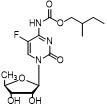
A kind of detection method of capecitabine related substances
A capecitabine and detection method technology, applied in measurement devices, instruments, scientific instruments and other directions, can solve problems such as difficulty in effective detection, impurity E cannot be effectively separated, etc., and achieves easy operation, accurate and reliable detection results. , the special effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
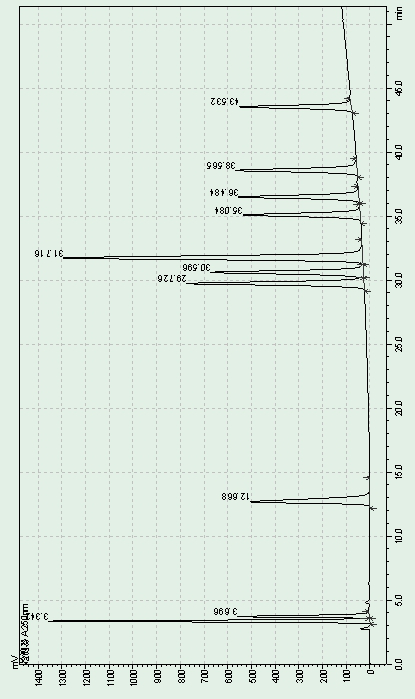
[0059] 1. Detection conditions
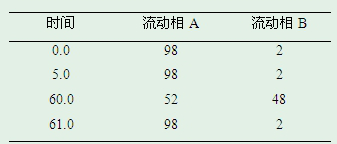
[0060] According to high performance liquid chromatography (Chinese Pharmacopoeia 2015 edition general rule 0512), Boltimate EXT-PFP was used, 2.7μm 4.6×250mm column was used, and methanol-acetonitrile-0.1% trifluoroacetic acid solution (4:1:15) was used as Mobile phase A; methanol-acetonitrile-0.1% trifluoroacetic acid solution (18:1:1) is mobile phase B, as follows:
[0061]
[0062] Gradient elution was performed, the flow rate was 1.0 mL per minute, the column temperature was 40°C, and the detection wavelength was 250 nm.
[0063] 2. Preparation of sample solution
[0064] Take an appropriate amount of capecitabine, dissolve and dilute it with a diluent (methanol:acetonitrile:water 4:1:15) to prepare a sample containing 0.3mg per 1ml, as the test solution.
[0065] Another appropriate amount of capecitabine reference substance was taken, dissolved with diluent and diluted to make a solution containing 0.15 μg per 1 mL, as the reference...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com