-FluorescenceFluorescence
The invention relates to the field of veterinary drug residue detection, in particular to an analysis method for efficiently simultaneously detecting thiamphenicol, florfenicol and florfenicol amine multiple residues in poultry eggs, poultry meat and pork. The analysis method includes steps of 1), extracting and purifying poultry egg, poultry meat and pork samples; 2), carrying out ultrahigh-performance liquid chromatography-fluorescence detection. The ultrahigh-performance liquid chromatography-fluorescence detection includes a ultrahigh-performance liquid chromatography conditions and b fluorescence detection. The ultrahigh-performance liquid chromatography conditions include that aqueous solution with 0.005 mol / L of NaH2PO4, 0.003 mol / L of sodium lauryl sulfate and 0.05% of triethylamine is used as mobile phase A, and acetonitrile is used as mobile phase B. The analysis method has the advantages that the residues of the thiamphenicol, the florfenicol and the florfenicol amine in thepoultry eggs (chicken eggs, duck eggs, goose eggs, pigeon eggs and quail eggs), the poultry meat (chicken, duck meat and goose meat) and the pork can be efficiently and accurately simultaneously detected by the aid of the analysis method, and the florfenicol amine is metabolite of the florfenicol; the requirements of the Ministry of Agriculture of China, the FDA (Food and Drug Administration) ofthe America and the European Union (EU) on methods for detecting veterinary drug residues can be met.
Owner:YANGZHOU UNIV
Method for determining other amino acids in L-valine raw material by high performance liquid chromatography
ActiveCN111505151ASolve the problem of short service life and unstable baseline of reversed phaseEasy to separateComponent separationSilica gelPhenylalanine
The invention provides a method for determining other amino acids in an L-valine raw material by high performance liquid chromatography. The method comprises the following steps of preparing L-valine,leucine, isoleucine, glycine, alanine and phenylalanine reference substance solutions; detecting through a high performance liquid chromatograph, comparing the appearance time and peak shape of L-valine, leucine, isoleucine, glycine, alanine and phenylalanine in a chromatogram of the test sample with those of L-valine, leucine, isoleucine, glycine, alanine and phenylalanine in a chromatogram of the reference substance solution, and judging whether the test sample solution contains other amino acids or not. According to the method, by screening an aminopropyl bonded silica gel chromatographiccolumn, the separation effects are found, and by screening the wavelengths, the maximum absorption of each amino acid is found, and the retention time of each amino acid peak on the chromatographic column is regulated and controlled by regulating the ratio of triethylamine, the pH value and the ratio of two phases by utilizing an acetonitrile / phosphate buffer solution binary mobile phase, so thatthe hydrolysis and shedding of bonded aminopropyl in the amino column are effectively prevented, and the problems of short reverse-phase service life and unstable baseline of the amino column are solved.
Owner:宜昌三峡普诺丁生物制药有限公司
A method of measuring the EGCG content in tea polyphenols
InactiveCN106168608AEfficient separationEasy to separateComponent separationRetention timeQuality control
A method of measuring the EGCG content in tea polyphenols is disclosed. The method includes (1) a step of setting high performance liquid chromatography conditions, (2) a step of preparing a contrast solution, (3) a step of preparing a solution of a sample to be detected, and (4) a step of measuring the content, namely a step of separately measuring the contrast solution and the solution of the sample to be detected, injecting into a liquid chromatograph, performing operation of the high-efficient liquid chromatograph, recording chromatograms and calculating the EGCG content by utilizing the chromatograms. The method adopts a gradient elution manner, and can effectively separate EGCG and EC which are two components difficult to separate in the tea polyphenols. The method is simple, convenient, high in sensitivity, good in reappearance, good in sample separation degree, good in symmetry, good in peak shapes, proper in retention time and more suitable for routine detection, and can be effectively used for EGCG content detection and quality control.
Owner:JIANGSU TIANSHENG PHARMA
Method for determining content of clindamycin phosphate vaginal tablets
ActiveCN112684056AEasy to separateHigh measurement accuracyComponent separationFluid phaseClindamycin Phosphate
The invention provides a method for determining the content of clindamycin phosphate vaginal tablets. The method adopts high performance liquid chromatography for detection, selects mobile phase components, adopts scientific proportioning, adopts gradient elution and sets elution conditions, and can rapidly and accurately detect the content of clindamycin hydrochloride for injection so as to achieve the purpose of simply, conveniently, rapidly and accurately controlling the product quality.
Owner:HAINAN HAISHEN TONGZHOU PHARM CO LTD
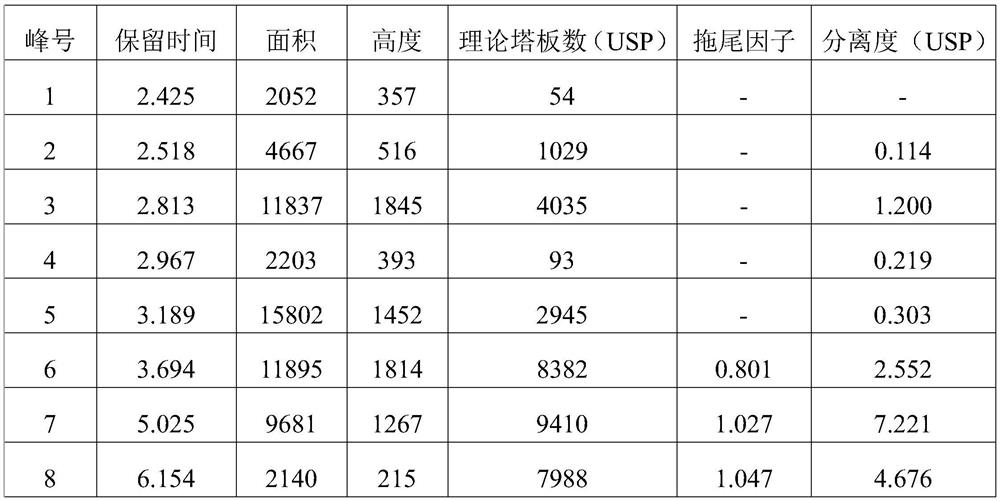
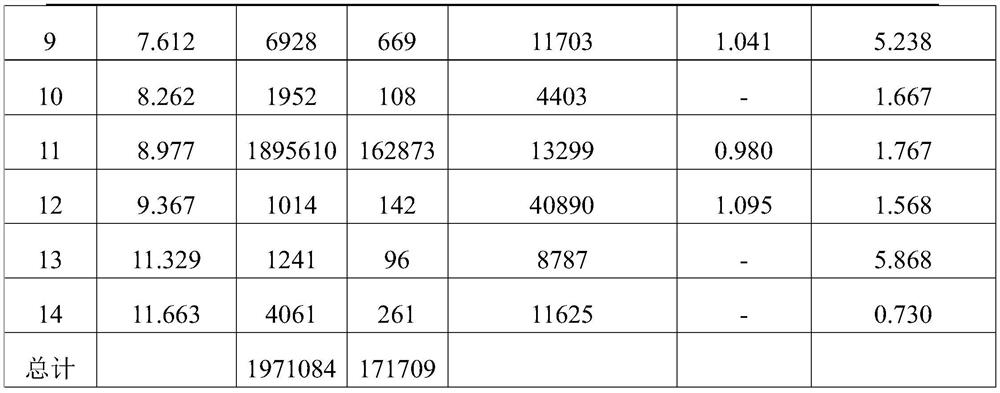
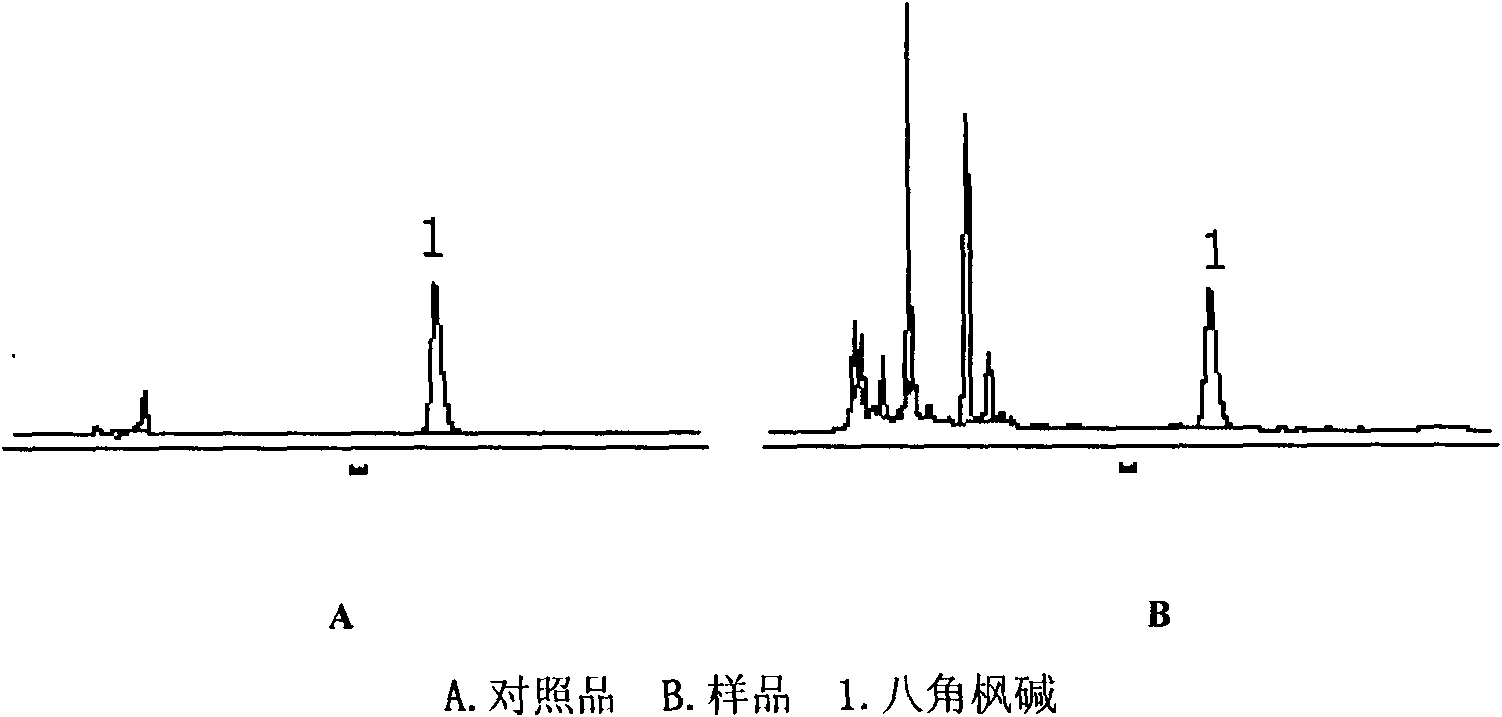
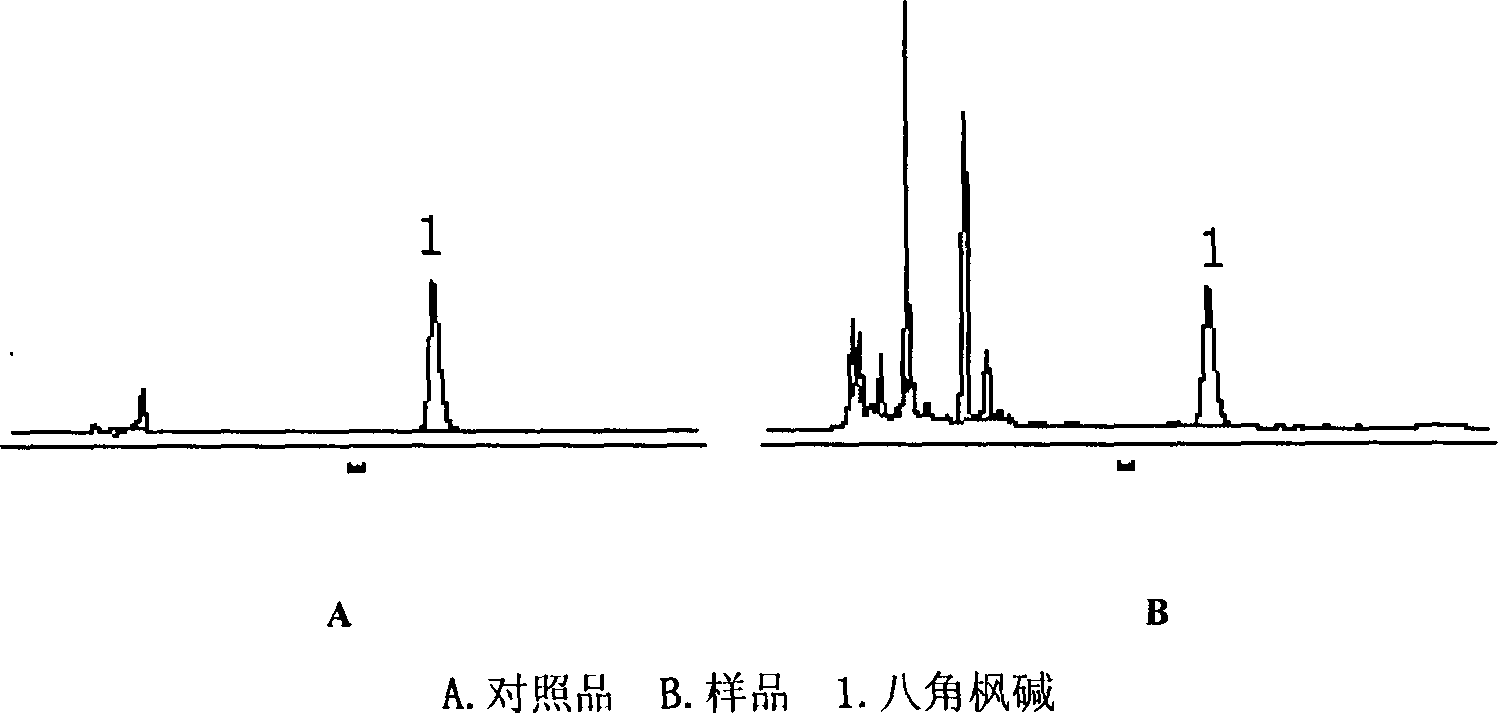
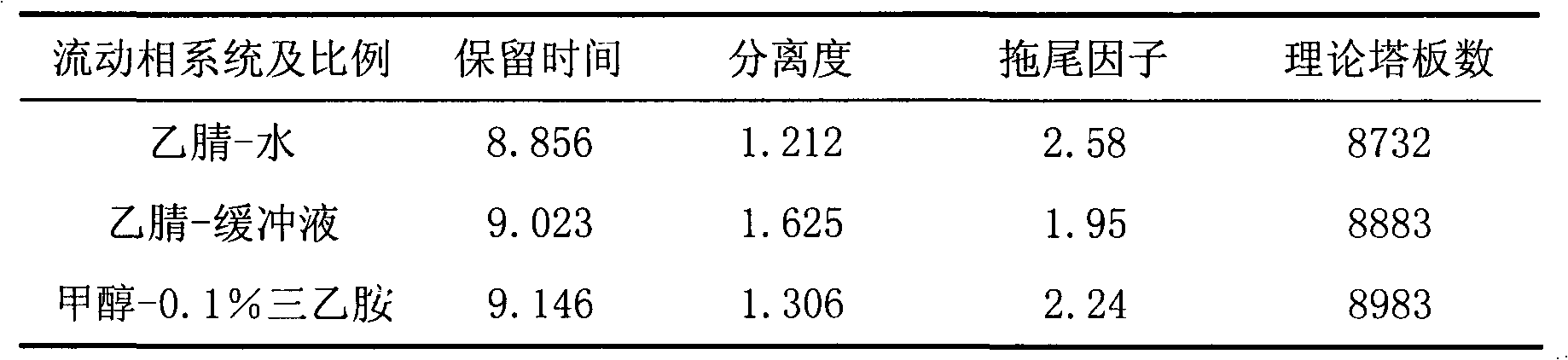
Content determination method for anabasine
ActiveCN102590368AEasy to separateModerate retention timeComponent separationPlant ingredientsRational useContent determination
The invention provides a content determination method for anabasine, which is used for determining content of the anabasine in alangium chinense which is a traditional Chinese medicinal material. The content determination method includes firstly extracting the traditional Chinese medicinal material alangium chinense by means of ultraphonic, obtaining a sample solution, and determining the content of the alangium chinense by means of high performance liquid chromatography. The content determination method has the advantages of being easy, simple and fast to operate, high in specialization, precision degree and recovery rate, exact in determination results, and the like, the purpose of completing the quality standard of the alangium chinense is achieved, and experimental basis is provided for safe, effective and rational use of the alangium chinense.
Owner:北京创立科创医药技术开发有限公司
Method for detecting impurity F and impurity G of omeprazole sodium
InactiveCN112816590APrevent leakageEfficient detectionComponent separationOmeprazole SodiumFluid phase
The invention provides a method for detecting an impurity F and an impurity G of omeprazole sodium. The omeprazole sodium impurity F and the omeprazole sodium impurity G are detected by adopting high performance liquid chromatography, optimization is carried out on the basis of raw material medicines, and the proportion of an organic phase is increased so that the omeprazole sodium impurity F and the omeprazole sodium impurity G can be effectively eluted, and the omeprazole sodium impurity F and the omeprazole sodium impurity G can be effectively detected. Main peak retention time, an area and a height are moderate, a theoretical plate number and a separation degree are good, a tailing factor is in the range of 0.9-1.95, the S / N is 10-30, the requirements are met, and a reference basis is provided for the quality control of omeprazole sodium.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Blood-production preparation quality test method and construction method of standard fingerprint spectrum thereof
ActiveCN103926351BThe pretreatment method is simpleImprove stabilityComponent separationTest samplePhosphoric acid
Owner:KANGSHOU PHARMACY CO LTD HUNAN
A fingerprint detecting method for a Shensu preparation
ActiveCN106645534AThe pretreatment method is simpleImprove stabilityComponent separationPhosphoric acidQuality control
The invention relates to the technical field of medicine quality control, particularly relates to a fingerprint detecting method for a Shensu preparation. The method includes preparing the Shensu preparation into a solution to be tested, adopting puerarin as a contrast, detecting through high performance liquid chromatography, and marking common peaks to obtain a fingerprint of the Shensu preparation. According to chromatographic conditions, a chromatographic column is a C18 reversed phase chromatographic column, a mobile phase A is acetonitrile, a mobile phase B is a phosphoric acid solution and gradient elution is adopted.
Owner:KANGSHOU PHARMACY CO LTD HUNAN
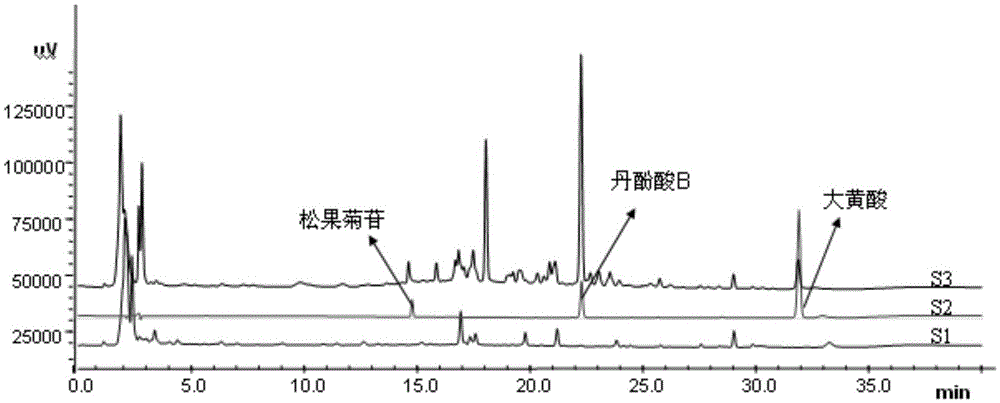
Method for detecting quality of salviae miltiorrhizae medicinal materials
PendingCN110646542AReduced resolutionQuality improvementComponent separationMedicinal herbsHplc fingerprint
The invention relates to a method for detecting the quality of salviae miltiorrhizae medicinal materials. An HPLC fingerprint measurement method of salviae miltiorrhizae medicinal materials is established. The method has the advantages of simple operation, stability, high precision and good reproducibility. In a common mode of the established HPLC fingerprint of the salviae miltiorrhizae medicinalmaterials, the analysis results of correlation coefficients show that the similarity of the salviae miltiorrhizae medicinal materials and the salviae miltiorrhizae medicinal materials in different processing methods is generally higher than 0.900, and it is intuitively shown that the chemical components of salviae miltiorrhizae medicinal materials and the salviae miltiorrhizae medicinal materialsin different processing methods are relatively stable, and the safety and effectiveness of the medicinal materials are improved.
Owner:贵州中医药大学
Detection method of residual solvents in (N-(3-chloro-4-(3-fluorobenzyloxy)phenyl-6-(3-(4-methyl-4-oxo-1-nitrogen-4-phosphorus hetero yclohexane-1-yl)propyl-1-alkynyl)quinazoline-4-amine, bis 4-methyl benzenesulfonate bulk drug
InactiveCN105388242ALow detection limitHigh precisionComponent separationN dimethylformamideColumn temperature
The invention relates to the technical field of analysis chemistry, and in particular relates to a detection method of residual solvents in a (N-(3-chloro-4-(3-fluorobenzyloxy)phenyl-6-(3-(4-methyl-4-oxo-1-nitrogen-4-phosphorus hetero yclohexane-1-yl)propyl-1-alkynyl)quinazoline-4-amine, bis 4-methyl benzenesulfonate bulk drug. According to the method, the (N-(3-chloro-4-(3-fluorobenzyloxy)phenyl-6-(3-(4-methyl-4-oxo-1-nitrogen-4-phosphorus hetero yclohexane-1-yl)propyl-1-alkynyl)quinazoline-4-amine, bis 4-methyl benzenesulfonate bulk drug is dissolved by DMSO, and then is injected in headspace, and the contents of four residual solvents, namely methyl alcohol, ethanol, isopropyl alcohol and N,N-dimethylformamide are detected by gas chromatography; and the column temperature of the gas chromatography is maintained for 5min at 35-50 DEG C, and then raised to 220+ / -10 DEG C at the speed of 20 DEG C / min and maintained for 5-9min. According to the detection method, by verification, methyl alcohol has a detection limit of 0.3ppm and a quantification limit of 1.6ppm, ethanol has a detection limit of 0.5ppm and a quantification limit of 2.5ppm, isopropyl alcohol has a detection limit of 0.5ppm and a quantification limit of 2.6ppm, and DMG has a detection limit of 8.5ppm and a quantification limit of 19.1ppm. After the same sample is repeatedly detected for six times, the RSD is less than or equal to 2%, and the added sample recovery rate is 97.4%-103.1%, which indicates that the accuracy and precision are good.
Owner:JIANGSU KANION PHARMA CO LTD
Method for determining content of nitidine chloride in radix zanthoxyli toothpaste
The invention discloses a method for determining the content of nitidine chloride in radix zanthoxyli toothpaste, and belongs to the field of chemical detection. The method is accurate, simple and convenient and can be used for controlling the content of nitidine chloride in the radix zanthoxyli toothpaste. The method comprises the following sub-steps sequentially: firstly, extracting nitidine chloride from radix zanthoxyli with ethanol, and collecting ethanol extract; secondly, determining the content of nitidine chloride in the ethanol extract with HPLC (high performance liquid chromatography). The method can be used for determining the content of nitidine chloride in the radix zanthoxyli toothpaste.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
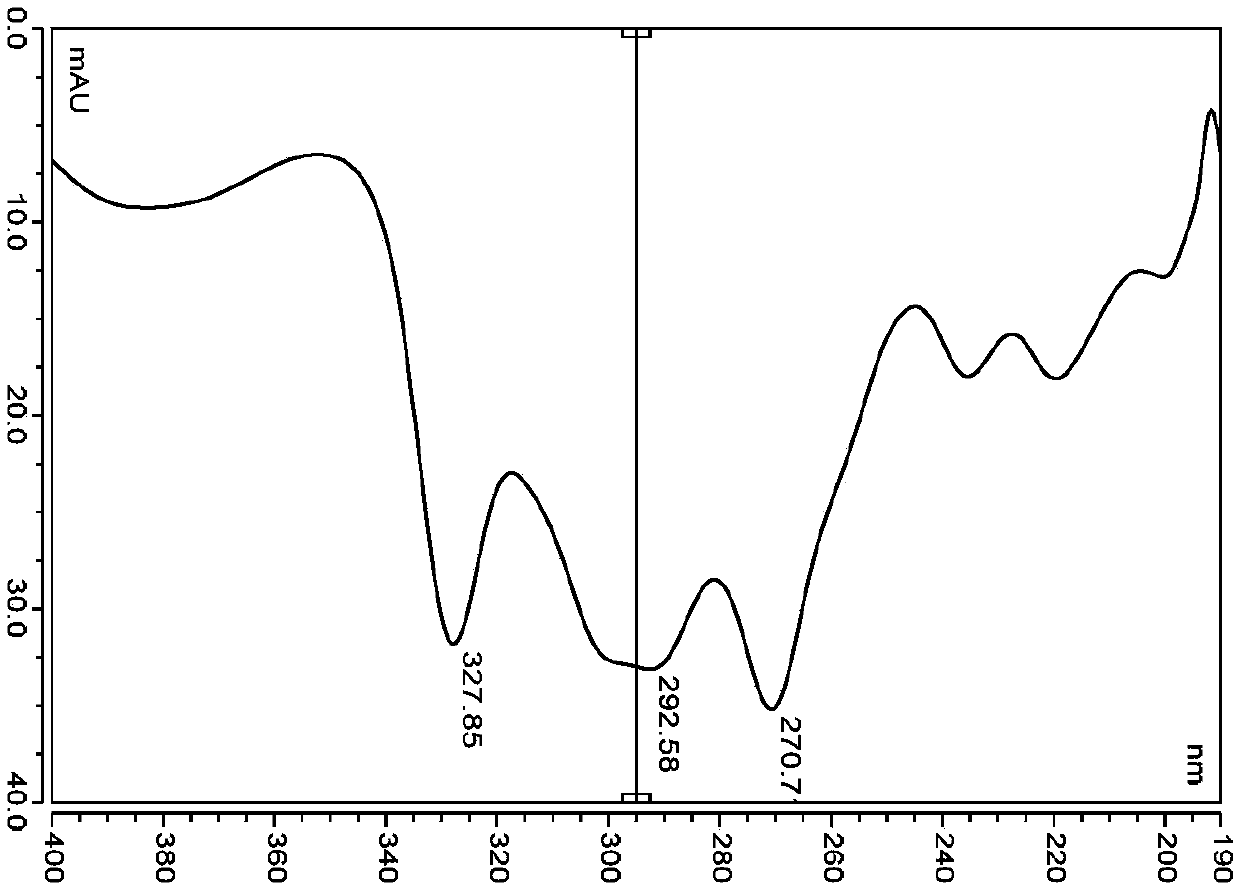
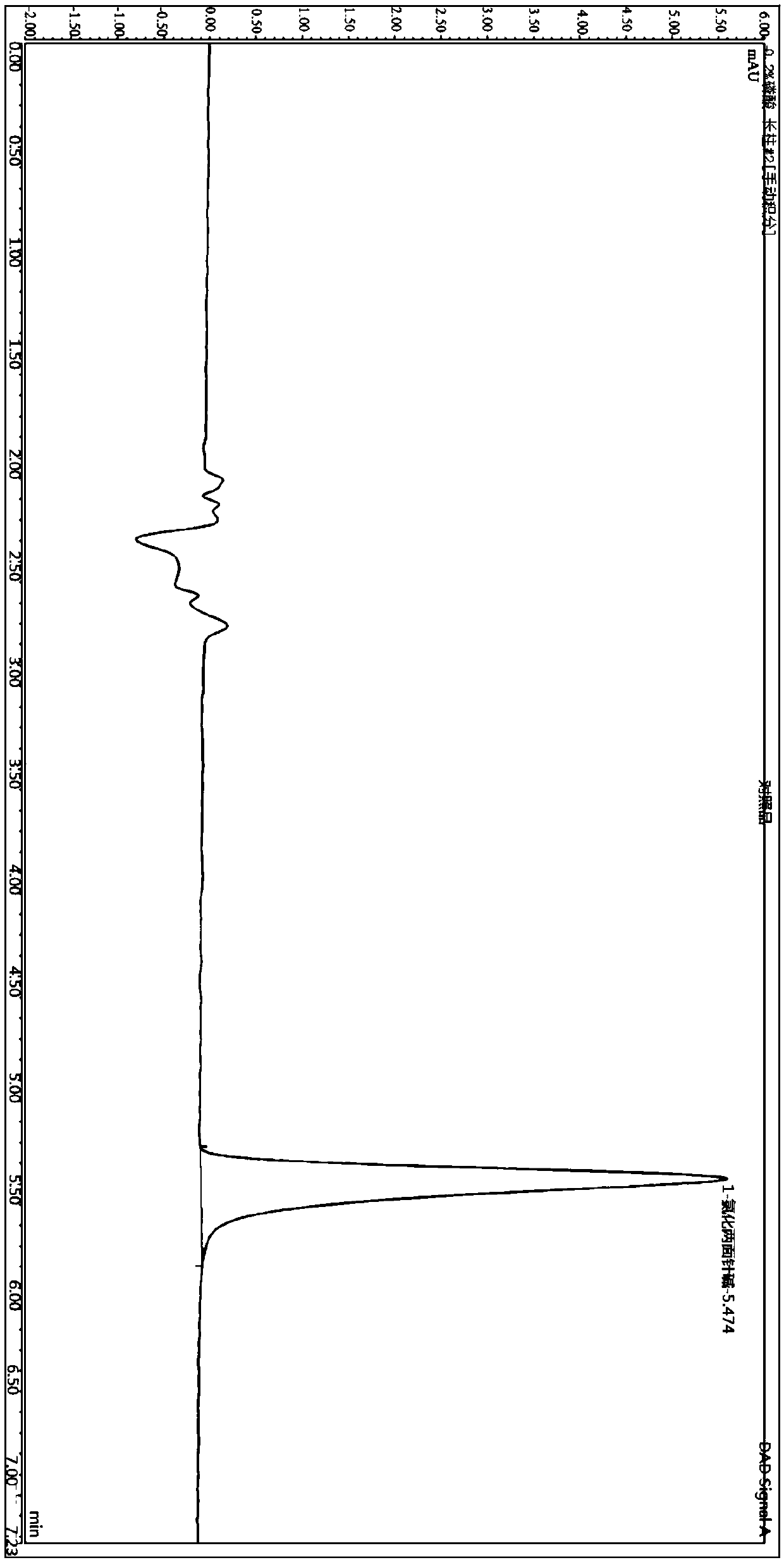
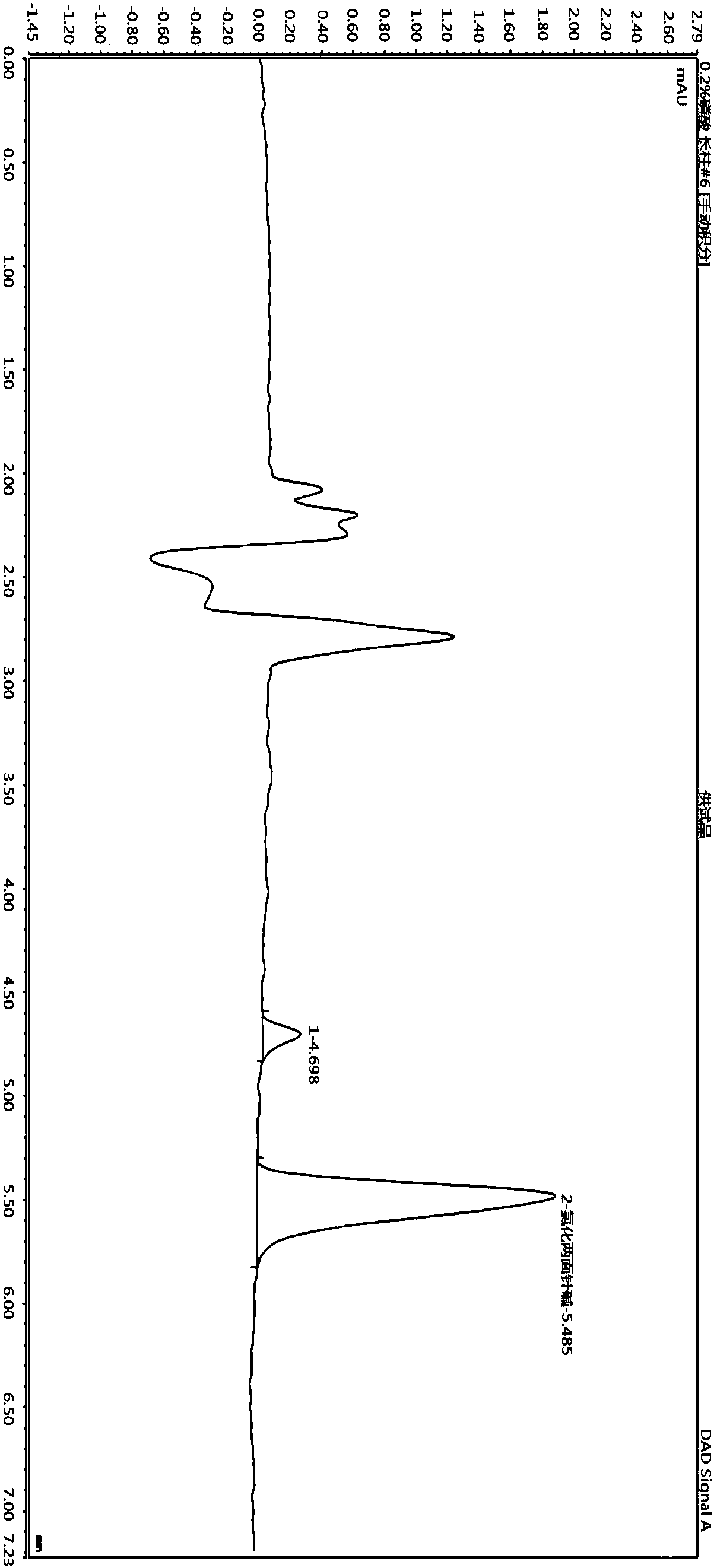
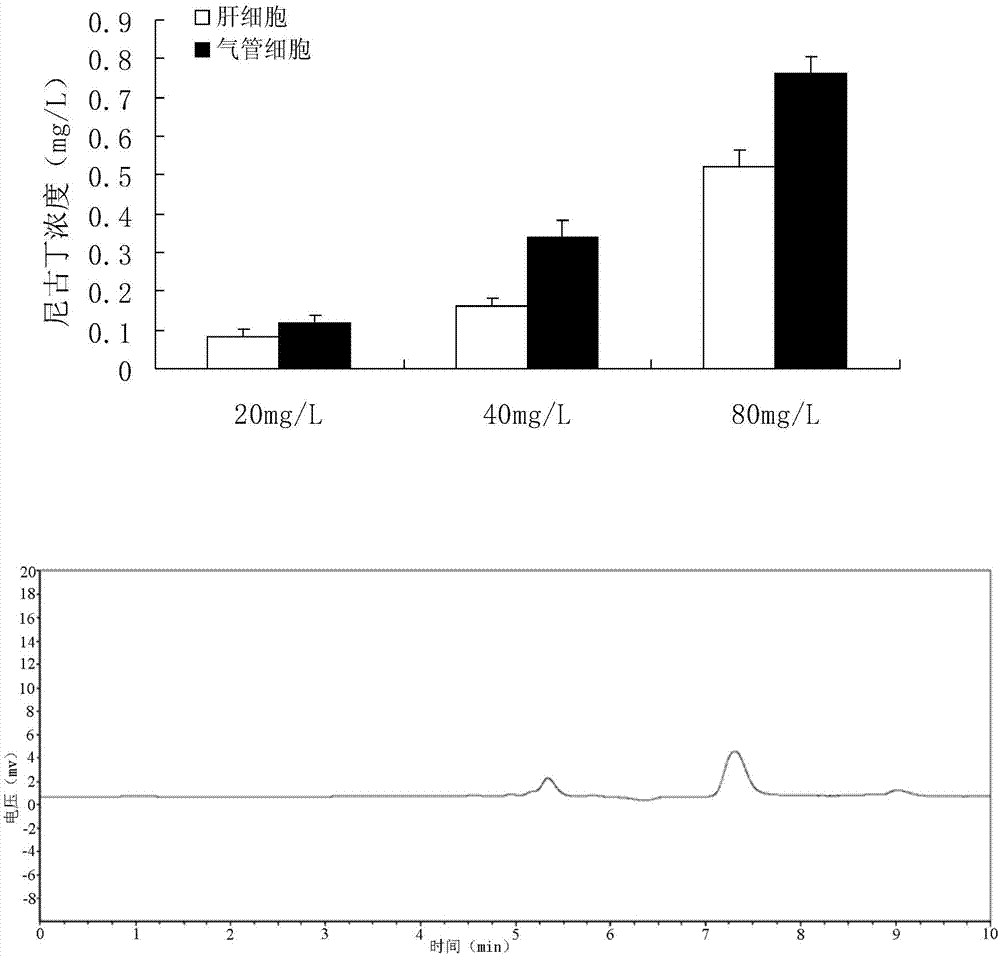
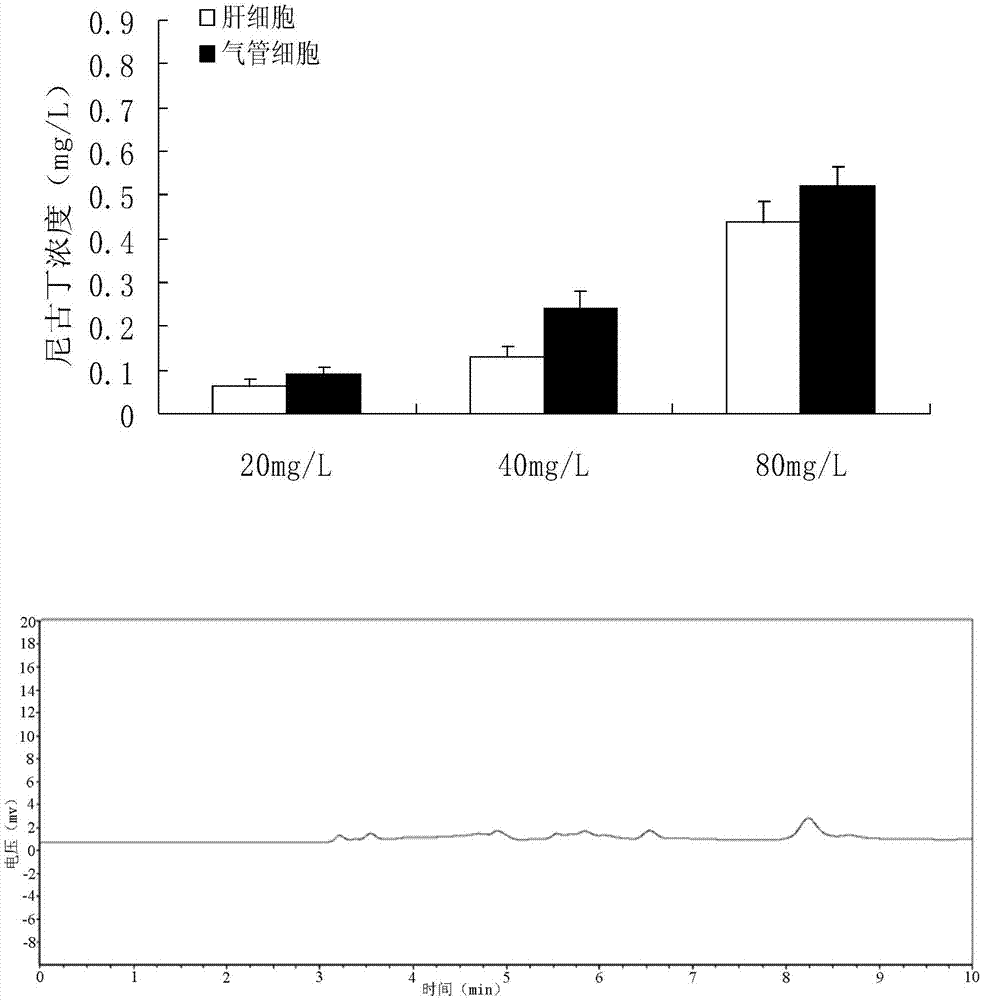
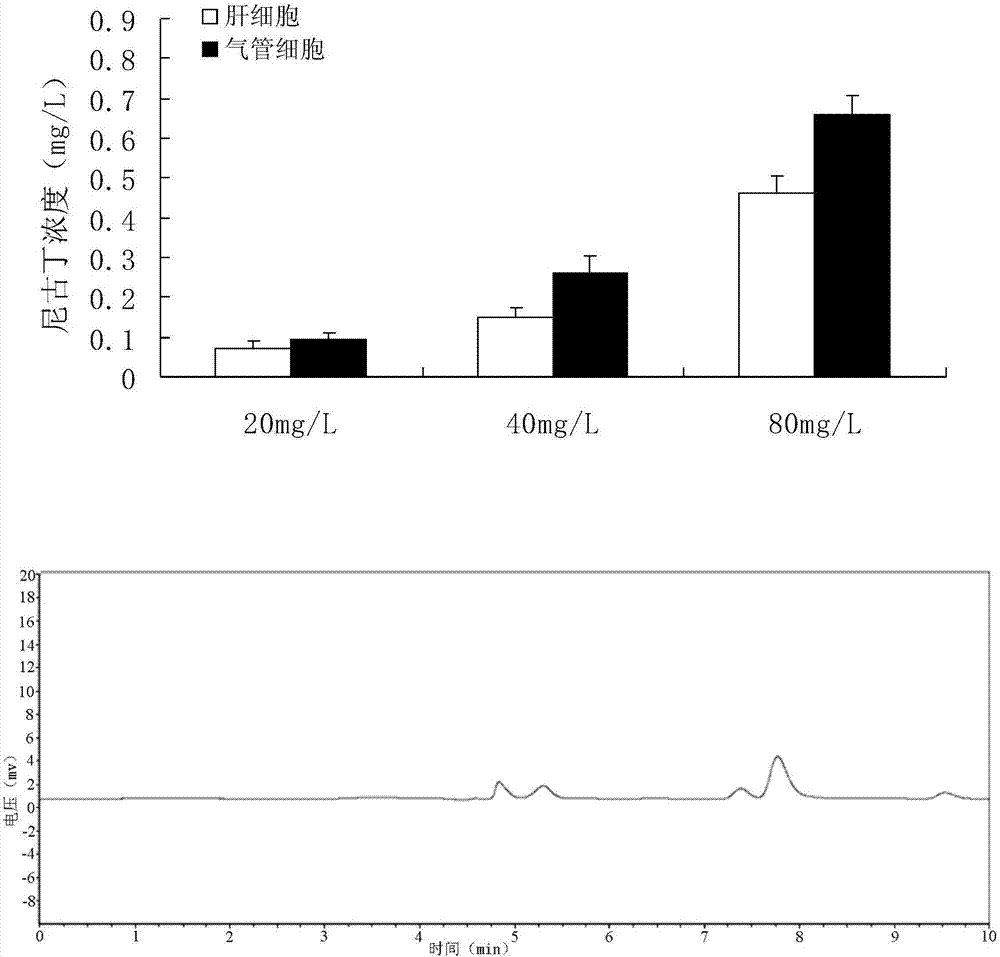
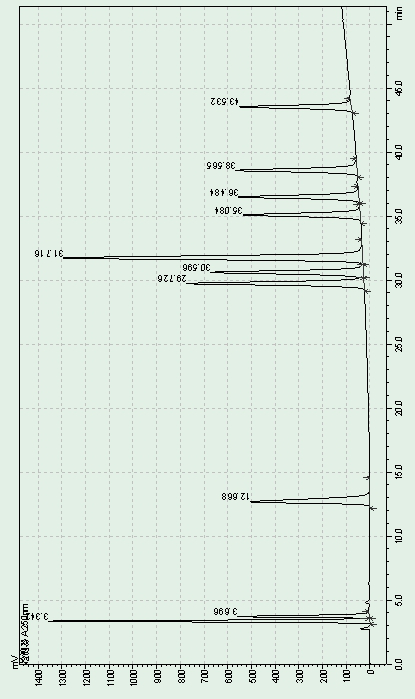
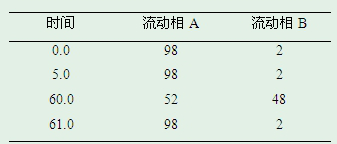
Quantitative detection method for nicotine in cell lysate
The invention discloses a quantitative detection method for nicotine in cell lysate. The quantitative detection method takes the cell lysate as an object to be detected; the cell lysate is prepared by adding a cell lysis solvent into cell sap / whole blood; the quantitative detection method sequentially comprises the following steps: adding a sodium hydroxide solution and ethyl ether into the cell lysate; after drying an obtained ethyl ether layer, re-dissolving and centrifuging to obtain supernatant I; carrying out chromatography treatment on the supernatant I to obtain supernatant II; carrying out high performance liquid chromatography system analysis on the supernatant II; substituting an obtained medicine peak area Y of the nicotine into a formula Y is equal to 74729X-354.61 and converting to obtain the concentration of the nicotine in the cell sap / whole blood to be detected.
Owner:CHINA JILIANG UNIV +1
A kind of detection method of capecitabine related substances
ActiveCN110398555BModerate retention timeEfficient separationComponent separationFluid phaseGradient elution
The invention discloses a detection method for capecitabine related substances: the method adopts high performance liquid chromatography for detection, and includes the following steps: Step 1. Prepare a capecitabine sample solution, the capecitabine The sample solution includes capecitabine test solution and capecitabine control solution; step 2, inject the sample solution obtained in step 1 into a high performance liquid chromatograph, and use mobile phase A and mobile phase B as mobile phases for gradient elution , and record the chromatogram. The detection method adopts a pentafluorophenyl bonded silica gel chromatographic column. The detection method is easy to operate, the retention time of the main peak of capecitabine is suitable, the defects of the prior art are overcome, the impurity D and the impurity E are effectively separated, other known impurities can be effectively detected, and the separation degree is high. It has strong specificity and accurate and reliable detection results, which provides a simple and reasonable detection method for the quality control and impurity research of capecitabine.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Quantitative detection method for tilmicosin residues in poultry meat, pork or poultry eggs
PendingCN113740450AMeet the Residue Testing RequirementsHigh recovery rateComponent separationChemistryPoultry meat
The invention relates to the field of veterinary drug residue detection, in particular to a quantitative detection method for tilmicosin residues in poultry meat, pork or poultry eggs. The method comprises the following steps of sequentially extracting the poultry meat, the pork or the poultry eggs by acetonitrile, degreasing by normal hexane, purifying an extracting solution by an HLB solid-phase extraction column, and blow-drying and concentrating an eluent by nitrogen; adding the pyridine and acetic anhydride into the concentrated and dried sample, filtering after derivative reaction, and detecting filtrate by GC-MS / MS detection. According to the present invention, the derivative product obtained by adopting the extraction method combining the liquid-liquid extraction, solid-phase extraction and gel chromatography is good in peak pattern and few in interference impurity peak, and the response value of the sample derivative product is high.
Owner:YANGZHOU UNIV
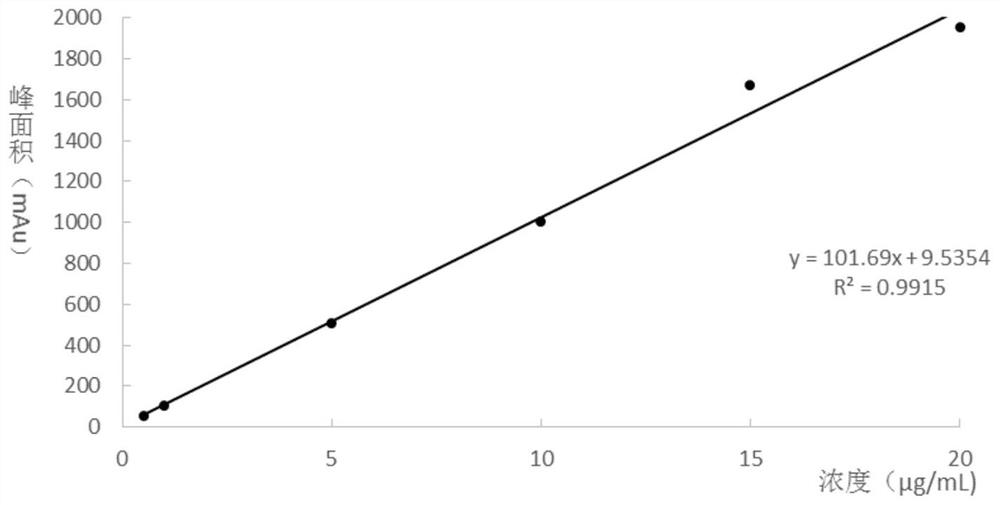
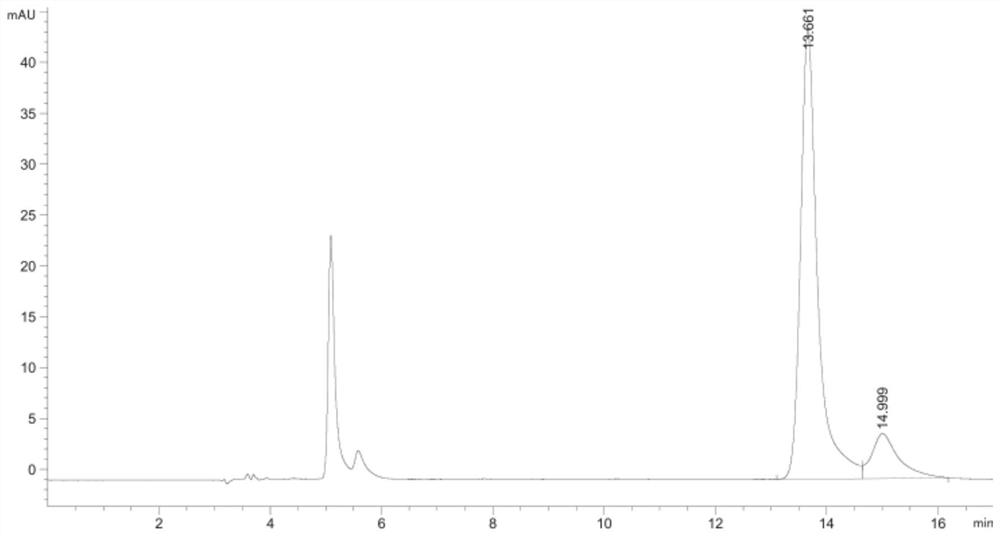
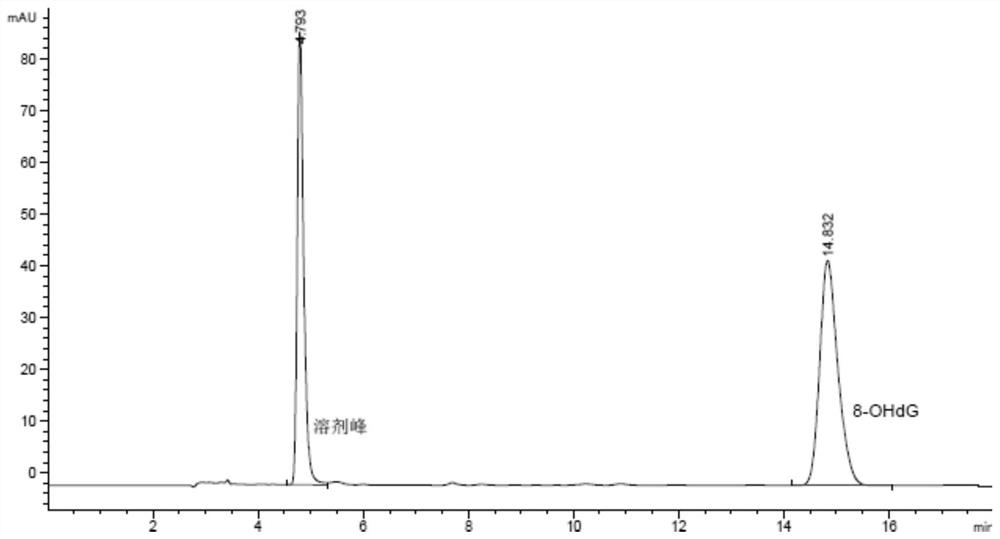
8-Hydroxydeoxyguanosine detection kit and its application
ActiveCN108732256BEasy to clogEliminate peak tailing phenomenonComponent separationFluid phaseTherapeutic effect
Owner:KEAISE MEDICINE WUHAN +1
Quality control method of traditional Chinese medicine composition for treating chronic renal failure
The invention relates to the technical field of a traditional Chinese medicine preparation, and particularly relates to a quality control method of a traditional Chinese medicine composition for treating chronic renal failure. The quality control method comprises the following steps: establishing a fingerprint spectrum of the traditional Chinese medicine composition by adopting a high performance liquid chromatography with the chromatographic condition of taking octadecyl silane as a filling agent, taking a 0.2% phosphoric acid solution as a mobile phase A and taking acetonitrile as a mobile phase B; preparing a test solution, namely, putting 0.1-1g of the traditional Chinese medicine composition for treating chronic renal failure into a 50ml of a centrifugal pipe, adding 5-20ml of ultrapure water, carrying out ultrasound treatment for 10-30 minutes, cooling, mixing evenly, then centrifuging, taking liquid supernatant, filtering by a 0.45micron of microporous filtering membrane, and taking subsequent filtrate to obtain a test solution; and determining, namely, sucking the test solution precisely, pouring into a liquid chromatograph, and recording a chromatogram. The quality control method is rapid, simple, and convenient; by adopting once extraction and simultaneous determining, the operation steps are reduced, the time and cost are saved, and thus the analysis efficiency is improved.
Owner:SHENZHEN TRADITIONAL CHINESE MEDICINE HOSPITAL
Rapid classification and identification of chemical components in Kudiezi injection based on uplc-q-tof-ms technology
ActiveCN104359968BEasy to separateSolve key problemsComponent separationMaterial analysis by electric/magnetic meansOrganic acidChemical composition
Based on UPLC‑Q‑TOF‑MS technology, the rapid classification and identification of chemical components in Kudiezi injection was realized, aiming to study flavonoids, organic acids, amino acids and nucleosides in Kudiezi injection. The UPLC‑Q‑TOF‑MS technology platform realizes the rapid classification and identification of chemical components in Kudiezi injection. In this study, the information of flavonoids, organic acids, amino acids and nucleosides in Kudiezi injection was firstly integrated, and the diagnostic fragments and neutral loss rules of these four categories of substances were found and summarized; at the same time, UPLC-Q ‑TOF‑MS technology performs mass spectrometry analysis on reference substances of different types of compounds to verify. Then, using the diagnostic fragmentation and neutral loss methods as screening and identification tools, a method for the rapid classification and identification of chemical components in Kudiezi injection was constructed.
Owner:TONGHUA HUAXIA PHARMA
Content determination method for anabasine
ActiveCN102590368BEasy to separateModerate retention timeComponent separationPlant ingredientsRational useContent determination
The invention provides a content determination method for anabasine, which is used for determining content of the anabasine in alangium chinense which is a traditional Chinese medicinal material. The content determination method includes firstly extracting the traditional Chinese medicinal material alangium chinense by means of ultraphonic, obtaining a sample solution, and determining the content of the alangium chinense by means of high performance liquid chromatography. The content determination method has the advantages of being easy, simple and fast to operate, high in specialization, precision degree and recovery rate, exact in determination results, and the like, the purpose of completing the quality standard of the alangium chinense is achieved, and experimental basis is provided for safe, effective and rational use of the alangium chinense.
Owner:北京创立科创医药技术开发有限公司
Establishment method of oyster powder fingerprint spectrum and fingerprint spectrum
ActiveCN112394117ACharacterizing qualityQuality improvementComponent separationBiotechnologyTest sample
The invention discloses an establishment method of an oyster powder fingerprint spectrum and the fingerprint spectrum. The establishment method of the fingerprint spectrum comprises the following steps of: 1, preparing a reference peak solution; 2, preparing an oyster powder test solution; 3, precisely sucking a certain amount of the reference peak solution and the test solution respectively, injecting the solutions into a liquid chromatograph, and recording a chromatogram; and 4, exporting the oyster powder fingerprint spectrum obtained in the step 3 from the instrument, importing the oysterpowder fingerprint spectrum into a traditional Chinese medicine chromatographic fingerprint similarity evaluation system, selecting chromatographic peaks existing in chromatograms of different batchesof oyster powder test samples as common peaks, generating a control fingerprint spectrum of the oyster powder by using an average value calculation method, and calculating the relative retention timeand the relative peak area of each common peak. The method for establishing a fingerprint spectrum has the advantages of simplicity, convenience, stability, high precision, good reproducibility and the like. The oyster powder fingerprint spectrum provided by the invention can comprehensively and objectively characterize the quality of oyster powder, and is beneficial to comprehensive monitoring of the quality of medicines.
Owner:QINGDAO NAT LAB FOR MARINE SCI & TECH DEV CENT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com